
DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-1
Course Title: Radiological Control Technician
Module Title: Physical Sciences
Module Number: 1.03
Objectives:
1.03.01 Define the following terms as they relate to physics:
a. Work
b. Force
c. Energy
1.03.02 Identify and describe four forms of energy.
1.03.03 State the Law of Conservation of Energy.
1.03.04 Distinguish between a solid, a liquid, and a gas in terms of shape and volume.
1.03.05 Identify the basic structure of the atom, including the characteristics of subatomic
particles.
1.03.06 Define the following terms:
a. Atomic number
b. Mass number
c. Atomic mass
d. Atomic weight
1.03.07 Identify what each symbol represents in the
A
Z
X notation.
1.03.08 State the mode of arrangement of the elements in the Periodic Table.
1.03.09 Identify periods and groups in the Periodic Table in terms of their layout.
1.03.10 Define the terms as they relate to atomic structure:
a. Valence shell
b. Valence electron
INTRODUCTION
This lesson introduces the RCT to the concepts of energy, work, and the physical states of
matter. Knowledge of these topics is important to the RCT as he or she works in environments
where materials can undergo changes in state, resulting in changes in the work environment.

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-2
References:
1. "Nuclides and Isotopes"; Fourteenth Edition, General Electric Company; 1989.
2. "Modern Physics"; Holt, Rinehart and Winston, Publishers; 1976.
3. "Chemistry: An Investigative Approach"; Houghton Mifflin Co., Boston; 1976.
4. "Chemical Principles with Qualitative Analysis"; Sixth ed.; Saunders College Pub.; 1986.
5. "Introduction to Chemistry" sixth ed., Dickson, T. R., John Wiley & Sons, Inc.; 1991.
6. "Matter"; Lapp, Ralph E., Life Science Library, Time Life Books; 1965.
7. "Physics"; Giancoli, Douglas C., second ed., Prentice Hall, Inc.; 1985.
8. DOE/HDBK-1015 "Chemistry: Volume 1 of 2"; DOE Fundamentals Handbook Series;
January 1993.

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-3
1.03.01 Define the following terms as they relate to physics:
a. Work
b. Force
c. Energy
WORK & FORCE
Physics is the branch of science that describes the properties, changes, and interactions of
energy and matter. This unit will serve as a brief introduction to some of the concepts of
physics as they apply to the situations that may be encountered by RCTs. Energy can be
understood by relating it to another physical concept
work.
The word work has a variety of meanings in everyday language. In physics, however, work is
specifically defined as a force acting through a distance. Simply put, a force is a push or a
pull. A more technical definition of force is any action on an object that can cause the
object to change speed or direction.
Units
Force is derived as the product of mass and acceleration (see equation below). The SI
derived unit of force is the newton (N). It is defined as the force which, when applied to a
body having a mass of one kilogram, gives it an acceleration of one meter per second
squared; that is:
N
kg × m
s
2
As we said before, work is what is accomplished by the action of a force when it makes
an object move through a distance. Mathematically, work is expressed as the product of a
displacement and the force in the direction of the displacement; that is:
W = Fd
where: W = Work
F = Force (newtons)
d = Distance (meters)

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-4
1.03.02 Identify and describe four forms of energy.
E
K
1
2
mv
2
For example, a horse works by exerting a physical force (muscle movement) to move a
carriage. As the horse pulls, the carriage moves forward in the direction that the horse is
pulling. Work is also done by an outside force (energy) to remove an electron from its
orbit around the nucleus of an atom.
The SI derived unit of work is the joule (J). One joule of work is performed when a force
of one newton is exerted through a distance of one meter. Thus:
J N × m
By this definition, work can only be performed when the force causes an object to be
moved. This means that if the distance is zero then no work has been performed, even
though a force has been applied. For example, if you stand at rest holding a bag of
groceries in your hands, you do no work on it; your arms may become tired (and indeed
energy is being expended by your muscles), but because the bag is not moved through a
distance (d = 0), no work is performed (W = 0).
ENERGY
Energy (E) is defined as the ability to do work. Energy and work are closely related, but they
are not the same thing. The relationship is that it takes energy to do work, and work can
generate energy. This energy will be found in various forms.
Kinetic Energy
Kinetic energy describes the energy of motion an object possesses. For example, a
moving airplane possesses kinetic energy.
where: m = mass
v = velocity

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-5
1.03.03 State the Law of Conservation of Energy.
Potential Energy
Potential energy (gravitational) indicates how much energy is stored as a result of the
position or the configuration of an object. For example, water at the top of a waterfall
possesses potential energy.
E
P
mgh
where: m = mass
g = free fall acceleration
h = vertical distance
Thermal Energy
Thermal energy, or heat, describes the energy that results from the random motion of
molecules. (Molecules are groups of atoms held together by strong forces called
chemical bonds.) For example, steam possesses thermal energy.
Chemical Energy
Chemical energy describes the energy that is derived from atomic and molecular
interactions in which new substances are produced. For example, the substances in a
dry cell provide energy when they react.
Other Forms of Energy
Other forms of energy, such as electrical and nuclear, will be described in later lessons.
Energy may also appear as acoustical (sound) or radiant (light) energy.
Law of Conservation of Energy
The Law of Conservation of Energy states that the total amount of energy in a closed
system remains unchanged. Stated in other terms, as long as no energy enters or leaves
the system, the amount of energy in the system will always be the same, although it can
be converted from one form to another.
For example, suppose a boulder lies at the bottom of a hill and bulldozer is used to push it
to the top. If the dozer puts a certain continuous force on the boulder to keep it moving

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-6
up the slope and moves it a distance, work has been done. The dozer is able to do this
work because its engine burns gasoline, creates heat. The heat is converted into the
kinetic energy of the moving bulldozer and the boulder in front of it. Some of this energy
is converted into heat and noise. Some is converted into the potential energy that the
dozer and the boulder have gained in going to the top of the hill. If the boulder is allowed
to roll back down the hill again, its potential energy will be converted partly into kinetic
energy and partly into heat. The heat is produced by friction as the boulder rolls.
Eventually the boulder will come to a stop, when all of its kinetic energy has been
converted into heat. It leaves a trail of heat that is soaked up in the surroundings.
Gasoline contains chemical energy that is released in the form of heat when a chemical
reaction (burning) with oxygen occurs. This energy comes from the breaking and making
of bonds between atoms. New products, carbon dioxide and water, are formed as the
gasoline combines with oxygen. The energy of the burning gasoline produces heat energy
which causes the gaseous combustion products to do work on the pistons in the engine.
The work results in the bulldozer moving, giving it kinetic energy.
Units of Energy
Energy is expressed in the same units as work, that is, joules (J). The joule is the SI unit
of energy. However, because energy can take on many different forms, it is sometimes
measured in other units which can be converted to joules. Some of these units are
mentioned below.
Thermal Energy
Thermal energy is often measured in units of calories (CGS) or British Thermal
Units or BTUs (English).
•A calorie is the amount of heat needed to raise the temperature of 1 gram
of water by 1 EC. One calorie is equal to 4.18605 joules.
•A BTU is the amount of heat needed to raise the temperature of 1 pound
of water by 1 EF. One BTU is equal to 1.055E3 joules.
Electrical Energy
Electrical energy is sometimes expressed in units of kilowatt-hours. One kw-hr is
equal to 3.6E6 joules
A very small unit used to describe the energy of atomic and subatomic size
particles is the electron volt (eV). One electron volt is the amount of energy
acquired by an electron when it moves through a potential of one volt. For
example, it takes about 15.8 eV of energy to remove an electron from an argon

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-7
Figure 1. Energy Conversion in an Automobile
atom. Superunits such as kiloelectron volt (keV) and megaelectron volt (MeV)
are used to indicate the energies of various ionizing radiations.
Work-Energy Relationship
When work is done by a system or object, it expends energy. For example, when the
gaseous combustion products in an automobile engine push against the pistons, the gas
loses energy. The chemical energy stored in the gasoline is used to do work so that the
car will move.
When work is done on a system or object, it acquires energy. The work done on the car
by the combustion of the gasoline causes the car to move, giving it more kinetic energy.
When energy is converted to work or changed into another form of energy, the total
amount of energy remains constant. Although it may appear that an energy loss has
occurred, all of the original energy can be accounted for.
Consider again the automobile engine. The energy stored in the gasoline is converted to
heat energy, some of which is eventually converted to kinetic energy. The remainder of
the heat energy is removed by the engine's cooling system. The motion of the engine
parts creates friction, heat energy, which is also removed by the engine's cooling system.
As the car travels, it encounters resistance with the air. If no acceleration occurs, the car
will slow down as the kinetic energy is converted to friction or heat energy. The contact
of the tires on the road converts some of the available kinetic energy to heat energy
(friction), slowing down the car. A significant amount of the energy stored in the
gasoline is dissipated as wasted heat energy.

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-8
1.03.04 Distinguish between a solid, a liquid, and a gas in terms of shape and volume.
Energy-mass relationship
Energy can also be converted into mass and mass converted into energy. This will be
discussed further in section 1.04 "Nuclear Sciences."
ENERGY AND CHANGE OF STATE
Matter is anything that has mass and takes up space. All matter is made up of atoms and
molecules which are the building blocks used to form all kinds of different substances. These
atoms and molecules are in constant random motion. Because of this motion they have
thermal energy. The amount of energy depends on the temperature and determines the state or
phase of the substance. There are three states of matter
solid, liquid and gas.
Any substance can exist in any of the three states, but there is generally one state which
predominates under normal conditions (temperature and pressure). Take water, for example.
At normal temperatures, water is in the liquid state. In the solid state, water is called ice. The
gaseous state of water is called steam or water vapor. It's all still water
just in different states.
Table 1 provides a summary of these three states in terms of shape and volume.
Table 1. States of Matter Compared
State Shape Volume
Solid definite definite
Liquid indefinite definite
Gas indefinite indefinite

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-9
Gas
Liquid
Solid
Figure 2. States of Matter
Solid State
A solid has definite shape and volume. The solid state differs from the liquid and
gaseous states in that:
• The molecules or ions of a solid are held in place by strong attractive forces.
• The molecules have thermal energy, but the energy is not sufficient to overcome
the attractive forces.
• The molecules of a solid are arranged in an orderly, fixed pattern.
The rigid arrangement of molecules causes the solid to have a definite shape and a
definite volume.
Liquid State
When heat is added to a substance, the molecules acquire more energy, which causes
them to break free of their fixed crystalline arrangement. As a solid is heated, its
temperature rises until the change of state from solid to liquid occurs.
The volume of a liquid is definite since the molecules are very close to each other, with
almost no space in between. Consequently, liquids can undergo a negligible amount of
compression. However, the attractive forces between the molecules are not strong
enough to hold the liquid in a definite shape. For this reason a liquid takes the shape of
its container.
High energy molecules near the surface of a liquid can overcome the attractive forces of
other molecules. These molecules transfer from the liquid state to the gaseous state. If

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-10
1.03.05 Identify the basic structure of the atom, including the characteristics of subatomic
particles.
+
Nucleus
Figure 3. Atomic Model
energy (heat) is removed from the liquid, the kinetic energy of the molecules decreases
and the attractive forces can hold the molecules in fixed positions. When compared with
the kinetic energy, the attractive forces are not strong enough to hold the molecules in
fixed positions, forming a solid.
Gaseous State
If the temperature of a liquid is increased sufficiently, it boils
that is, molecules change to
the gaseous state and escape from the surface. Eventually, all of the liquid will become a
gas. A gas has both indefinite shape and indefinite volume. A large space exists between
gas molecules because of their high thermal energy. This allows for even more
compression of a substance in the gaseous state.
THE ATOM
The Bohr Model
As stated previously, the fundamental building block
of matter is the atom. The basic atomic model, as
described by Ernest Rutherford and Niels Bohr in
1911, consists of a positively charged core surrounded
by negatively-charged shells. The central core, called
the nucleus, contains protons and neutrons. Nuclear
forces hold the nucleus together. The shells are
formed by electrons which exist in structured orbits
around the nucleus. Below is a summary of the three
primary subatomic particles which are the constituent
parts of the atom.

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-11
Protons
• Positively charged (+1)
• Mass: 1.6726E-24 gm or 1.007276470 amu
• Each element is determined by the number of protons in its nucleus. All atoms of the
same element have the same number of protons.
Neutrons
• Neutrally charged (0)
• Mass: 1.6749E-24 gm or 1.008665012 amu
• The number of neutrons determines the isotope of an element. Isotopes are atoms which
have the same number of protons (therefore, of the same element) but different number of
neutrons. This does not affect the chemical properties of the element.
Electrons
• Negatively charged (-1)
• Small mass: 9.1085E-28 gm or 0.00054858026 amu (. 1/1840 of a proton)
Because the mass of an electron is so small as compared to that of a proton or neutron,
virtually the entire mass of an atom is furnished by the nucleus.
• The number of electrons is normally equal to the number of protons. Therefore, the atom
is electrically neutral.
• The number of electrons in the outermost shell determines the chemical behavior or
properties of the atom.
THE ELEMENTS
Even though all atoms have the same basic structure, not all atoms are the same. There are
over a hundred different types of atoms. These different types of atoms are known as
elements. The atoms of a given element are alike but have different properties than the atoms
of other elements.
Elements are the simplest forms of matter. They can exist alone or in various combinations.
Different elements can chemically combine to form molecules or molecular compounds. For
example, water is a compound, consisting of water molecules. These molecules can be

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-12
decomposed into the elements hydrogen and oxygen. The elements hydrogen and oxygen are
fundamental forms of matter. They cannot be further separated into simpler chemicals.
Chemical Names
Currently, there are 109 named elements. Table 2 lists the elements and their symbols.
Some have been known for many centuries, while others have only been discovered in the
last 15 or 20 years. Each element has a unique name. The names of the elements have a
variety of origins. Some elements were named for their color or other physical
characteristics. Others were named after persons, places, planets or mythological figures.
For example, the name chromium comes from the Greek word chroma, which means
"color." Chromium is found naturally in compounds used as pigments. The elements
curium, einsteinium, and fermium were named after famous nuclear physicists.
Germanium, polonium and americium, were named after countries. Uranium, neptunium
and plutonium are named in sequence for the three planets Uranus, Neptune and Pluto.
Chemical Symbols
For convenience, elements have a symbol which is used as a shorthand for writing the
names of elements. The symbol for an element is either one or two letters taken from the
name of the element (see Table 2). Note that some have symbols that are based on the
historical name of the element. For example, the symbols for silver and gold are Ag and
Au respectively. These come from the old Latin names argentum and aurum. The
symbol for mercury, Hg, comes from the Greek hydrargyros which means "liquid silver."

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-13
Table 2. List of Elements by Name
Element Symbol Z Element Symbol Z Element Symbol Z
Actinium Ac 89 Hafnium Hf 72 Promethium Pm 61
Aluminum Al 13 Hassium Hs 108 Protactinium Pa 91
Americium Am 95 Helium He 2 Radium Ra 88
Antimony Sb 51 Holmium Ho 67 Radon Rn 86
Argon Ar 18 Hydrogen H 1 Rhenium Re 75
Arsenic As 33 Indium In 49 Rhodium Rh 45
Astatine At 85 Iodine I 53 Rubidium Rb 37
Barium Ba 56 Iridium Ir 77 Ruthenium Ru 44
Berkelium Bk 97 Iron Fe 26 Rutherfordium Rf 104
Beryllium Be 4 Krypton Kr 36 Samarium Sm 62
Bismuth Bi 83 Lanthanum La 57 Scandium Sc 21
Bohrium Bh 107 Lawrencium Lw 103 Seaborgium Sg 106
Boron B 5 Lead Pb 82 Selenium Se 34
Bromine Br 35 Lithium Li 3 Silicon Si 14
Cadmium Cd 48 Lutetium Lu 71 Silver Ag 47
Calcium Ca 20 Magnesium Mg 12 Sodium Na 11
Californium Cf 98 Manganese Mn 25 Strontium Sr 38
Carbon C 6 Meitnerium Mt 109 Sulfur S 16
Cerium Ce 58 Mendelevium Md 101 Tantalum Ta 73
Cesium Cs 55 Mercury Hg 80 Technetium Tc 43
Chlorine Cl 17 Molybdenum Mo 42 Tellurium Te 52
Chromium Cr 24 Neodymium Nd 60 Terbium Tb 65
Cobalt Co 27 Neon Ne 10 Thallium Tl 81
Copper Cu 29 Neptunium Np 93 Thorium Th 90
Curium Cm 96 Nickel Ni 28 Thulium Tm 69
Dubnium Db 105 Niobium Nb 41 Tin Sn 50
Dysprosium Dy 66 Nitrogen N 7 Titanium Ti 22
Einsteinium Es 99 Nobelium No 102 Tungsten W 74
Erbium Er 68 Osmium Os 76 Uranium U 92
Europium Eu 63 Oxygen O 8 Vanadium V 23
Fermium Fm 100 Palladium Pd 46 Xenon Xe 54
Fluorine F 9 Phosphorus P 15 Ytterbium Yb 70
Francium Fr 87 Platinum Pt 78 Yttrium Y 39
Gadolinium Gd 64 Plutonium Pu 94 Zinc Zn 30
Gallium Ga 31 Polonium Po 84 Zirconium Zr 40
Germanium Ge 32 Potassium K 19
Gold Au 79 Praseodymium Pr 59

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-14
1.03.06 Define the following terms:
a. Atomic number
b. Mass number
c. Atomic mass
d. Atomic weight
Atomic Number
The number of protons in the nucleus of an element is called the atomic number. All
atoms of a particular element have the same atomic number. Atomic numbers are
integers. For example, a hydrogen atom has one proton in the nucleus. Therefore, the
atomic number of hydrogen is 1. A helium atom has two protons in the nucleus, which
means that its atomic number is 2. Uranium has 92 protons in the nucleus and, therefore,
has an atomic number of 92. Atomic number is often represented by the symbol Z.
Mass Number
The total number of protons plus neutrons in the nucleus of a particular isotope of an
element is called the mass number. It is the integer nearest to the mass of the atom of
concern. Since a proton has a mass of 1.0073 amu, we will give a proton a mass number
of 1. The mass number of a neutron would also be 1, since its mass is 1.0087 amu. So,
by adding the number of protons and the number of neutrons we can determine the mass
number of the atom of concern.
For example, a normal hydrogen atom has 1 proton, but no neutrons. Therefore, its mass
number is 1. A helium atom has 2 protons and 2 neutrons, which means that it has a mass
number of 4. If a uranium isotope has 146 neutrons then it has a mass number of 238 (92
+ 146), while if it only has 143 neutrons its mass number would be 235.
The mass number can be used with the name of the element to identify which isotope of
an element we are referring to. If we are referring to the isotope of uranium that has a
mass number of 238, we can write it as Uranium-238. If we are referring to the isotope of
mass number 235, we write it as Uranium-235. Often, this expression is shortened by
using the chemical symbol instead of the full name of the element, as in U-238 or U-235.
Atomic Mass
The actual mass of an atom of a particular isotope is called its atomic mass. The units
are expressed in Atomic Mass Units (AMU). AMUs are based on 1/12 of the mass of a
Carbon-12 atom (1.660E-24 gm). In other words, the mass of one C-12 atom is exactly
12 amu.

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-15
1.03.07 Identify what each symbol represents in the
A
Z
X notation.
For example, the mass of a hydrogen atom is 1.007825 amu (1 proton + 1 electron =
1.00727647 + 0.00054858026) . The mass of a Uranium-238 atom in amu is 238.0508,
while the mass of a U-235 atom is only 235.0439. Notice that atomic masses are very
accurate and are written as decimals.
Atomic Weight
The weighted average of the isotopic masses of an element, based on the percent
abundance of its naturally occurring isotopes, is called the atomic weight. The atomic
weight is expressed in AMU and is used mainly in calculations of chemical reactions.
Since AMUs are based on Carbon-12, one may wonder why the Periodic Table (see
Figure 5) shows the atomic weight of Carbon as 12.011, and not exactly 12. The
explanation is simple and will help to clarify the difference between the atomic weight of
an element and the atomic mass of an isotope of that element.
Carbon, as it occurs in nature, is a mixture of two isotopes: about 98.9% of all carbon
atoms are C-12, while the abundance of C-13 atoms is 1.1% (a total of 100%). The
presence of these heavier Carbon atoms explains why the atomic weight of carbon is
slightly more than 12. The atomic weight of an element is a "weighted average" (no pun
intended). This average is determined by finding the sum of the mass of each isotope
multiplied by its percent abundance. If the atomic mass of C-12 is 12.00, and the atomic
mass of C-13 is 13.00, we can determine the atomic weight of carbon:
12.00(0.989) + 13.00(0.011) = 11.868 + 0.143 = 12.011 amu
With the understanding of these concepts, we can discuss the Periodic Table of the
Elements and the information it provides.
NUCLIDE NOTATION
The format for representing a specific combination of protons and neutrons is to use its nuclear
symbol. This is done by using the standard chemical symbol, with the atomic number written
as a subscript at the lower left of the symbol, and the mass number written as a superscript at
the upper left of the symbol:

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-16
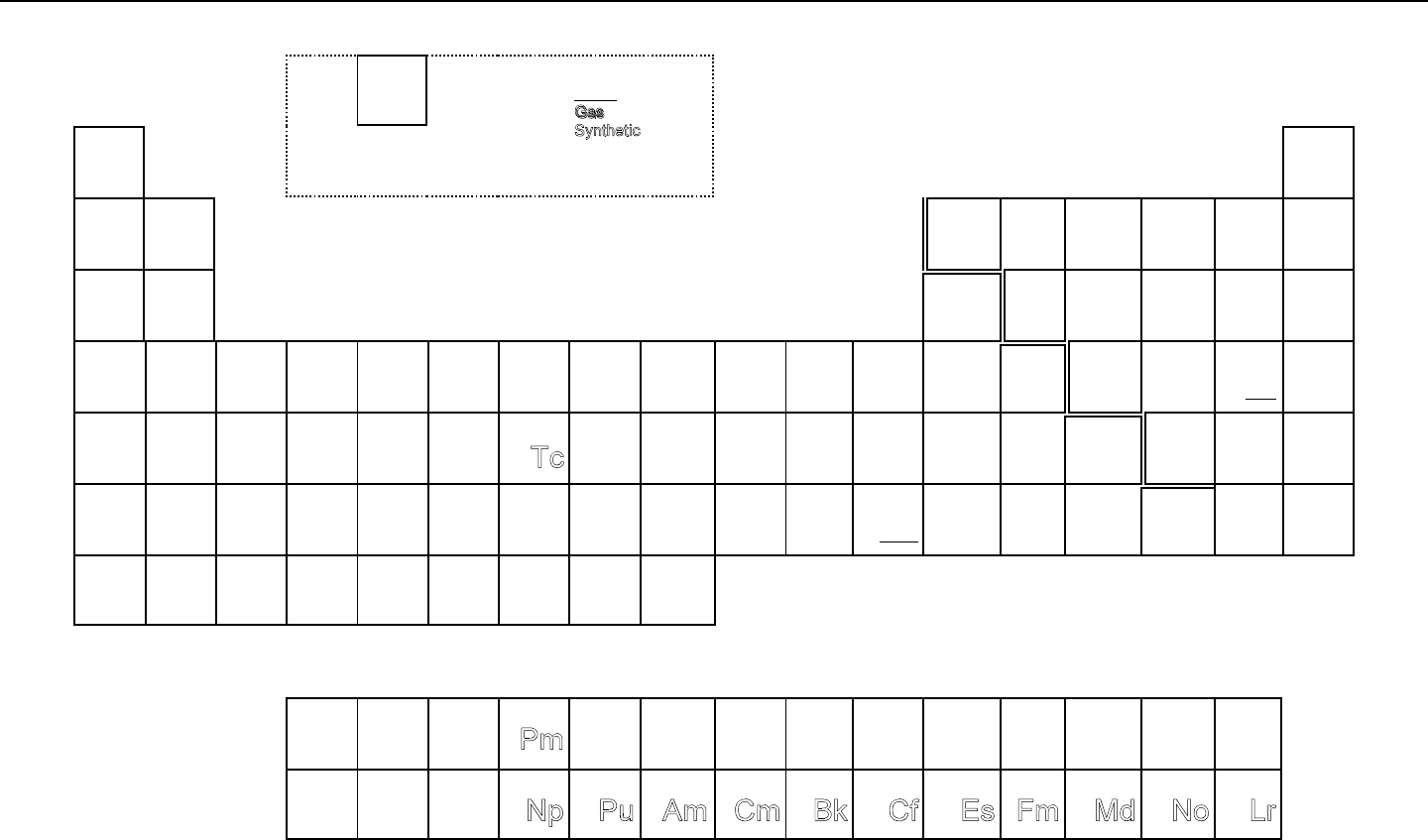
1.03.08 State the mode of arrangement of the elements in the Periodic Table.
1.03.09 Identify periods and groups in the Periodic Table in terms of their layout.
A
Z
X
where: X = Symbol for element
Z = Atomic number: number of protons
A = Mass number: number of protons (Z) plus number of neutrons (N);
therefore: A = Z + N
For example, the notation for Uranium-238 would be
23
9
8
2
U.
MODERN PERIODIC TABLE
The modern Periodic Table (see Figure 5) is an arrangement of the elements in order of
increasing atomic number. A comparison of the properties for selected elements will
illustrate that there is a predictable, recurring pattern, or periodicity. This observation is
summarized in the Periodic Law, which states that the properties of the elements are repetitive
or recurring functions of their atomic numbers.
Data about each element in the Periodic Table are presented in a column and row format. The
rows or horizontal sections in the Periodic Table are called periods. The columns or
vertical sections are called groups or families because they "behave" chemically similar; that
is they have similar chemical properties.
Since the number of electrons is equal to the number of protons, the structure of the Periodic
Table directly relates to the number and arrangement of electrons in the atom (see Table
3). Figure 4 below gives a simple illustration of the electron shells described in the Bohr
model of the atom.
Electrons orbit around the nucleus in structured shells, designated sequentially as 1 through 7
(K through Q) from inside out. Shells represent groups of energy states called orbitals. The
higher the energy of the orbital the greater the distance from the nucleus. The lowest energy
state is in the innermost shell (K).
The number of orbitals in a shell is the square of the shell number (n). The maximum number
of electrons which can occupy an orbital is 2. Therefore, each shell can hold a maximum of
2n
2
electrons. For example, for the L shell the maximum number of electrons would be 8:

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-17
Figure 4. Electron Shells
L-shell: n = 2 YYY 2(2
2
) = 8
DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-18
Figure 5. Periodic Table of the Elements
Atomic
Weight
83.80
36 Kr
Symbol
Solid
Liquid
NOBLE
GASES
1.0080
1
H
H
IIA
Atomic
Number
IIIB IVB VB VIB
4.00260
He
He
6.941
3 Li
9.01218
4 Be
Metalloid
Line
10.81
5 B
12.011
6 C
14.0067
7
N
N
15.9994
8
O
O
18.9984
9
F
F
20.179
10
Ne
Ne
22.98977
11 Na
24.305
12 Mg
IIIA IVA VA VIA VIIA
VIIIA
IB IIB
26.98154
13 Al
28.0855
14 Si
30.97376
15 P
32.06
16 S
32.06
17
Cl
Cl
39.948
18
Ar
Ar
39.0983
19 K
40.08
20 Ca
41.9559
21 Sc
47.90
22 Ti
50.9415
23 V
51.996
24 Cr
54.9380
25 Mn
55.847
26 Fe
58.933
27 Co
58.70
28 Ni
63.546
29 Cu
65.38
30 Zn
69.72
31 Ga
72.59
32Ge
74.9216
33 As
78.96
34 Se
79.904
35 Br
83.80
36
Kr
Kr
85.4678
37 Rb
87.62
38 Sr
88.9059
39 Y
91.22
40 Zr
92.9064
41 Nb
95.94
42 Mo
(98)
43
101.07
44 Ru
102.905
45 Rh
106.4
46 Pd
107.868
47 Ag
112.41
48 Cd
114.82
49 In
118.69
50Sn
121.75
51 Sb
127.60
52 Te
126.9045
53 I
131.30
54
Xe
Xe
132.9054
55 Cs
137.33
56 Ba
138.9055
57 La
\
178.49
72 Hf
180.9479
73 Ta
183.85
74 W
186.207
75 Re
190.2
76 Os
192.22
77 Ir
195.09
78 Pt
196.966
79 Au
200.59
80 Hg
204.37
81 Tl
207.2
82Pb
208.9804
83 Bi
(209)
84 Po
(210)
85 At
(222)
86
Rn
Rn
(223)
87 Fr
226.0254
88 Ra
227.0278
89 Ac
\\
(261)
104 Rf
(262)
105Db
(263)
106Sg
(264)
107Bh
(265)
108Hs
(266)
109Mt
\
140.12
58 Ce
140.9077
59 Pr
144.24
60 Nd
(145)
61
150.4
62Sm
151.96
63 Eu
157.25
64 Gd
158.925
65 Tb
162.50
66 Dy
164.9304
67 Ho
167.26
68 Er
168.9342
69 Tm
173.04
70 Yb
174.967
71 Lu
\\
232.0381
90 Th
231.0359
91 Pa
238.029
92 U
237.0482
93
(244)
94
(243)
95
(247)
96
(247)
97
(251)
98
(252)
99
(257)
100
(258)
101
(259)
102
(260)
103

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-19
Table 3. Electron Configuration of the Elements
Z Element K L M N O Z Element K L M N O P Q
1 Hydrogen 1 55 Cesium 2 8 18 18 8 1
2 Helium 2 56 Barium 2 8 18 18 8 2
3 Lithium 2 1 57 Lanthanum 2 8 18 18 9 2
4 Beryllium 2 2 58 Cerium 2 8 18 20 8 2
5 Boron 2 3 59 Praseodymium 2 8 18 21 8 2
6 Carbon 2 4 60 Neodymium 2 8 18 22 8 2
7 Nitrogen 2 5 61 Promethium 2 8 18 23 8 2
8 Oxygen 2 6 62 Samarium 2 8 18 24 8 2
9 Fluorine 2 7 63 Europium 2 8 18 25 8 2
10 Neon 2 8 64 Gadolinium 2 8 18 25 9 2
11 Sodium 2 8 1 65 Terbium 2 8 18 27 8 2
12 Magnesium 2 8 2 66 Dysprosium 2 8 18 28 8 2
13 Aluminum 2 8 3 67 Holmium 2 8 18 29 8 2
14 Silicon 2 8 4 68 Erbium 2 8 18 30 8 2
15 Phosphorus 2 8 5 69 Thulium 2 8 18 31 8 2
16 Sulfur 2 8 6 70 Ytterbium 2 8 18 32 8 2
17 Chlorine 2 8 7 71 Lutetium 2 8 18 32 9 2
18 Argon 2 8 8 72 Hafnium 2 8 18 32 10 2
19 Potassium 2 8 8 1 73 Tantalum 2 8 18 32 11 2
20 Calcium 2 8 8 2 74 Tungsten 2 8 18 32 12 2
21 Scandium 2 8 9 2 75 Rhenium 2 8 18 32 13 2
22 Titanium 2 8 10 2 76 Osmium 2 8 18 32 14 2
23 Vanadium 2 8 11 2 77 Iridium 2 8 18 32 15 2
24 Chromium 2 8 13 1 78 Platinum 2 8 18 32 16 2
25 Manganese 2 8 13 2 79 Gold 2 8 18 32 18 1
26 Iron 2 8 14 2 80 Mercury 2 8 18 32 18 2
27 Cobalt 2 8 15 2 81 Thallium 2 8 18 32 18 3
28 Nickel 2 8 16 2 82 Lead 2 8 18 32 18 4
29 Copper 2 8 18 1 83 Bismuth 2 8 18 32 18 5
30 Zinc 2 8 18 2 84 Polonium 2 8 18 32 18 6
31 Gallium 2 8 18 3 85 Astatine 2 8 18 32 18 7
32 Germanium 2 8 18 4 86 Radon 2 8 18 32 18 8
33 Arsenic 2 8 18 5 87 Francium 2 8 18 32 18 8 1
34 Selenium 2 8 18 6 88 Radium 2 8 18 32 18 8 2
35 Bromine 2 8 18 7 89 Actinium 2 8 18 32 18 9 2
36 Krypton 2 8 18 8 90 Thorium 2 8 18 32 18 10 2
37 Rubidium 2 8 18 8 1 91 Protactinium 2 8 18 32 20 9 2
38 Strontium 2 8 18 8 2 92 Uranium 2 8 18 32 21 9 2
39 Yttrium 2 8 18 9 2 93 Neptunium 2 8 18 32 22 9 2
40 Zirconium 2 8 18 10 2 94 Plutonium 2 8 18 32 24 8 2
41 Niobium 2 8 18 12 1 95 Americium 2 8 18 32 25 8 2
42 Molybdenum 2 8 18 13 1 96 Curium 2 8 18 32 25 9 2
43 Technetium 2 8 18 13 2 97 Berkelium 2 8 18 32 27 8 2
44 Ruthenium 2 8 18 15 1 98 Californium 2 8 18 32 28 8 2
45 Rhodium 2 8 18 16 1 99 Einsteinium 2 8 18 32 29 8 2
46 Palladium 2 8 18 18 0 100 Fermium 2 8 18 32 30 8 2
47 Silver 2 8 18 18 1 101 Mendelevium 2 8 18 32 31 8 2
48 Cadmium 2 8 18 18 2 102 Nobelium 2 8 18 32 32 8 2
49 Indium 2 8 18 18 3 103 Lawrencium 2 8 18 32 32 9 2
50 Tin 2 8 18 18 4 104 Rutherfordium 2 8 18 32 32 10 2
51 Antimony 2 8 18 18 5 105 Dubnium 2 8 18 32 32 11 2
52 Tellurium 2 8 18 18 6 106 Seaborgium 2 8 18 32 32 12 2
53 Iodine 2 8 18 18 7 107 Bohrium 2 8 18 32 32 13 2
54 Xenon 2 8 18 18 8 109 Meitnerium 2 8 18 32 32 15 2

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-20
1.03.10 Define the terms as they relate to atomic structure:
a. Valence shell
b. Valence electron
The highest occupied energy level in a ground-state atom is called its valence shell. Therefore,
the electrons contained in it are called valence electrons. The rows or periods in the Periodic
Table correspond to the electron shells. The elements contained in first period have their
valence electrons in the first energy level or K-shell. The elements contained in the second
period have their outer or valence shell electrons in the second energy level or L-shell, and so
on. The pattern continues down the table.
The number of electrons in the valence shell determines the chemical properties or "behavior"
of the atom. The valence shell can have a maximum of eight electrons, except for the K-shell
which can only have two. Atoms are chemically stable when the valence shell has no
vacancies; that is, they "prefer" to have a full valence shell. Atoms of elements toward the
right of the Periodic Table seem to lack only one or two electrons. These will "look" for ways
to gain electrons in order to fill their valence shell. Atoms of elements on the left side of the
table seem to have an excess of one or two electrons. These will tend to find ways to lose
these excess electrons so that the full lower shell will be the valence shell.
The outcome is that certain atoms will combine with other atoms in order to fill their valence
shells. This combination that occurs is called a chemical bond, and results in the formation of
a molecule. The bond is accomplished by "sharing" or "giving up" valence electrons, thus
forming a molecule whose chemical properties are different than those of the individual
element atoms.
A good example is table salt. Salt is a 1:1 combination of sodium and chlorine; that is, a salt
molecule is formed when one sodium atom bonds with one chlorine atom. If we look at Table
3, we can see that sodium (Na) has 1 electron in its outermost shell. Chlorine (Cl) needs one
electron to complete its valence shell. The sodium atom "gives up" its extra electron to the
chlorine atom who then "thinks" that its valence shell is full. Because the sodium atom has
one less electron, the atom now has a net positive charge; that is, it has one less electron than it
has protons. The chlorine atom now has a net negative charge because it has one more
electron than it has protons. The opposite charges of the two ions attract and form an ionic
bond. The bond results in a sodium chloride molecule (NaCl). However, this is just one type
of chemical bond between atoms. There are several other types of chemical bonds that can
occur, but which are beyond the scope of this lesson.
Note the rightmost column in the Periodic Table. These elements are known as the noble or
inert gases because they all have a full valence shell (see also the underlined elements in Table
3). This means that they "feel" no need to bond with other atoms. Noble gases are thus
considered chemically inert and very rarely interact with other elements.

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-21
The Quantum Mechanical Model
Over the years, the Bohr model of the atom was found to be inadequate as the principles
of quantum mechanics evolved. A newer model, known as the quantum mechanical
model, describes the electrons arranged in energy levels corresponding to the "electron
shells" of the Bohr model. In the quantum mechanical model the electron is not viewed
as particle in a specific orbit, but rather as an electron cloud in which the negative charge
of the electron is spread out within the cloud. These energy levels are referred to as
orbitals to emphasize that these are not circular "orbits" like those of the Bohr model but
rather electron clouds. An electron cloud is a representation of the volume about the
nucleus in which an electron of a specific energy is likely to be found.
The quantum mechanical model further states that the energy levels are subdivided into
sublevels, referred to by the letters s, p, d, f, etc. An energy level can contain one or more
sublevels or orbitals, and a maximum of two electrons can reside in each sublevel. For
example, the first energy level contains one s sublevel which can accommodate a
maximum of two electrons.

DOE-HDBK-1122-99
Module 1.03 Physical Sciences Study Guide
1.03-22
This page intentionally left blank.
