
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP
Page 2 of 168
REVISION HISTORY
Guidance Version (Publish Date)
TPB-GN-005-013 (Version 13; Updated 31 July 2024)

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 3 of 168
TABLE OF CONTENTS
CHAPTER A GENERAL OVERVIEW ............................................................................. 11
1 FOREWORD .............................................................................................................. 11
1.1 Scope of This Guidance Document .............................................................. 11
1.2 Therapeutic Product Registration .................................................................. 13
2 APPLICANT AND REGISTRANT RESPONSIBILITIES .............................................. 15
3 WHETHER A THERAPEUTIC PRODUCT IS SUBJECT TO PATENT ....................... 16
4 PROTECTION OF CONFIDENTIAL SUPPORTING INFORMATION AND
REGISTRATION EXCLUSIVITY ................................................................................ 18
CHAPTER B REGISTRATION PROCESS ..................................................................... 19
5 PRE-SUBMISSION PREPARATION .......................................................................... 20
5.1 Product Types .............................................................................................. 20
5.2 Application Types ......................................................................................... 20
5.3 Evaluation Routes ........................................................................................ 23
5.4 Pre-Submission Consultation Mechanisms ................................................... 23
5.4.1 Pre-Submission Notification ............................................................ 24
5.4.2 Pre-Submission Meeting ................................................................. 24
6 APPLICATION SUBMISSION .................................................................................... 25
6.1 PRISM Application Form .............................................................................. 25
6.2 Application Dossier ....................................................................................... 26
6.2.1 Submission Requirements .............................................................. 27
6.2.2 Language and Translation............................................................... 29
6.2.3 Certifying Non-Original Documents ................................................. 31
7 APPLICATION SCREENING ..................................................................................... 32
8 APPLICATION EVALUATION .................................................................................... 33
8.1 Evaluation Stages ......................................................................................... 34
9 REGULATORY DECISION ........................................................................................ 36
10 POST-APPROVAL CHANGES ................................................................................... 38
11 TARGET PROCESSING TIMELINES ........................................................................ 38
12 FEES .......................................................................................................................... 38
12.1 Screening Fee .............................................................................................. 38
12.2 Evaluation Fee .............................................................................................. 39
12.2.1 Changes to Application Types and Re-routing of Evaluation During
Screening ........................................................................................ 40
CHAPTER C NEW DRUG APPLICATION SUBMISSION ............................................... 42
13 APPLICATION TYPES ............................................................................................... 42
14 EVALUATION ROUTES ............................................................................................. 43
14.1 Full Evaluation Route .................................................................................... 43
14.2 Abridged Evaluation Route ........................................................................... 43
14.2.1 Priority Review ................................................................................ 44
14.3 Verification Evaluation Route ........................................................................ 45
14.3.1 NDA-3 Applications ......................................................................... 47
15 DOCUMENTARY REQUIREMENTS .......................................................................... 47
15.1 Administrative Documents ............................................................................ 48
15.2 CTD Overview and Summaries..................................................................... 65
15.3 Quality Documents ....................................................................................... 65
15.3.1 Body of Data – Drug Substance ...................................................... 65
15.3.2 Body of Data – Drug Product .......................................................... 69
15.4 Non-clinical Documents ................................................................................ 74
15.5 Clinical Documents ....................................................................................... 74
15.6 Documentary Requirements for Each Evaluation Route ............................... 75
15.6.1 Full Evaluation Route ...................................................................... 75

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 4 of 168
15.6.2 Abridged Evaluation Route .............................................................. 76
15.6.3 Verification Evaluation Route .......................................................... 76
CHAPTER D GENERIC DRUG APPLICATION SUBMISSION ....................................... 81
16 APPLICATION TYPES ............................................................................................... 81
16.1 Generic Product ............................................................................................ 81
16.2 Singapore Reference Product ....................................................................... 82
17 EVALUATION ROUTES ............................................................................................. 83
17.1 Abridged Evaluation Route ........................................................................... 83
17.2 Verification Evaluation Route ........................................................................ 83
18 DOCUMENTARY REQUIREMENTS .......................................................................... 85
18.1 Administrative Documents ............................................................................ 86
18.2 CTD Overview and Summaries................................................................... 100
18.3 Quality Documents ..................................................................................... 100
18.3.1 Body of Data – Drug Substance .................................................... 100
18.3.2 Body of Data – Drug Product ........................................................ 104
18.4 Non-clinical and Clinical Documents ........................................................... 111
18.5 Documentary Requirements for Each Evaluation Route ............................. 112
18.5.1 Abridged Evaluation Route ............................................................ 112
18.5.2 Verification and Verification-CECA Evaluation Routes .................. 112
18.6 Documentary Requirements for Second Brand Registration of Chemical
Therapeutic Products .................................................................................. 117
18.6.1 Definition ....................................................................................... 117
18.6.2 Documentary Requirements .......................................................... 117
CHAPTER E BIOSIMILAR PRODUCT APPLICATION SUBMISSION .......................... 119
19 APPLICATION TYPES ............................................................................................. 119
19.1 Biosimilar Product ....................................................................................... 120
19.2 Singapore Reference Biological Product ..................................................... 120
20 EVALUATION ROUTES ........................................................................................... 121
20.1 Abridged Evaluation Route ......................................................................... 122
20.2 Verification Evaluation Route ...................................................................... 122
20.2.1 NDA-3 Applications ....................................................................... 124
21 DOCUMENTARY REQUIREMENTS ........................................................................ 124
21.1 Administrative Documents .......................................................................... 125
21.2 CTD Overviews and Summaries ................................................................. 125
21.3 Quality Documents ..................................................................................... 125
21.4 Non-clinical and Clinical Documents ........................................................... 127
21.4.1 Non-clinical Documentation ........................................................... 128
21.4.2 Clinical Documentation ................................................................. 128
21.5 Documentary Requirements for Each Evaluation Route ............................. 130
21.5.1 Abridged Evaluation Route ............................................................ 130
21.5.2 Verification Evaluation Route ........................................................ 131
CHAPTER F POST-APPROVAL PROCESS ................................................................ 135
22 APPLICATION TYPES ............................................................................................. 135
23 VARIATION APPLICATION PROCESS ................................................................... 137
23.1 Pre-Submission Consultation Mechanisms ................................................. 137
23.1.1 Pre-Submission Notification .......................................................... 138
23.2 Application Submission ............................................................................... 138
23.2.1 PRISM Application Form ............................................................... 138
23.2.2 Variation Application Dossier ........................................................ 138
23.3 Application Screening ................................................................................. 142
23.4 Application Evaluation and Regulatory Decision ......................................... 143
23.5 Target Processing Timelines ...................................................................... 147
23.6 Fees ........................................................................................................... 147
23.6.1 Screening Fee ............................................................................... 147
23.6.2 Evaluation Fee .............................................................................. 148
23.6.3 Application Fee ............................................................................. 150

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 5 of 168
CHAPTER G MAJOR VARIATION (MAV) APPLICATION SUBMISSION ..................... 151
24 MAV-1 APPLICATIONS ........................................................................................... 151
24.1 Evaluation Routes ...................................................................................... 151
24.1.1 Full Evaluation Route .................................................................... 152
24.1.2 Abridged Evaluation Route ............................................................ 152
24.1.3 Verification Evaluation Route ........................................................ 152
24.2 Documentary Requirements ....................................................................... 154
24.2.1 Administrative Documents............................................................. 155
24.2.2 CTD Overviews and Summaries ................................................... 156
24.2.3 Quality Documents ........................................................................ 156
24.2.4 Non-clinical and Clinical Documents ............................................. 156
24.2.5 Documentary Requirements for Each Evaluation Route ................ 157
25 MAV-2 APPLICATIONS ........................................................................................... 159
25.1 Evaluation Routes ...................................................................................... 159
25.2 Eligibility Criteria ......................................................................................... 159
25.3 Documentary Requirements ....................................................................... 160
25.4 ‘Me-too’ Reclassification ............................................................................. 161
CHAPTER H MINOR VARIATION (MIV) APPLICATION SUBMISSION ....................... 162
26 APPLICATION TYPES ............................................................................................. 162
27 APPLICATION SUBMISSION .................................................................................. 163
27.1 MIV-1 Applications ...................................................................................... 164
27.1.1 Submitting multiple/consequential changes ................................... 164
27.2 MIV-2 Applications ...................................................................................... 165
27.2.1 MIV-2 Notification .......................................................................... 165
27.2.2 MIV-2 Do-and-Tell ......................................................................... 165

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 6 of 168
LIST OF APPENDICES
APPENDIX 1 Patent Declaration Forms
APPENDIX 2A Application Checklist (ICH CTD) for NDA and GDA
APPENDIX 2B Application Checklist (ICH CTD) for MAV
APPENDIX 3A Application Checklist (ACTD) for NDA and GDA
APPENDIX 3B Application Checklist (ACTD) for MAV
APPENDIX 4 Sample Verification Document for Translator
APPENDIX 5 Target Processing Timelines
APPENDIX 6 Guideline on Submission for Non-Prescription Therapeutic Products
APPENDIX 7 Points to Consider for Singapore Labelling
APPENDIX 7A Guidance on Electronic Labelling for Therapeutic Products
APPENDIX 8 Guideline on the Registration of Human Plasma-derived Therapeutic
Products
APPENDIX 9 Guideline on the Registration of Human Therapeutic Products
Containing Materials of Animal Origin
APPENDIX 9A Annex 1 Checklist For The Registration Of Human Therapeutic
Products Containing Materials Of Animal Origin
APPENDIX 10 Product Interchangeability and Biowaiver Request for Chemical
Generic Drug Applications
APPENDIX 11 Guideline on Drug Master File (DMF)
APPENDIX 11A [obsolete]
APPENDIX 11B Sample Letter of Access for DMF
APPENDIX 12 Online MIV Self-guided Tool and Enquiry Form
APPENDIX 13 Guideline on Minor Variation Applications (MIV-1 & MIV-2) for
Chemical Therapeutic Products
APPENDIX 13A Part A: Checklist on Dossier Requirements for MIV-1 Applications for
Chemical Therapeutic Products
APPENDIX 13B Part B: Checklist on Dossier Requirements for MIV-2
(Notification)Applications for Chemical Therapeutic Products
APPENDIX 13C Part C: Checklist on Dossier Requirements for MIV-2 (Do-and-Tell)
Applications for Chemical Therapeutic Products
APPENDIX 14 Guideline on Minor Variation Applications (MIV-1 & MIV-2) for
Biological Therapeutic Products

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 7 of 168
APPENDIX 14A Part A: Checklist on Dossier Requirements for MIV-1 Applications for
Biological Therapeutic Products
APPENDIX 14B Part B: Checklist on Dossier Requirements for MIV-2 (Notification)
Applications for Biological Therapeutic Products
APPENDIX 14C Part C: Checklist on Dossier Requirements for MIV-2 (Do-and-Tell)
Variation
APPENDIX 15 [obsolete]
APPENDIX 16 [obsolete]
APPENDIX 16A [obsolete]
APPENDIX 17 Guideline on PRISM Submission
APPENDIX 18 Confirmation of Quality Dossiers with Reference Agencies Approval
APPENDIX 18A Dossier Clarification Supplement

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 8 of 168
ABBREVIATIONS AND ACRONYMS
ACPM
ACRA
Advisory Committee on Prescription Medicines
Accounting and Corporate Regulatory Authority
ACTD
ASEAN Common Technical Document
ACTR
ASEAN Common Technical Requirements
ASEAN
Association of Southeast Asian Nations
ATC
Anatomical Therapeutic Chemical
BA
BCS
Bioavailability
Biopharmaceutics Classification System
BE
Bioequivalence
BP
British Pharmacopoeia
BSE
Bovine Spongiform Encephalopathy
CECA
Comprehensive Economic Cooperation Agreement
CEP
Certificate of Suitability (Ph. Eur. monograph)
CHMP
Committee for Medicinal Products for Human Use (formerly Committee
for Proprietary Medicinal Products) (EU)
CMC
CMI
Chemistry, Manufacturing and Controls
Consumer Medicine Information
CMS
Concerned Member State
COA
COO
Certificate of Analysis
Country of Origin (Finished product manufacturer)
CPP
Certificate of Pharmaceutical Product
CTD
Common Technical Document
DCP
Decentralised Procedure
DMF
DP
DS
Drug Master File
Drug Product
Drug Substance
EDQM
EIR
European Directorate for the Quality of Medicines
Establishment Inspection Report
EMA
European Medicines Agency (EU)
FDA
Food and Drug Administration (US)
FTA
Free Trade Agreement
GDA
Generic Drug Application

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 9 of 168
GMP
GSL
Good Manufacturing Practice
General Sale List medicine
HDPE
High-Density Polyethylene
HP
Health Product
HPA
Health Products Act
HPRG
Health Products Regulation Group
HSA
Health Sciences Authority (Singapore)
ICH
International Council for Harmonisation (of Technical Requirements for
Registration of Pharmaceuticals for Human use)
INN
IPOS
International Non-proprietary Names
Intellectual Property Office of Singapore
JP
MAH
Japanese Pharmacopoeia
Marketing Authorisation Holder
MAV
Major Variation
MHRA
Medicines and Healthcare Products Regulatory Agency (UK)
MIV
MRP
Minor Variation
Mutual Recognition Procedure
NDA
New Drug Application
OTC
Over-The-Counter
P
Pharmacy-Only Medicine
PD
Pharmacodynamics
Ph. Eur.
European Pharmacopoeia
PI
Package Insert (Singapore), Product Information
PIC/S
Pharmaceutical Inspection Convention and Pharmaceutical Inspection
Co-operation Scheme
PIL
Patient Information Leaflet
PK
PMDA
Pharmacokinetics
Pharmaceuticals and Medical Devices Agency (Japan)
PMF
Plasma Master File
POM
Prescription-Only Medicine
PRISM
Pharmaceutical Regulatory and Information System
QOS
Quality Overall Summary
RMP
Risk Management Plan
RMS
Reference Member State

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 10 of 168
SRBP
Singapore Reference Biological Product
SPC
Summary of Product Characteristics
TGA
Therapeutic Goods Administration (Australia)
TP
Therapeutic Product
TSE
Transmissible Spongiform Encephalopathy
USP
United States Pharmacopeia
WHO
World Health Organisation

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 11 of 168
CHAPTER A GENERAL OVERVIEW
1 FOREWORD
This guidance document outlines the regulatory processes and requirements for
therapeutic product registration and should be read in conjunction with the relevant
legislation in Singapore, including:
• Health Products Act 2007; and
• Health Product (Therapeutic Products) Regulations 2016.
The Health Products Act (HPA) provides for the legislative basis for regulating the
manufacture, import, supply, presentation and advertisement of therapeutic products,
one of the health products categories regulated under the Act.
1.1 Scope of This Guidance Document
This guidance document describes the procedures and requirements for submitting an
application to register a therapeutic product, or to make a variation application to a
registered therapeutic product.
Under the First Schedule of the HPA, a therapeutic product means any substance that:
(a) is intended for use by and in humans for a therapeutic, preventive, palliative or
diagnostic purpose, including any of the following purposes:
(i) for preventing, diagnosing, monitoring, treating, curing or alleviating any
disease, disorder, ailment, injury, handicap or abnormal physical or mental
state, or any symptom thereof;
(ii) for investigating, modifying, or replacing any physiological process;
(iii) for influencing, controlling or preventing conception; or
(iv) for inducing anaesthesia.
(b) has as its constituent any of the following active ingredients:
(i) any chemical or botanical element, naturally occurring chemical or botanical
material or chemical product obtained by chemical change or synthesis;
(ii) any metabolite from a micro-organism;

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 12 of 168
(iii) any macromolecule extracted from an organism; or
(iv) any substance derived from a biological system, including any of the following:
(A) a whole cell or micro-organism, such as a whole virus or bacterium used
as a vaccine;
(B) a part of a micro-organism, such as a sub-unit vaccine;
(C) a plasma-derived product; or
(D) a biotechnology-derived substance, such as a protein or polypeptide;
(c) exerts an inherent effect either pharmacologically, chemically or by other
physiological means, leading to its use for a therapeutic preventive, palliative or
diagnostic purpose; and
(d) is not any of the following:
(i) a medical device;
(ii) a cell, tissue or gene therapy product;
(iii) whole blood or any blood component;
(iv) any Chinese proprietary medicine;
(v) any homoeopathic medicine;
(vi) any medicated oil or balm;
(vii) any quasi-medicinal product; or
(viii) any traditional medicine.
To avoid doubt, items d(vi), (v), (vi), (vii) and (viii) have the same meaning as defined
in the Medicines Act 1975 in paragraph 2 of the Medicines (Traditional Medicines,
Homoeopathic Medicines & Other Substances) (Exemption) Order.
In making an application for a therapeutic product, applicants should ensure that the
submission requirements as specified in this guidance document are duly fulfilled. In
a situation where an applicant proposes an alternative to any of the specified
requirements, such a proposal should be accompanied by scientific justification and
discussed with HSA prior to making the submission to avoid potential rejection of the
application. Information on pre-submission consultation can be found in Chapter B;
5.4.
HSA may also request for additional information to supplement the specified
submission requirements if this is deemed necessary for the assessment of the safety,

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 13 of 168
efficacy and quality of the product for which an application is made. Information on the
submission requirements can be found in the following Chapters of this guidance.
Within this document, the term ‘quality’ is used to describe chemical, pharmaceutical
and biological data, while the term ‘non-clinical’ is used to describe preclinical,
pharmacological and toxicological data.
Applicants are advised to check HSA's website for the latest version of this guidance
document and other related therapeutic product registration guidelines.
1.2 Therapeutic Product Registration
A therapeutic product registered under the HPA is specific to the product with respect
to its:
• proprietary or brand name;
• pharmaceutical formulation;
• pharmaceutical dosage form (i.e. physical presentation) and strength; and
• indication(s) and dosing regimen.
Different formulations, dosage forms and strengths of the same chemical or biologic
entity are considered as different products and will require separate registrations for
the individual product.
Forensic Classification
Upon satisfying the regulatory requirements for quality, safety and efficacy, a
therapeutic product may be registered under one of the following forensic
classifications, which determines the level of control for access:
• Prescription-Only Medicine (POM);
• Pharmacy-Only Medicine (P); or
• General Sale List medicine (GSL).
Prescription-Only Medicines (POM) control is required in the following situations:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 14 of 168
(a) The product poses a direct
1
or indirect
2
danger to human health, even when used
correctly, if used without medical supervision;
(b) The product is frequently and widely used incorrectly and, as a result, is likely to
present a direct or indirect danger to human health;
(c) The product requires further investigation into its activity and/or side effects; and/or
(d) The product is normally prescribed by a doctor or dentist to be administered
parenterally.
The following also needs to be taken into consideration when deciding whether a
product should be classified as a POM:
(a) Whether the product contains a substance which is listed in either the Narcotic
Drug Convention or the Psychotropic Substances Convention;
(b) Whether the product is likely to lead to medicinal abuse or addiction if used
incorrectly or to be used for illegal purposes;
(c) Whether the product contains a substance which, by reason of its novelty or
properties, has the potential to fall within point (b) above;
(d) Whether the product, by reason of its pharmaceutical characteristics, is reserved
for treatments which can only be administered in a hospital;
(e) Whether the product is used in the treatment of conditions which must be
diagnosed in a hospital or in an institution with special diagnostic facilities; and/or
(f) Whether the product is intended for outpatients but may produce serious side
effects, which would require medical supervision throughout the treatment.
Pharmacy-Only Medicines (P) control is required for products that possess
characteristics which are not sufficiently critical to warrant POM control but for which
the following apply:
(a) Consultation with a pharmacist is necessary to confirm the appropriate choice of
therapy;
(b) The contraindications, drug interactions, precautions or warnings need
reinforcement by a pharmacist or are not easily recognised by the purchaser; or
(c) Special precaution is needed in the storage and handling of the product.
1
Direct danger: Adverse reactions for which there is no preventive action or which are serious, severe or of high
frequency
2
Indirect danger: Masking of an underlying condition that requires medical attention e.g. cancer, heart disease

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 15 of 168
General Sale List Medicines (GSL) control is sufficient in the following situations:
(a) The product is reasonably safe and can be sold or supplied without the need for
supervision by a registered doctor, dentist or pharmacist;
(b) The contraindications, drug interactions, precautions and warnings are easily
recognised by the consumer; and
(c) The hazard to health, the risk of misuse, the risk of misdiagnosis, or the need to
take special precaution in the storage and handling the product is small.
2 APPLICANT AND REGISTRANT RESPONSIBILITIES
The applicant of a product registration refers to the local company that is submitting a
therapeutic product application in Singapore. The applicant company may authorise
officers, permanent employees, or designated external parties, all of whom are
referred to as the “applicant representative”, to submit the therapeutic product
application.
According to Section 30(10) of the HPA, an applicant, in making an application for the
registration of a therapeutic product, must ensure that all information contained in the
application is truthful and is not misleading. An applicant must inform HSA of any
emerging information that may affect the benefit-versus-risk assessment of the
therapeutic product to which the application relates, as soon as the applicant becomes
aware of such information.
The applicant is responsible for submitting the application and all the accompanying
supporting documents (including but not limited to the dossier, responses to HSA’s
queries and commitment letters).
HSA may require a statutory declaration by the applicant verifying any information
contain in or relating to the application.
HSA may register the product subject to post-approval commitments. In such
circumstances, the applicant will be required to furnish a letter of commitment stating
the undertakings concerned. Upon the approval of an application to register a
therapeutic product, the product is registered and is assigned a registration number

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 16 of 168
and entered in the Register of Health Products. The applicant of the product
registration becomes the product registrant.
The product registrant should refer to Part 8 of the HPA and Part 6 of the HP (TP
Regulations) 2016 for duties imposed on registrants.
It should be emphasised that product registrants must comply with the registration
conditions and the post-approval commitments specified in the registration. The
registration conditions can be viewed at Enquire@PRISM.
For submission of documents to fulfil registration conditions, please use this form
(Submission of Documents to Fulfil Therapeutic Product Registration Conditions -
https://go.gov.sg/fulfil-tp-reg-conditions ).
3 WHETHER A THERAPEUTIC PRODUCT IS SUBJECT TO PATENT
An applicant for registration of a therapeutic product is required to make a declaration
on whether the therapeutic product for which registration is sought is subject to a
subsisting restraining patent, pursuant to Regulation 23 of the Health Products
(Therapeutic Products) Regulations, hereafter referred to as the Regulations. A
“restraining patent” refers to a patent mentioned in regulation 23(1)(a) of the
Regulations.
The declaration must be made in the form specified in Appendix 1 – Form 1 of this
guidance document and furnished at the time of making the application, as well as at
any other such time as HSA may require. A second declaration is required prior to the
grant of registration.
A registration application may be declared as one of the following categories:
• Category A1: where no restraining patent is in force in respect of the therapeutic
product to which the application relates;
• Category A2: where a restraining patent is in force in respect of the therapeutic
product to which the application relates and the applicant is either the proprietor of
the restraining patent, or if the applicant is not the proprietor of the restraining

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 17 of 168
patent, the proprietor has consented to or acquiesced in the grant of the
registration;
• Category A3: where a restraining patent is in force in respect of the therapeutic
product to which the application relates, the applicant is not the proprietor of the
restraining patent and the proprietor has not consented to or acquiesced in the
grant of the registration, and the applicant is requesting for the grant of registration
after the restraining patent expires. A Category A3 declaration is applicable only to
an application that is made within 18 months of the expiry of the restraining patent
from the point of application submission. Such an application may not be made
earlier than 18 months before the restraining patent expires. Applicants who
deviate from this guideline may be required to withdraw their application and
resubmit it at the appropriate juncture;
• Category B: where a restraining patent is in force in respect of the therapeutic
product to which the application relates, the applicant is not the proprietor of the
restraining patent and the proprietor has not consented to or acquiesced in the
grant of the registration, and in the applicant’s opinion and to the best of his belief
the restraining patent is invalid or will not be infringed by the performing of the act
for which the registration is sought.
Where an application is declared as a Category B application, HSA will require the
applicant to serve a notice on the proprietor of the restraining patent in the form
specified in Appendix 1 – Form 2 of this guidance document. An applicant may also
be required to serve a notice where HSA considers it appropriate.
Where the proprietor of the restraining patent has made an application to a court
pursuant to regulation 23(8)(a) and furnishes a written notice to HSA under regulation
23(8)(b) of the Regulations, the written notice must be accompanied by the following:
• Evidence of the application made under regulation 23(8)(a) of the Regulations;
and
• A declaration made in the form specified in Appendix 1 – Form 3 of this
guidance document that the aforementioned application relates to a restraining
patent.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 18 of 168
The information contained in this section serves solely as guidance on the requirement
for submission of declaration on patent-related information in respect of an application
for product registration. HSA does not provide advice on the category under which an
application should be declared or whether a therapeutic product is subject to a
subsisting restraining patent. An applicant requiring such assistance should seek
appropriate legal advice.
4 PROTECTION OF CONFIDENTIAL SUPPORTING INFORMATION AND
REGISTRATION EXCLUSIVITY
Regulation 26 and 29 of the Regulations provide for protection of confidential
supporting information relating to innovative therapeutic product applications and
exclusivity of safety and efficacy data, respectively.
Confidential information received in support of the registration of an innovative
therapeutic product is protected for a period of 5 years from the date of receipt, during
which HSA will not use the information to determine whether to grant any other
registration applications. In this regard, confidential supporting information refers to
trade secrets and information that has commercial value that would be, or is likely to
be, diminished by disclosure.
A 5-year period of exclusivity is granted for a therapeutic product for which safety and
efficacy data has been generated in support of its registration. During the exclusivity
period, a subsequent similar therapeutic product will not be able to rely on such data
generated for the earlier therapeutic product to obtain registration.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP
Page 19 of 168
CHAPTER B REGISTRATION PROCESS
A company seeking to market a therapeutic product in Singapore must obtain
marketing approval from HSA through making an application for product
registration. The registration process involves a series of steps, as shown in Figure
1.
PRE-SUBMISSION
PREPARATION
APPLICATION
SUBMISSION
APPLICATION
SCREENING
APPLICATION
EVALUATION
REGULATORY
DECISION
POST-APPROVAL
CHANGES
NON-ACCEPTANCE /
WITHDRAWAL
ACCEPTANCE
APPROVAL
NON-APPROVAL /
WITHDRAWAL
Figure 1 Registration Process for a Therapeutic Product

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 20 of 168
5 PRE-SUBMISSION PREPARATION
The following are important considerations for an applicant to register a therapeutic
product:
(a) Knowing which type of application to apply for;
(b) Knowing which evaluation route to choose; and
(c) Understanding the requirements as specific in this guidance.
5.1 Product Types
A therapeutic product could contain either chemical or biological entity(ies) as the
active ingredient(s).
A chemical entity refers to any chemical element, naturally occurring chemical
material or chemical product obtained by chemical change or synthesis (including
macromolecules produced by chemical synthesis, such as peptides/oligo-
nucleotides), or any metabolites from a micro-organism (such as antibiotics).
A biological entity refers to any macromolecule extracted from an organism (such
as proteins, nucleic acids, proteoglycans, cytokines and growth factors), or any
substance derived from a biological system, including any of the following:
(a) A whole cell or micro-organism, such as a whole virus or bacterium used as a
vaccine;
(b) A part of a micro-organism, such as a sub-unit vaccine;
(c) A plasma-derived product; or
(d) A biotechnology-derived substance, such as a protein or polypeptide.
5.2 Application Types
In applying for a new product registration for a therapeutic product in Singapore,
there are two categories of applications – a new drug application (NDA) and a
generic drug application (GDA):

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 21 of 168
NDA New Drug Application
NDA-1:
For the first strength of a product containing a new
3
chemical or biological
entity.
NDA-2:
(a) For the first strength of a product
(i) containing a new combination of registered chemical or biological
entities;
(ii) containing registered chemical or biological entity(ies) in a new
dosage form (e.g. tablets, capsules, injectables), new presentation
(e.g. single-dose vials, multi-dose vials, pre-filled syringe, starter
packs), or new formulation (e.g. preservative-free);
(iii) containing registered chemical or biological entity(ies) for use by a
new route of administration; or,
(iv) containing registered chemical or biological entity(ies) for new
indication(s), dosage recommendation(s) and/or patient
population(s).
(b) For products that do not fall under the descriptions for NDA-1, NDA-3 or
GDA.
NDA-3:
For subsequent strength(s) of a product that has been registered or has
been submitted as an NDA-1 or NDA-2. The product name, active
ingredient, dosage form, presentation, indication, dosing regimen and
patient population should be the same as that for the NDA-1 or NDA-2.
3
i.e. not a currently registered entity in Singapore. Currently registered therapeutic products can be found in
the Register of Therapeutic Products at www.hsa.gov.sg.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 22 of 168
GDA
Generic Drug Application
A generic drug application applies to a therapeutic product that contains one or more
chemical entities, and that is essentially the same as a current registered product
with respect to its qualitative and quantitative composition of active ingredients,
pharmaceutical dosage form and clinical indication.
Follow-on biologic products (also known as biosimilar products) are not eligible for a
GDA and are required to be submitted via a NDA.
GDA-1:
For the first strength of a generic chemical product.
GDA-2:
For subsequent strength(s) of the generic chemical product that has been
registered or submitted as GDA-1. The product name and dosage form
should be the same as that for the GDA-1.
In cases where multiple strengths of a generic product are submitted together, the
strength of the product used in the BE study is considered as GDA-1. The remaining
strength(s) should be submitted as GDA-2.
Figure 2 is a schematic diagram illustrating the various types of applications:
Post-Approval
Process, Chapter F
GDA – 2
IS PRODUCT
REGISTERED?
First strength of
product?
Fulfils definition of
a generic product?
NDA – 1
Contains new
chemical or
biological entity?
NO
YES
YES
NO
NDA – 2
NDA – 3
GDA – 1
NO
NO
YES
YES
Figure 2 Schematic Diagram of Application Routes for Drug Registration

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 23 of 168
5.3 Evaluation Routes
There are four types of evaluation routes for registering a new therapeutic product:
Full route:
Applies to any new product that has not been approved by
any drug regulatory agency at the time of application
submission to HSA.
Abridged route:
Applies to any new or generic product that has been
evaluated and approved by at least one drug regulatory
agency.
Verification route:
Applies to any new or generic product that has been
evaluated and approved by HSA’s reference drug regulatory
agencies, which are EMA
4
, FDA, Health Canada, MHRA
5
,
Swissmedic and TGA.
Verification-CECA
route:
Applies to any generic product manufactured in India which
has been evaluated and approved by HSA’s reference drug
regulatory agencies, which include EMA
4
, FDA, Health
Canada, MHRA
5,
Swissmedic and TGA.
Applicants should refer to Chapters C, D and E for detailed information about the
selection of appropriate evaluation routes for NDA, GDA and Biosimilar product
applications, respectively.
5.4 Pre-Submission Consultation Mechanisms
There is a range of mechanism that enable companies to self-help, which includes
the use of guidelines, flow charts, frequently asked questions (FAQ) and self-help
tools as alternatives to pre-submission meeting.
For more information on TPB’s pre-submission consultation mechanisms, refer to
the website: Pre-submission Consultation Mechanisms
4
For products approved via the Centralised Procedure
5
For products approved via the national procedure or where MHRA acted as the RMS for the MRP or
Decentralised Procedures on or prior to 31 January 2020

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 24 of 168
5.4.1 Pre-Submission Notification
A pre-submission meeting is not compulsory for making an application to HSA.
Nonetheless, the applicant is required to notify HSA at least two months prior to the
intended submission date for applications submitted via the full evaluation route.
The notification should include information on the product name (if available), active
ingredient(s), summaries of the quality, non-clinical and clinical data (e.g.
Overviews), planned submissions in other countries, and planned date of
submission to HSA.
5.4.2 Pre-Submission Meeting
An applicant may request for a pre-submission meeting to seek HSA’s advice on
specific issues relating to the data package for supporting an application
submission, if the issue could not be addressed by the self-help mechanisms
provided at Pre-submission Consultation Mechanisms.
A pre-submission meeting is reserved for scientific discussion and does not provide
for screening or checking of the submission dossier for the applicant. To ensure the
correctness of the application type and the completeness of the dossier, please
refer to the documentary requirements sections in this guidance.
Before making a request for pre-submission meeting, the applicant must ensure
that at least one of the criteria below is met:
i. The product is a novel therapeutic product developed using new or emerging
technologies or methodologies; or
ii. The product is developed in the absence of, or deviates from local or
international regulatory guidance.
The following required documents must be submitted together with the request for
pre-submission meeting:
1. Proposed agenda for the meeting;
2. Summary information which may include Chemistry, Manufacturing and
Controls (CMC)/ non-clinical/ clinical information of the product and
proposed application; and
3. Specific scientific issues that require advice.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 25 of 168
HSA may reject the pre-submission meeting request if:
• The product does not fulfil the pre-submission meeting criteria above; or
• The request is not accompanied by the required documents; or
• The issues can be addressed via email instead of having a pre-submission
meeting.
Overview of the Process
Advice given at pre-submission meetings will be based on information current at
the time of the consultation and have no bearing on the eventual outcome of the
application concerned.
6 APPLICATION SUBMISSION
The submission of an application comprises two key steps – (i) online submission
of the application form via PRISM and (ii) submission of the technical dossier.
6.1 PRISM Application Form
All applications must be made online via PRISM. Please refer to Appendix 17
Guideline on PRISM submission for further details.
Meeting to be
held on
scheduled
time and date
Request for pre-
submission
meeting with the
documents 40
working days
(WD) prior to
proposed
meeting date
HSA issues a
response 20
WD upon
receipt of
request
Request
granted?
No further
action needed
HSA confirms
meeting
arrangement
with applicant
HSA issues
advice
Y
N

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 26 of 168
6.2 Application Dossier
The technical dossier accompanying the application should be submitted within 2
working days of the PRISM application submission to prevent delays in the
processing of the application. The date of receipt of the actual technical dossier
by HSA will be taken as the submission date where the processing time starts.
Application dossiers should be organised in a CTD format. The CTD provides a
common format for the preparation of a well-structured submission dossier. It uses
a modular framework described in ICH Topic M4 and ASEAN guidelines on the
Common Technical Document for Registration of Pharmaceuticals for Human use:
Organisation of the Dossier. This guidance document should be read in conjunction
with the current version of the ICH CTD and the ASEAN CTD (ACTD) guidance
documents.
Either the ICH CTD or the ACTD format is acceptable for making a submission to
HSA. Table 1 summarises the organisation of the respective format:
Table 1 Format of the ICH CTD and ACTD
Documents
Location in
ICH CTD
ACTD
Administrative Documents and
Product Information
Module 1
Part I
Common Technical Document
Overview and Summaries
Module 2
Incorporated in
Parts II, III and IV
Quality documents
Module 3
Part II
Non-clinical documents
Module 4
Part III
Clinical documents
Module 5
Part IV
Application checklists for both ICH CTD and ACTD dossiers are provided in
Appendix 2A and 3A, respectively, to guide applicants on the submission
requirements and to ensure completeness of the dossier. Each application must
be accompanied by a checklist duly completed by the applicant and attached
in PRISM.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 27 of 168
Applicants should note that the CTD format cannot be changed once the application
is submitted. Any subsequent variation applications for the product should follow
the same format.
6.2.1 Submission Requirements
The complete application dossier – i.e. Modules 1 to 5 of the ICH CTD or Parts I to
IV of the ACTD – must be submitted in an electronic format.
Applicants must organise the dossier (i.e. folders and subfolders) according to the
CTD format and to include bookmarks in all documents to facilitate the retrieval of
documents.
Files containing the below scripts will not be accepted due to cybersecurity reasons:
As a general guide, folder or file names should not be named with “xxx.P (e.g.
“3.2.P”).
All documents required under Module 1/Part I must be submitted in softcopy in
PRISM. Colour scanned copy of the original documents should be submitted and
original hardcopy of documents are not required. However, HSA reserves the rights
to request for the submission of the original or certified true copy of the submitted
document if there is any doubt that the submitted scanned document is not an
accurate reflection of the original document.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 28 of 168
Please refer to section 6.2.3 for more information on certifying non-original
documents if the original documents cannot be provided.
To submit Modules 2 to 5/Parts II to IV, applicants have the option to submit via the
following methods:
1. Upload into PRISM section 7 (Supporting Attachments)
2. Submit in a CD/DVD
3. Submit via a cloud-based file exchange software (EasiShare)
Note: We are unable to accept submission of dossier via any digital portable
devices (e.g. flash memory sticks).
Submitting via a CD/DVD
The CD/DVD should be properly labelled or accompanied by a letter with the follo
wing information:
• PRISM application number;
• PRISM submission date;
• Product name;
• Application type;
• Contents of the CD/DVD (e.g. Module 2, 3 and 5); and
• Applicant’s email address.
Upon receipt of the CD/DVD, HSA will issue an acknowledgement email to the app
licant via the email address provided with the CD/DVD submission.
Applicants must ensure the access to the content of CD/DVD. For protected files,
password(s) must be provided as appropriate.
Upon acceptance of the application for evaluation, applicants will be notified if addi
tional copies of clinical documents (in CD/DVD) will be required.
Submitting via cloud-based file exchange software (EasiShare)
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 29 of 168
Refer to the guidance “Key Points to Note when Preparing Documents for Therape
utic Product Application Dossier Submission via EasiShare” available at:
https://www.hsa.gov.sg/therapeutic-products/register/overview/application-dossier
6.2.2 Language and Translation
All documents submitted in support of an application to HSA must be in English.
For documents in their original language which is not English, a certified translation
or a verified translation may be acceptable.
Translation
type
Type of
Documents
Requirements
Certified
Translation
▪ Official
certificates
issued by
the drug
regulatory
agency of a
country
▪ Proof of
approval
issued by
the drug
regulatory
agency of a
country
Notarisation & Authentication
(a) Notarisation
▪ These documents must be notarised by a
notary public in country where document is
issued.
▪ Details of particulars to be included by
notary:
(i) The name of the notary;
(ii) A statement that the notary is duly
admitted to practice in the place of issue
of the certificate;
(iii) The names of the signatories and the
capacity in which they have executed the
document, whether on their own behalf or
in an official or representative capacity;
(iv) A statement authenticating the
signatures of the parties and, where
appropriate, indicating that evidence has
been produced to the notary proving the

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 30 of 168
capacity in which they have executed the
document;
(v) The place and date of issue of the
notarial certificate; and
(vi) The signature and seal of the notary.
(b) Authentication
▪ These documents must be authenticated
(i.e. the origin of the document is attested
to) by one of the following government
bodies:
(i) The Ministry of Foreign Affairs of the
country in which the document was
issued; or
(ii) The Singapore Embassy/Consulate in
the country where the document was
issued.
Applicants are advised to consult the Singapore
Embassy/Consulate in the country where the
document originated regarding the local
requirements for document legalisation, as
these may deviate from the process as outlined
in the preceding paragraph.
Verified
Translation
▪ Technical
documents
(e.g.
package
insert,
submission
dataset)
Verification Document
▪ A verification document must be provided
by the translator of the document into the
English language.
▪ The verification document must state that
the translation into English is accurate.
▪ Details of particulars to be included in
verification document:
(i) the name of translator;

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 31 of 168
(ii) a statement that he/she is well versed in
English and the relevant foreign
language; and
(iii) a reference to the document being
translated.
Refer to the sample verification document for
translator enclosed in Appendix 4.
With Singapore acceding to the Apostillle Convention on 16 September 2021, for
certified translated document issued by a country which acceded to the Apostille
Convention, an apostille certificate can be submitted in lieu of a
notarised/authenticated certified translation.
6.2.3 Certifying Non-Original Documents
If the softcopy official document (e.g. CPP, GMP certificate) submitted to HSA in
PRISM is not a scan of the original document, the document must be certified prior
to submission. A certified true copy certifies that the photocopy presented is a true
and accurate copy of the original document. Acceptable certification of documents
to support therapeutic product applications to HSA can be done by the Company
Director or Company Secretary as registered with ACRA or above, or by an
independent authority such as a lawyer, notary public, Commissioner for
Oaths/Declarations/Affidavits, Justice of Peace, the original issuer of the document
or Embassy/Consulate. A notarised and authenticated copy is the same as a
certified true copy.
A certified true copy of an approval letter requires certification by the drug regulatory
agency that issued the approval letter, a notary public or the Singapore
Embassy/Consulate in the country where the approval letter was issued.
Certification of an approval letter is not required if the approval letter is available on
the drug regulatory agency’s website. In this instance, applicants can provide the
internet address (URL) for validation by HSA.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 32 of 168
7 APPLICATION SCREENING
Following a submission made via PRISM and the receipt of the application dossier
by HSA, the application will be screened to ensure the correctness of the application
type and the completeness of the dossier. The date of receipt of the application
dossier (i.e. the technical dossier [e.g. in a CD/DVD] including the application
checklist) will be taken as the submission date and the start of the screening
timeline.
During screening, if an application is identified to be more appropriately submitted
under a different application type, the applicant will be informed of this change and
the necessary actions to effect this change via an Input Request. More information
on the change in application type is described in section 12.2.1 Changes to
Application Types and Re-routing of Evaluation During Screening.
For applications submitted without the following documents, the applicant will
be requested to withdraw the application as screening cannot proceed:
• Entire dossier sections (drug substance, drug product, clinical)
• Drug Master File (DMF) (if applicable)
#
• For applications supported by DMF, a copy of the acknowledgment email
from HSA on the receipt of the Letter of Access (if applicable)
• Application checklist in MS WORD format (Appendix 2 or 3)
• Assessment reports (for verification route)
# Please refer to Appendix 11 for information on the documentary requirements in support
of a DMF submission
Applicants should ensure that the dossier is compiled according to the required
format. Failure to adhere to the required CTD format will lead to the non-acceptance
of the dossier without screening.
If deficiencies are identified in an application dossier, a screening query stating the
deficiencies will be issued via Input Request to the applicant. The stop-clock starts
when an Input Request is sent and ends upon receipt of a complete and satisfactory
response to the query. The total number of Input Requests sent during screening is

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 33 of 168
capped at two. Applicants will be given 20 working days to respond to each Input
Request, starting from the date the Input Request is sent.
The application will only be accepted when all deficiencies have been adequately
addressed and HSA is satisfied that the dossier is complete for evaluation. An
acceptance notice will then be issued via PRISM and the date of acceptance of the
application will be taken as the start of the evaluation timeline. For full and abridged
applications, applicants may be required to submit additional copies of the dossier
in CD/DVD format after acceptance.
If the applicant fails to address the deficiencies raised during screening, the
application will not be accepted for evaluation. An Input Request will be issued to
the applicant to withdraw the application. If the application is subsequently re-
submitted, it will be processed as a new application.
8 APPLICATION EVALUATION
Once the application is accepted, the evaluation stage begins. Evaluation queries
may be issued via Input Request to the applicant if clarification or additional
information is required.
The stop-clock starts whenever HSA issues a query and ends upon the receipt of a
complete and satisfactory response from the applicant.
In situations where the applicant is unable to provide a complete response within
the specified timeframe, the applicant should notify HSA as soon as possible after
receiving HSA’s queries. The application will be considered withdrawn if the
applicant fails to observe the specified response deadline.
Applicants are reminded that the submission of additional supporting data not
requested by HSA following the acceptance of the application will not be
NOTE: The screening process only checks for the completeness of the application
dossier for evaluation. The acceptance of the dossier for evaluation does not denote the
adequacy of the data for regulatory approval.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 34 of 168
considered, unless prior arrangement with HSA is made for the submission
concerned. During the evaluation process, HSA may assess that the application is
more suitably evaluated via an alternative route, in which case the application will
be re-routed to the appropriate route. Any re-routing of the application will be
discussed with the applicant.
HSA may engage external evaluators, experts and advisory committees in the
evaluation process, when necessary. These experts include scientists and
clinicians from both local and overseas institutions. All external evaluators and
experts are bound by agreement to protect the information made available to them.
The identity of the external evaluators is kept confidential.
8.1 Evaluation Stages
The progress status of the evaluation is available for certain application types and
evaluation routes. Table 2 describes the applicable product applications and the
stages of the evaluation:
Table 2 Product Applications Applicable for Notification of Stages During
Evaluation
Stages of
Notification to
Applicant
1
st
Stage
2
nd
Stage
3
rd
Stage
4
th
Stage
Application
Type
Evaluation
Route
Evaluation Status
Acceptance
for
Evaluation
Active
Evaluation
in Progress
Evaluation at
Midway
Completed
Evaluation
NDA-1
NDA-2
NDA-3
Full or
Abridged
Application
is accepted
for
evaluation
and has
entered the
evaluation
queue.
Application
is under
active
evaluation.
Applicants
can expect
to receive
Application is
approximately
midway
through the
evaluation.
Evaluation is
completed for
the application.
Application is
now
undergoing the
GDA-1
GDA-2
Abridged,
Verification,
or

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 35 of 168
Verification-
CECA
This marks
the start of
the
evaluation
timeline.
the first set
of
evaluation
queries (if
any) from
us towards
the end of
this stage.*
Applicants are
expected to
submit the
response to
evaluation
queries.
regulatory
decision phase,
after which a
regulatory
decision
#
will
be issued.
Applicants can
still expect
further queries
from HSA
during this
stage.
* For applications without any evaluation queries, recommended changes to product labels
will be communicated to the applicant during the regulatory decision phase.
#
The issuance of a regulatory decision marks the end of the evaluation timeline for a product
application.
Applicants may view the evaluation stage via Track@PRISM. The following
screenshots illustrate the change in stages of a pending application:
Enter PRISM application to
view stage of evaluation.
Choose these options from
the drop-down lists.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 36 of 168
Applicants are also notified via system-generated emails whenever an evaluation
stage change occurs.
After the application is accepted for evaluation, applicants can expect to receive the
first evaluation Input Request by:
Type of Applications
Evaluation Route
No. of working days
NDA
Full
160
NDA
Abridged
120
GDA
Abridged
150
Note: excluding any stop-clock time between acceptance and issuance of first evaluation Input
Request.
9 REGULATORY DECISION
A regulatory decision is made following the conclusion of the benefit-risk
assessment by HSA based on the data submitted in support of the application.
Applicants will be notified of one of the following outcomes:
• Approval – the application satisfies the registration requirements for quality,
safety and efficacy;
• Approvable – when the application can be approved subject to adequate
response to minor deficiencies;
• Non-approvable – when the application has major deficiencies; or
• Rejection – when the response provided by the applicant fails to address the
major deficiencies specified in HSA’s non-approvable decision.
Active Evaluation
The evaluation stage
is seen here.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 37 of 168
‘Approval’ and ‘rejection’ are final decisions issued by HSA.
For an ‘approvable’ application, the applicant will be informed of the conditions for
approval and is required to fulfil these conditions within a stipulated timeframe prior
to the grant of a final approval.
For a ‘non-approvable’ application, the applicant will be informed of the deficiencies
leading to the non-approvable decision. If the applicant wishes to address the
specified deficiencies, the response should be based on the original data set
submitted to HSA and furnished within the stipulated timeframe. New data not
previously reviewed by HSA during the evaluation of the application concerned will
not be accepted.
An application will be considered withdrawn if the applicant fails to reply within the
stipulated timeframe subsequent to an ‘approvable’ or a ‘non-approvable’ decision.
Once the application is withdrawn, it is considered closed and the applicant will be
required to make a new application if he wishes to pursue the regulatory approval
for the product concerned.
Upon an ‘approval’ regulatory decision, the product will be added to the Register of
Therapeutic Products.
HSA may register the product subject to post-approval commitments. In such
circumstances, the applicant will be required to furnish a letter of commitment
stating the undertakings concerned.
Applicants must take note of the registration conditions and the post-approval
commitments specified in the registration. The registration conditions can be viewed
at Enquire@PRISM.
For submission of documents to fulfil registration conditions, please use this form
(Submission of Documents to Fulfil Therapeutic Product Registration Conditions -
https://go.gov.sg/fulfil-tp-reg-conditions ).

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 38 of 168
10 POST-APPROVAL CHANGES
Upon the registration of a product, product registrants are responsible for ensuring
the product’s quality, efficacy and safety through its life cycle.
HSA must be notified of any changes to the product’s quality, efficacy and safety
as per Chapter F of this guidance.
11 TARGET PROCESSING TIMELINES
Please refer to Appendix 5 for information on target processing timelines for the
different application types and evaluation routes.
12 FEES
As the fees may be subject to revision from time to time, applicants are advised to
visit the HSA website for updated information on fees
.
Payment can be made via GIRO or other electronic payment modes such as eNets
or eCredit card.
Regardless of payment mode selection, the collection of both screening and
evaluation fee for applications submitted via the full evaluation route occurs upon
issuance of the screening outcome.
12.1 Screening Fee
A screening fee is payable at the time of online submission via PRISM and is non-
refundable once the application is submitted via PRISM.
For payment via GIRO, the screening fee will be debited upon the successful
submission of an online application.
NOTE: Applicants are strongly encouraged to apply for eGIRO for the convenience of
payment (apply eGIRO).

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 39 of 168
For payment via other electronic payment modes (i.e. eNETs or eCredit card), the
screening fee must be paid before the application is considered successfully
submitted online.
12.2 Evaluation Fee
An evaluation fee is payable upon the acceptance of the dossier for evaluation and
is non-refundable once the application is accepted.
For payments via GIRO, the evaluation fee will be debited upon the acceptance of
the application.
For payments via other electronic payment modes (i.e. eNETs or eCredit card), the
evaluation fee will be collected together with the screening fee. In the event that the
application is not accepted for evaluation, the fee collected will be refunded to the
applicant’s mode of payment.
Applicants may opt for the progressive payment scheme for payment of evaluation
fee. This is an opt-in scheme eligible for applicants who make payment via GIRO
and is only applicable to the application types listed in Table 3:
Table 3 Product Applications Applicable for Progressive Payment Scheme
Percentage of Evaluation Fee Payable at Each Stage
Application
Type
Evaluation
Route
Evaluation Status
Acceptance for
Evaluation
Active Evaluation
in Progress
Evaluation
at Midway
Completed
Evaluation
NDA-1
NDA-2
NDA-3
Full or
Abridged
30%
40%
20%
10%
GDA-1
GDA-2
Abridged,
Verification
or
Verification
-CECA

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 40 of 168
Once the application is submitted, the selected payment scheme (full or
progressive) cannot be amended. Applicants who wish to change their selected
payment scheme will have to withdraw and re-submit the application(s); and any
upfront payment made (e.g. screening fee) is non-refundable.
For applications under the progressive payment scheme, in the event that the
application is withdrawn during the evaluation stage, any fees that had been
charged, but not debited from the GIRO account would remain payable. Any paid
fee is non-refundable.
12.2.1 Changes to Application Types and Re-routing of Evaluation During
Screening
If an application type or evaluation route is incorrectly selected, applicants will be
informed via an Input Request. Such changes may result in a different evaluation
fee upon acceptance of the application.
In the situation where the applicant decides not to pursue the application due to the
changes, the screening fee is not refundable.
For applications which require withdrawal and resubmission, the screening fee is
not refundable.
12.2.1.1 Change of Sub-Type within the Same Application Type
This refers to a change in the sub-type of the selected application type (e.g. from
NDA-1 to NDA-2, NDA-2 to NDA-3, or GDA-1 to GDA-2).
The applicant will be informed of the change via an Input Request. However,
applicants should not amend the application type field in the PRISM application
form. The change will be effected by HSA at the point of acceptance of the
application.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 41 of 168
In the situation where the applicant decides not to pursue the application due to the
said change, the applicant must withdraw the application prior to acceptance to
avoid the evaluation fee being charged.
12.2.1.2 Change of Application between Different Application Types
This refers to a change in the application type between GDA to NDA or vice versa.
The applicant will be required to withdraw and resubmit the application if the
applicant intends to pursue the application.
12.2.1.3 Change of Evaluation Route
This refers to a change in evaluation route (e.g. Full to Abridged, Verification to
Abridged, Abridged to Verification, etc.).
The applicant will be required to withdraw and resubmit the application if the
applicant intends to pursue the application.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 42 of 168
CHAPTER C NEW DRUG APPLICATION SUBMISSION
This chapter applies to new drug applications for products containing new chemical
and biological entities. Applicants are advised to refer to Chapter E for new drug
applications for biosimilar products.
13 APPLICATION TYPES
NDA
New Drug Application
NDA-1:
For the first strength of a product containing a new
6
chemical or biological
entity.
NDA-2:
(a) For the first strength of a product
(i) containing a new combination of registered chemical or biological
entities;
(ii) containing registered chemical or biological entity(ies) in a new
dosage form (e.g. tablets, capsules, injectables), new presentation
(e.g. single-dose vials, multi-dose vials, pre-filled syringe, starter
packs), or new formulation (e.g. preservative-free);
(iii) containing registered chemical or biological entity(ies) for use by a
new route of administration; or
(iv) containing registered chemical or biological entity(ies) for new
indication(s), dosage recommendation(s) and/or patient
population(s).
(b) For products that do not fall under the descriptions for NDA-1, NDA-3 or
GDA.
NDA-3:
For subsequent strength(s) of a product that has been registered or has
been submitted as an NDA-1 or NDA-2. The product name, active
ingredient, dosage form, presentation, indication, dosing regimen and
patient population should be the same as that for the NDA-1 or NDA-2.
6
i.e. not a currently registered entity in Singapore.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 43 of 168
14 EVALUATION ROUTES
There are three evaluation routes for an NDA – full, abridged and verification
evaluation routes. The eligibility criteria are different for each evaluation route.
Applicants should be familiar with the criteria for each evaluation route because
each route has different documentary requirements.
Figure 3 is a schematic diagram illustrating the evaluation routes for NDAs:
14.1 Full Evaluation Route
Full evaluation applies to a product that has not been approved by any drug
regulatory agency at the time of submission.
For a submission under the full evaluation route, the applicant is required to notify
HSA at least two months prior to the intended submission date of the application
dossier. The notification should include information on the product name (if
available), active ingredient(s), summaries of the quality, non-clinical and clinical
data (e.g. Module 2.4 Non-clinical Overview, Module 2.5 Clinical Overview),
planned submissions in other countries, and the planned date of submission to
HSA.
14.2 Abridged Evaluation Route
Abridged evaluation applies to a product that has been approved by at least one
drug regulatory agency at the time of submission.
Approved by
HSA’s reference
agencies and
meets
verification
criteria?
Is the product
registered with
any drug
regulatory
agency?
NO
FULL ROUTE
ABRIDGED
ROUTE
YES
NO
YES
VERIFICATION
ROUTE
NDA
Figure 3 Schematic Diagram of Evaluation Routes for NDAs

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 44 of 168
14.2.1 Priority Review
For NDAs submitted via the abridged evaluation route, the applicant may request
for priority review for a life-saving drug if there are unmet medical needs. The
following are the criteria that will be considered for granting a priority review:
(a) The drug is intended for the treatment of a serious life-threatening condition and
demonstrates the potential to address local unmet medical needs, as defined
by:
(i) the absence of a treatment option; or
(ii) the lack of safe and effective alternative treatments, such that the drug would
be a significant improvement compared to available marketed products, as
demonstrated by
(A) evidence of increased effectiveness in treatment, prevention, or
diagnosis;
or
(B) elimination or a substantial reduction of a treatment-limiting drug
reaction.
(b) Disease conditions that are of local public health concern will be given primary
consideration for priority review. Currently these include:
(i) cancer; and
(ii) infectious diseases: dengue, tuberculosis, hepatitis and malaria.
The request for priority review should be made at the point of the application
submission and accompanied by justifications (attached in PRISM; see section 15.1
– Introduction (CTD/PRISM section 1.3)) for requesting for a priority review and how
the product is expected to benefit patients, as substantiated by the following
evidence:
• The seriousness of the disease condition, local and worldwide mortality rates,
anticipated morbidity and debilitation as a consequence of the disease;
• Local epidemiology data and disease burden;
• The unmet needs, current available treatment options and standard therapies,
and the inadequacy of current therapies;

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 45 of 168
• The extent to which the product is expected to have a major impact on medical
practice, its major benefit, and how it addresses the unmet needs; and
• Clinical evidence supporting the claims of significant improvement compared to
available treatments.
HSA reserves the right to deny a request for priority review if it is deemed
appropriate. The decision for the granting of priority review would be conveyed to
the applicant at the point of acceptance of the application for evaluation.
14.3 Verification Evaluation Route
Therapeutic products with similar indication(s), dosing regimen(s), patient group(s),
and/or direction(s) for use that have been approved by at least two of HSA’s
reference drug regulatory agencies may be eligible for submission via the
verification evaluation route. HSA’s reference drug regulatory agencies are:
• EMA via the Centralised Procedure
• FDA
• Health Canada
• MHRA via
- the national procedure, or
- as the Reference Member State (RMS) via the Mutual Recognition
Procedure or Decentralised Procedure on or prior to 31 January 2020
• Swissmedic
• TGA
However, approval by these reference drug regulatory agencies does not oblige
HSA to approve the application. HSA may also re-categorise applications to other
evaluation routes if the applications did not meet the eligibility criteria and/or
submission requirements.
The applicant must confirm one of the reference drug regulatory agencies as the
primary reference agency. The chosen primary reference agency is defined as the
reference drug regulatory agency from which the qualifying supporting documents
(as outlined in this guidance) will be submitted.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 46 of 168
The pre-requisite requirements for the verification route include:
• The product has received full marketing approval by the reference agencies
following a complete independent scientific assessment (i.e. the approval is not
granted on the basis of less comprehensive data than normally would require or
subject to post-approval conditions that require submission of additional data to
confirm the product’s benefit-risk profile);
• The application must be submitted to HSA within three years from the date of
approval by the chosen primary reference agency;
• A declaration letter issued by the product owner/applicant must be provided
stating that all aspects of the drug product’s quality, including but not limited to
the formulation, manufacturing site(s), release and shelf life specifications and
primary packaging, are identical to that currently approved by the chosen
primary reference agency at the time of submission. However, a different
container closure system type (e.g. Alu/Alu blister vs. HDPE bottle) may be
proposed to meet ASEAN stability requirements;
• If a Drug Master File is submitted, then a separate declaration letter issued by
the applicant must also be provided to state that the DMF submitted to HSA is
identical to that submitted to the chosen primary reference agency;
• The product does not need an independent assessment by HSA to contextualise
the benefit-risk profile due to local disease epidemiology, medical practice
and/or public health considerations. Examples of products that may require such
contextualised assessment are anti-infectives, vaccines, etc.; and
• The product and its intended use – i.e. indication(s), dosing regimen(s) and
patient group(s) – have not been rejected, withdrawn, or approved via appeal
process or are not pending deferral by a drug regulatory agency for safety and/or
efficacy reasons.
The proposed indication(s), dosing regimen(s), patient group(s) and/or direction(s)
for use should be the most stringent among those approved by the reference drug
regulatory agencies. In the event that the chosen primary reference agency does
not bear the most stringent indication(s), dosing regimen(s), patient group(s) and/or
direction(s) of use, the clinical assessment report from the reference drug regulatory
agency that does meet these requirements should be submitted. Reports from the

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 47 of 168
public domain are acceptable. The proposed PI/PIL should be identical to that
bearing the most stringent indication(s), dosing regimen(s), patient group(s) and/or
direction(s) of use (with the exception of country-specific information).
14.3.1 NDA-3 Applications
For the NDA-3 application type, the verification evaluation route may be applied to
the registration of subsequent strengths of a currently-registered product in
Singapore. To qualify for the verification evaluation route for an NDA-3 application:
• if the product has been evaluated and approved by at least one of HSA’s
reference drug regulatory agencies, then the NDA-3 must be submitted within
two years from the date of approval by that reference drug regulatory agency;
or
• if the product has been evaluated and approved by at least two of HSA’s
reference drug regulatory agencies, then the NDA-3 must be submitted within
three years from the date of approval by the chosen primary reference agency.
All other eligibility criteria for the verification evaluation route as stated in section
14.3 above will apply to NDA-3 applications except for the following:
• The proposed indication(s), dosing regimen(s), patient group(s), and/or
direction(s) for use must be identical to the corresponding approved NDA-1
and/or NDA-2 product(s); and
• The proposed PI/PIL should also be consistent with that currently approved for
the corresponding NDA-1 and/or NDA-2 product(s).
15 DOCUMENTARY REQUIREMENTS
Table 4 outlines the CTD Modules/Parts required for NDAs submitted under each
evaluation route:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 48 of 168
Table 4 Dossier Submission Requirements for NDAs
Documents
Location in
Module/Part required for
ICH
CTD
ACTD
Full
NDA
Abridged NDA
Verification NDA
Administrative
Documents
Module
1
Part I
Yes
Yes
Yes
Common
Technical
Document
Overview and
Summaries
Module
2
Incorporate
d in Parts
II, III and
IV
Yes
Yes
Yes
Quality
documents
Module
3
Part II
Yes
Yes
Yes
Non-clinical
documents
Module
4
Part III
Yes
ICH: No
#
ACTD: Overview
only
ICH: No
#
ACTD: Overview
only
Clinical
documents
Module
5
Part IV
Yes
Study report(s) of
pivotal studies
and synopses of
all studies (phase
I-IV) relevant to
requested
indication, dosing
and/or patient
group
Study report(s) of
pivotal studies
and synopses of
all studies (phase
I-IV) relevant to
requested
indication, dosing
and/or patient
group
#
Non-clinical overview included in Module 2 of the ICH CTD.
15.1 Administrative Documents
The administrative documents relate to Module 1 of the ICH CTD or Part I of the
ACTD and are applicable to all evaluation routes for NDAs. The following sections
are to be submitted:
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 49 of 168
Cover Letter (to attach under CTD/PRISM section 1.2 - Introduction)
To include a cover letter stating the product name, and the number of CD/DVDs
submitted in the application dossier.
Applicants should provide a concise and precise summary of the application in the
Cover Letter.
Applicants should ensure that the application dossier is complete. The omission of
any documents within the dossier or any deviation from the guidelines must be
appropriately justified.
Requests for priority review should be stated in the Cover Letter, with the
justification document appended in this section.
Comprehensive Table of Contents (CTD/PRISM section 1.1)
The comprehensive table of contents is a complete list of all documents provided
in the application dossier listed by Module/Part. The location of each document
should be identified by the Module/Part number.
Labelling, Package Insert and Patient Information Leaflet (CTD/PRISM section 1.4)
All proposed labels are to be submitted for registration in Singapore. Applicants are
required to provide the artwork/drafts of the proposed Singapore product labels, PI
and/or PIL for the product. The submission of the proposed PI or PIL is dependent
on the forensic classification of the product to be registered, as described in Table
5:
NOTE: Applicants must complete the relevant checklist found in Appendix 2A or
Appendix 3A and attach the completed checklist under PRISM section 1.2
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 50 of 168
Table 5 Submission of Proposed PI or PIL According to Forensic
Classification in Singapore
Forensic Classification in
Singapore
POM
P
GSL
Package Insert (PI), also known as
prescribing information, SPC, or
product monograph
Required
Optional
Optional
Patient Information Leaflet (PIL), also
known as consumer medicine
information (CMI)
Optional,
unless
warranted
Required
Required
One PI and/or PIL should be registered for each product application. If multiple
manufacturing sites are proposed for registration, information for all sites should be
included in one PI and/or PIL. If there are different strengths or dosage forms, the
submission of one common PI/PIL for all strengths or dosage forms is encouraged.
If separate PI/PILs are to be registered for different strengths or dosage forms, the
content should be consistent across the PI/PILs, except for strength/dosage form-
specific information.
All artwork and drafts should be legible. The draft artwork of the outer carton and
inner/blister labels should be consistent with the format, design and colour that are
to be printed. Separate labels must be submitted for each different pack size of the
drug product.
Handwritten information is not acceptable, with the exception of statements such
as ‘batch number and expiry dates will be printed’ or similar on the outer carton or
inner/blister labels. Movable text boxes/pictures placed over other hidden
information/text are also not acceptable.
The product labels, PI and/or PIL must be in English. If non-English text is included
in the labelling, applicants must provide an official statement to declare that the non-
English text is complete, accurate and unbiased information and is consistent with
the English text.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 51 of 168
Appendix 7 contains specific details on the product labelling requirements for
Singapore.
Approved SPC/PI/PIL (CTD/PRISM section 1.5)
In this section, the applicant should submit the following:
(a) The approved SPC, PI and/or PIL from the drug regulatory agency that issued
the proof of approval; and
(b) the approved SPC, PI and/or PIL from all of HSA’s reference drug regulatory
agencies, where applicable.
The country from which the submitted SPC, PI and/or PIL originates should be
appropriately indicated (e.g. in the document file name).
Assessment Report from Reference Agencies (CTD/PRISM section 1.6)
This section refers only to applications submitted under the verification evaluation
route. Assessment reports and supporting documents issued by the primary
reference agency and inserted into this section must be unredacted and unedited.
Applicants should refer to section 15.6.3 for specific details on the required
documents.
Description of Batch Numbering System (CTD/PRISM section 1.7)
Detailed information on the system of assigning unique codes to different
production batches of the product should be provided to allow for batch
identification. Where applicable, examples of the batch numbering system should
be included to illustrate how the batch number enables identification.
Proof of Approval (CTD/PRISM sections 1.8, 1.9)
Proof of approval is not required for NDAs undergoing a full evaluation.
For an abridged evaluation of an NDA, proof of approval from a competent drug
regulatory agency is required.
A competent drug regulatory agency refers to a national regulatory authority
participating in the World Health Organization’s Certification Scheme on the Quality

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 52 of 168
of Pharmaceutical Products Moving in International Commerce, and listed as such
on the World Health Organization’s website.
Proof of approval must come in the form of:
• a Certificate of Pharmaceutical Product (CPP) that is valid at the time of
submission; or
• an official approval letter that certifies the product’s registration status in the
country at the point of submission to HSA); and
• the SPC, PI and/or PIL approved by the drug regulatory agency that issued the
proof of approval.
For a verification evaluation of an NDA, proof of approval from at least two (or at
least one or two for NDA-3, depending on the eligibility criteria stated in section
14.3.1 NDA-3 Applications) of HSA’s reference drug regulatory agencies, including
the chosen primary reference agency, is required.
If the SPC is in a non-English language, applicants should refer to section 6.2.2
Language and Translation for more information on acceptable translations.
Note that all aspects of the product’s quality and intended direction(s) for use in
Singapore should be the same as those approved by the drug regulatory agency
that issued the proof of approval.
If information such as the product formula, manufacturing sites, etc. are present in
the CPP, the information should be consistent with that proposed for the Singapore
market.
Electronic CPPs issued by the regulatory authority are acceptable.
NOTE: CPPs that indicate that the product is not licensed in the exporting country (including
scenario where the product is licensed for “solely for export only”) are not acceptable proof
of approval.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 53 of 168
Approval letters should either be an original copy or a certified true copy and in
English. Applicants should refer to sections 6.2.2 Language and Translation and
6.2.3 Certifying Non-Original Documents for more details.
Reference to drug regulatory authority websites in the form of website screenshot
and URL (for the website) for confirmation of the approval status of the products by
that regulatory authority are acceptable, provided that the product’s identity and
product’s ownership can be confirmed from that website, and the information stated
on the website is in English.
HSA reserves the right to request for an original hardcopy of the Certificate of
Pharmaceutical Product (CPP), if deemed appropriate.
If the brand name (trade name) of the product registered in the country which issued
the proof of approval is different from that proposed in Singapore, the applicant is
required to submit a declaration letter from the product owner to declare that both
products marketed under the different brand names are identical in all aspects of
quality, safety and efficacy except for the brand name.
Authorisation Letters (CTD/PRISM section 1.10)
All submitted authorisation letters should be on the authorising company’s (i.e.
product owner’s) letterhead, dated and signed by the designated authorised person
in the company.
If the product owner is not the local applicant, manufacturer and/or batch releaser;
or the product owner’s address is different from that of the local applicant,
manufacturer and/or batch releaser, then the following authorisation letter(s) must
be submitted:
(a) from Product Owner to the Applicant (Company) (1.10.1) – this letter authorises
the local applicant to apply for and be the product registrant for a specific
therapeutic product (product name to be stated as in PRISM) and be responsible
for all matters pertaining to the registration of this product in Singapore.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 54 of 168
(b) from Product Owner to Manufacturer (1.10.2) – this letter authorises the
specified manufacturer to produce, pack and/or label the drug product intended
for Singapore. If there are multiple drug product manufacturers, then the
applicant may opt to submit one authorisation letter which clearly states all of
the manufacturers (names and addresses) and their responsibilities relating to
the drug product (such as the manufacturing operation of each manufacturer in
relation to the product being submitted). For biologic drug products, an additional
authorisation letter from the product owner to the drug substance manufacturer
is required.
(c) from Product Owner to Batch Releaser (1.10.3) – this letter authorises the
specified company to batch release the drug product. If there are multiple sites
responsible for the batch release of the product, then the applicant may opt to
submit one authorisation letter which clearly states all of the batch releasers
(names and addresses) and their responsibilities.
The applicant may also issue an authorisation letter to authorise the specified
secondary packager located in Singapore to pack and/or label the drug product
intended for Singapore.
Applicants are to ensure that all names and addresses in the authorisation letters
are consistent with the information provided in PRISM and the dossier. For
manufacturers and batch releasers, the actual site address of the named company
should be stated in the letter(s) – i.e. do not state the office address. Any
discrepancy found will delay the registration process.
All authorisation letters should also state specific product details, including the
product name, dosage form and strength as stated in the PRISM application form.
Applicants also have the option to combine the authorisation letters as stated above
into one document, provided that all names, addresses and responsibilities are
clearly stated.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 55 of 168
GMP Certification/Proof of GMP Compliance (CTD/PRISM section 1.11)
(a) Local Manufacturers
(i) Drug substance manufacturer
(ii) Finished product manufacturer
A valid TPML is required at the point of product application submission.
The valid and active TPML number of the manufacturer must be stated in the
product application, and the submission of additional documentary evidence for
the manufacturer is not required.
(b) Overseas Manufacturers and Batch Releasers
Documentary evidence must be provided to certify that the manufacturer(s)
complies with current applicable GMP standards.
The proof of GMP compliance has to be submitted for all the following sites
mentioned in the product submission:
(i) Drug substance manufacturer
7
An applicant may submit any of the following types of GMP compliance evidence
to support the application:
• Valid PIC/S GMP certificate with the drug substance (DS) of interest stated.
If the DS of interest is not specified on the GMP certificate, a Written
Confirmation
8
for the DS of interest from the PIC/S authority which issued
the GMP certificate is to be supplemented;
7 With effect from 1 October 2024, it is mandatory to provide evidence of GMP compliance for chemical drug
substance manufacturers.
8 Written Confirmation using the European Union (EU) template or any other official document from
the PIC/S authority is acceptable.
A valid Active Ingredient Manufacturer’s Licence (AIML) is required at the
point of application submission. If the site is also involved in the manufacturing
of the finished product, the Therapeutic Product Manufacturer’s Licence
(TPML) may be submitted.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 56 of 168
• GMP inspection report, with the DS of interest included in the scope, together
with the close-out letter (where applicable) for PIC/S authorities which do not
issue GMP certificates;
• Valid Active Pharmaceutical Ingredient (API) Registration Certificate
covering the DS of interest listed on EudraGMDP, the database of the
European Community of manufacturing authorisations and of certificates of
good manufacturing practice;
• Certificate of Pharmaceutical Product (CPP) – Active Pharmaceutical
Ingredient (API) issued by FDA for the DS of interest;
• Other evidence such as a manufacturing licence issued by a PIC/S authority
covering the DS of interest and demonstrating that the site complies with
GMP requirements.
For applications supported by a valid CEP for the DS of interest, HSA leverages the
GMP compliance assessment under the EDQM Inspection Program for the site(s)
specified in the CEP, and submission of proof of GMP compliance for the drug
substance manufacturer is optional.
Proof of GMP compliance is required for micronisation and sterilisation sites of the
drug substance if these operations are performed by a different manufacturer. The
GMP compliance evidence should indicate the specific manufacturing operation,
i.e., micronisation or sterilisation.
(ii) Finished product manufacturer performing bulk production (including
solvent/diluent and drug product intermediate), primary and secondary
packaging activities and batch releaser
An applicant may submit any of the of the following documents as supporting
evidence:
• GMP certificate issued by a drug regulatory agency;
• CPP that bears the manufacturer’s name(s) and address(es) and states that
the certifying authority conducts periodic inspection of the manufacturing
plant in which the manufacturing activity and dosage form is produced; or

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 57 of 168
• Reference to Eudra GMP.
Applicants making reference to the Eudra GMP as proof of GMP compliance
should provide a screen capture of the Eudra GMP for the specific finished
product manufacturing site, as well as the URL to the website. Applicants should
note that the names and addresses of all manufacturers should be consistent
throughout the application – i.e. GMP certificate, Letter of Authorisation, CTD
section S.2.1 and P.3.1 and PRISM.
Certain accreditation documents/certificates issued by other drug regulatory
agencies (for example, PMDA Accreditation Certificate of Foreign Drug
Manufacturer, FDA Establishment Licence, Health Canada Establishment
Licence) are not acceptable proof of GMP compliance.
Proof of GMP compliance must be valid at the time of submission to HSA and
must be in English. Applicants should refer to section 6.2.2 Language and
Translation.
If the submitted proof of GMP compliance is no longer valid or has less than 1
month’s validity at the time of acceptance of the application for evaluation, HSA
reserves the right to request for a commitment letter from the applicant to submit
an updated and valid proof of GMP compliance by a specified date post-
acceptance.
It should be noted that diluents used for reconstituting the drug product and are
packaged together with the drug product will be considered as part of the final
drug product. Thus, manufacturer(s) of the supplied diluent(s) must follow the
same requirements applicable to the drug product, e.g. provide proof of GMP
compliance.
For products manufactured in the USA or Canada, if the applicant is unable to
obtain any proof of GMP compliance (in the form of a CPP or GMP certificate)
from either FDA/Health Canada or other drug regulatory agencies, the applicant
is required to submit the latest Establishment Inspection Report (EIR) issued by
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 58 of 168
FDA or Inspection Exit Notice issued by Health Canada, and any other relevant
supporting documents
9
as proof of GMP compliance. The applicant is also
required to provide the following information if not found in the inspection
reports:
• the last date of audit by FDA/Health Canada;
• the approved dosage forms;
• any licensing conditions or restrictions;
• the scope of the inspection; and/or
• objective evidence and the date of a satisfactory close-out of the latest
inspection conducted by FDA/Health Canada.
For products manufactured in Switzerland, if the applicant is unable to obtain
any proof of GMP compliance (in the form of a CPP or GMP certificate) from
either Swissmedic or other drug regulatory agencies, the Manufacturer’s
Licence issued by Swissmedic is an acceptable documentary GMP evidence.
(c) GMP Conformity Assessment for Overseas Finished Product Manufacturers by
HSA
If the drug product is manufactured by a new overseas drug product manufacturing
site not previously registered with HSA before 1
st
April 2004, a GMP Conformity
Assessment will be conducted by HSA. Thus, when applicable, applicants must
also submit the application form to request for GMP Evidence Evaluation or for an
Overseas GMP Audit with the required documents to the Therapeutic Products
Branch (as part of the product registration application) as stipulated in the Guidance
Notes on GMP Conformity Assessment of an Overseas Manufacturer. The relevant
GMP Conformity Assessment application form should be completed and submitted
as a supporting document in the product registration application. Hardcopy
submission is not required.
9
Any other supporting document which declares GMP compliance of the manufacturing site in the US and
signed by an official of the FDA.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 59 of 168
In general, a GMP Conformity Assessment is applicable to each specific
manufacturer, dosage form, manufacturing activities and applicant company. This
also includes contract manufacturers who perform certain manufacturing activities,
such as terminal sterilisation of the drug product, mixing of excipient with drug
substance (drug product intermediate), etc.
In general, applicant companies with active product registration that included the
finished product manufacturer(s) performing the same manufacturing activities
(excluding parametric release) for the same dosage form would not be required to
undergo another GMP Conformity Assessment by HSA when they submit a new
product application which included the same finished product manufacturer(s).
For drug product manufacturing sites that use parametric release (e.g. where a
terminally sterilised product is released based on the review of manufacturing
process data instead of sterility testing), a GMP Conformity Assessment is required
for overseas drug product manufacturers. Eligibility criteria for such applications for
overseas manufacturing sites are:
a) Country of origin must be a PIC/S country; and
b) Parametric release is approved by the local authority.
If the above criteria are met, then an approval letter and recent documentary
evidence of approval status (e.g. GMP Certificate) for parametric release issued by
the local authority should be provided. The manufacturing site and the product
proposed for parametric release should be clearly stated on these documents.
For a local manufacturing site that would like to apply for parametric release,
applicants are advised to contact HSA prior to submission as pre-approval
inspection is required.
HSA reserves the right to request for a GMP Conformity Assessment if deemed
necessary, or to request for additional or updated documents as evidence of GMP
compliance during the course of the registration process. HSA also reserves the
right to conduct an audit of any overseas manufacturer irrespective of the

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 60 of 168
documentary GMP evidence that is approved by HSA or any other PIC/S member
authorities, if deemed appropriate.
If in doubt whether a GMP Conformity Assessment by HSA is required for the
manufacturing sites included in the submission, applicants are encouraged to
complete and submit applications to request for GMP Conformity Assessment with
the product registration application, and HSA will advise accordingly whether the
GMP Conformity Assessment would be required.
Please note that GMP Conformity Assessment application forms submitted without
a valid pending product registration application would not be considered.
Patent Declaration (CTD/PRISM section 1.12)
A signed and dated patent declaration form is required for each NDA. Applicants
should refer to section 3 for information on patent linkage and Appendix 1 – Form
1 for the Patent Declaration Form.
Guiding notes on filling the Patent Declaration form are provided below:
(a) Section 1 ‘Applicant Particulars’ state the name and address of the local
company.
(b) Section 2 ‘Product Particulars’ state the product name, name and strength of
active ingredient and dosage form. All product particulars should be consistent
with that stated in the product labels and other relevant documents as submitted
in PRISM.
(c) Section 3 ‘Application Category’ declare the patent category (A1, A2, A3, or B)
that the application falls under.
(d) Section 4 ‘Information for Category A1 Applications’ applicable if category A1
is selected in Section 3.
(e) Section 5 ‘Information for Category A2 Applications’ applicable if category A2
is selected in Section 3. Check the box which is relevant and provide details of
the restraining patent in force.
(f) Section 6 ‘Information for Category A3 Applications’ applicable if category A3
is selected in Section 3. Provide details of the restraining patent in force.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 61 of 168
(g) Section 7 ‘Information for Category B Applications’ applicable if category B is
selected in Section 3. Check the box which is relevant and provide details of the
restraining patent in force.
(h) Section 8 ‘Declaration’ the patent declaration must be signed by the person
authorised to make the declaration on behalf of the company named in Section
1. The authorised person is ordinarily an officer of the company such as
Company Director or Company Secretary as registered with ACRA, or
equivalent. Evidence of such authorisation is to be submitted together with the
declaration.
Evidence of authorisation for Section 8 of the form can be in the form of:
• An ACRA printout
10
(BizFile) listing the Company Directors/Secretary;
• A resolution of board of directors;
• A resolution of a general meeting of the company; or
• An extract of the relevant portion of the company’s articles of association.
Declaration forms must bear the signatures of the authorised person in the
company.
The patent declaration form needs to be submitted twice – at the time of submitting
the application for registration and prior to the issuance of the regulatory decision
for registration (upon request by HSA), if the dossier was deemed satisfactory with
respect to the product’s safety, efficacy and quality aspects.
Declaration on Rejection, Withdrawal and Deferral (CTD/PRISM section 1.13)
The document required for this section is a declaration letter issued by the product
owner or applicant that states that the application submitted to HSA and the
directions of use including indication(s), dosing regimen(s) and patient population(s)
10
The required information on the company’s business profile should be obtained directly from ACRA’s
website (BizFile).
NOTE: The applicant should ensure that the information provided in the patent
declaration form and the evidence of authorisation are current at the point of application
submission.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 62 of 168
• have not been rejected or withdrawn;
• have not been approved via an appeal process; and
• are not pending deferral
by any drug regulatory agency. If any of the above conditions apply to the
application, details and reasons must be provided to HSA.
Declaration for NDA Verification (CTD/PRISM section 1.14)
This section applies only to the verification evaluation route.
A declaration letter issued by the product owner/applicant must be provided to state
that all aspects of the product’s quality are identical to that currently approved by
the chosen primary reference agency at the time of submission. Quality aspects
include, but are not limited to, formulation, manufacturing site(s), release and shelf
life specifications, and primary packaging. However, a different container closure
system type (e.g. Alu/Alu blister vs. HDPE bottle) may be proposed to meet ASEAN
stability requirements.
If a Drug Master File is submitted, then a separate declaration letter issued by the
applicant must also be provided to state that the DMF submitted to HSA is identical
to that submitted to the chosen primary reference agency.
Registration Status in Other Countries (CTD/PRISM section 1.15)
The registration status of the product in other countries should be entered into
PRISM section 4.9 – refer to Appendix 17 for further details.
In the event that the PRISM text space does not allow the input of the full details of
the indication(s) and/or reason(s), a brief description may be entered. The full
details should then be attached in softcopy (PDF) in PRISM section 7 (Supporting
Attachments). The document should be in the format shown in Table 6:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 63 of 168
Table 6 Example of a Table of Information on Registration Status in Other
Countries for CTD Section 1.15
Country
Application
status
Status
Date
Approved application
indication/dosing
regimen details
#
Approved
forensic
classification
+
Country 1
Approved
12 Jan
2005
Adjuvant treatment of
colorectal cancer stage III
(Dukes C) following
complete resection of
primary tumour.
POM
Country 2
Approved
2 Feb 2006
Adjuvant treatment of
colorectal cancer following
surgery
POM
Country 3
Withdrawn
by applicant
14 Apr
2002
Indication submitted
‘Adjuvant treatment of
colorectal cancer’.
Withdrawn due to
insufficient long term
efficacy data (only phase II
data submitted).
Re-submitted on 16 June
2005 with completed
phase III data for ‘Adjuvant
treatment of colorectal
cancer following surgery’.
POM

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 64 of 168
Country
Application
status
Status
Date
Approved application
indication/dosing
regimen details
#
Approved
forensic
classification
+
Country 4
Approved
21 Nov
2004
Adjuvant treatment of
colorectal cancer stage III
(Dukes C) following
complete removal of
primary tumour.
Notice of Compliance with
Conditions issued on 16
April 2003 based on
promising efficacy results
with condition to furnish
confirmatory efficacy data.
POM
Country 5
Pending
Submitted:
15 Jun
2005
Adjuvant treatment of
colorectal cancer stage III
(Dukes C) following
surgery.
POM
#
Applicable to information on reference agencies, Country of Origin, and all
rejections/withdrawals/deferrals
+
Applicable to information on reference agencies and Country of Origin.
Confirmation of Reference Agency’s Approval of Chemistry & Manufacturing
Control (CMC) Aspects (CTD/ PRISM section 1.16)
For applications submitted under the abridged evaluation route and for which
approval was obtained from at least one of HSA’s reference agencies not more than
5 years before the date of submission to HSA, a copy of the completed Dossier
Clarification Supplement should be submitted in PRISM (refer to Appendix 18
Confirmation of Quality Dossiers with Reference Agency’s Approval for more
information).

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 65 of 168
15.2 CTD Overview and Summaries
The ICH or ASEAN CTD overview and summary documents are to be inserted into
Module 2 of the ICH CTD or into the relevant sections in Part II, III and IV of the
ACTD. The ICH or ASEAN Quality Overall Summary can be submitted either
in Word or PDF format.
Overview and
Summaries
Location in CTD
ICH CTD
ACTD
Quality Overall Summary
Module 2, section 2.3
Part II, section B
Non-clinical Overview &
Summaries
Module 2, section 2.4 &
2.6
Part III, sections B & C,
respectively
Clinical Overview &
Summaries
Module 2, section 2.5 &
2.7
Part IV, sections B & C,
respectively
15.3 Quality Documents
The quality documents relate to Module 3 of the ICH CTD or Part II of the ACTD.
In addition to the ICH or ACTD technical content requirements, the following
explanatory notes pertain to requirements specific to Singapore:
15.3.1 Body of Data – Drug Substance
The ICH M4Q Technical Guidelines and ASEAN Common Technical Requirements
(ACTR) provide details on the information to be included in the drug substance
sections of an application dossier.
All of the drug substance sections of the CTD – i.e. S.1 to S.7, are required in the
application. Alternatively, applicants may submit either a Drug Master File (DMF) or
NOTE: If a drug product contains more than one drug substance, the information within
Module 3.2.S (ICH CTD) or Part II.S (ACTD) must be provided in its entirety for each
drug substance.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 66 of 168
Certificate of Suitability of Monographs of the European Pharmacopoeia (CEP) in
lieu of the entire CTD drug substance sections.
Drug Master File (DMF)
A Drug Master File (DMF) is a compilation of information on facilities, processes or
components used in the manufacturing, processing, packaging and storing of a
drug that is submitted to the HSA. A DMF contains confidential information and is
submitted solely at the discretion of the DMF holder.
A DMF is submitted in support of a therapeutic product application and will be
reviewed only in connection with the review of an application. As such, a separate
approval will not be issued for a DMF.
Appendix 11 describes the DMF process and documentary requirements for DMF
submission.
Plasma Master File (PMF)
A Plasma Master File (PMF) contains information on the collection and control of
source materials, and is required where a human plasma-derived product is used
either as a starting material or as an excipient. The PMF may be submitted either
as a stand-alone document or as part of the application dossier. Appendix 8
describes the PMF data requirements for submission.
If the PMF is a stand-alone document, it should be filed separately from the
application dossier for pre-marketing evaluation. The applicant may cross-reference
a PMF of a human plasma-derived product that is used in currently registered
products, where applicable.
Certificates of Suitability (CEP)
A Certificate of Suitability is a document issued by the European Directorate for the
Quality of Medicines and Healthcare (EDQM) that certifies the quality of a drug
substance in compliance to the Ph. Eur. A CEP may be submitted in lieu of the CTD
S Section or a DMF.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 67 of 168
If reference is made to a CEP, the applicant must submit a copy of the valid CEP,
including all annexes, and a copy of the Letter of Access from the CEP holder to
authorise the applicant to refer to the CEP in support of their application.
The following additional requirements apply for CEP-based submissions:
(a) If Ph. Eur. standard is claimed for the drug substance, the relevant CTDs should
be submitted:
(i) S.2.1;
(ii) S.4.1 and S.4.4 from both the drug substance and drug product
manufacturers; and
(iii) S.6 and S.7 should be provided if the re-test period/shelf life is not stated
on the CEP.
(b) If other standards are claimed for the drug substance, the relevant CTDs
should be submitted:
(i) S.2.1;
(ii) S.4.1 to S.4.5 from both drug substance and drug product manufacturers;
and
(iii) S.6 and S.7 should be provided if re-test period/shelf life is not stated on
the CEP.
If there is a CEP for animal-derived material used in the drug product, the applicant
may submit the CEP together with the documents stipulated in Annex 1- section 1.1
of Appendix 9 Guideline on the Registration of Human Medicinal Products
Containing Materials of Animal Origin.
It is the applicant’s responsibility to submit the latest CEP updates, with annexes,
as soon as they are available from EDQM.
Control of Drug Substance (CTD section 3.2.S.4)
NOTE: HSA reserves the right to request for any additional information about the CEP-
certified drug substance if it is deemed appropriate.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 68 of 168
As the drug product manufacturer is responsible for the quality control of the drug
substance that is used in the drug product, information on the control of the drug
substance, i.e., 3.2.S.4.1 to 3.2.S.4.5, must be provided from both the drug
substance and drug product manufacturers.
Batch analysis data should be provided by the drug substance and drug product
manufacturers, and, if available, from the same drug substance batches. Analytical
results should be provided from a minimum of two batches from each proposed
drug substance manufacturer and should be sufficient to support the
specification(s) as well as to demonstrate consistency in manufacturing. The
batches submitted should preferably be of production scale or at least pilot scale.
Stability Data of Drug Substance (CTD section 3.2.S.7)
At the time of submission, the minimum stability data required are as follows:
• At least 12 months of long term data and 6 months of accelerated data from at
least three primary batches of the drug substance; and
• The batches should be at least of pilot scale and manufactured by a method
that simulates the final commercial process.
Where multiple drug substance manufacturers are proposed for registration, if it can
be demonstrated that the submitted data is representative of the proposed sites, it
may be acceptable to extrapolate the stability data from one site to the other sites,
and stability data from each site may not be required at the point of submission. All
the following criteria must be met for the stability data to be considered
representative:
• The drug substance is manufactured using the same synthetic route and
process. Scientific justification should be provided to demonstrate equivalence
between the sites if differences exist;
• The drug substance is controlled by the same set of specifications;
• The drug substance is packaged in the same container closure system; and
• The drug substance is of comparable quality to the drug substance used in the
stability batches.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 69 of 168
If any of the above criteria are not met, site-specific stability data are required to
support the application.
In addition, a commitment to conduct stability studies for one production batch of
drug substance is required for each site that is not represented in the submitted
stability studies.
Stability data from a site not proposed for registration may also be provided as
supporting data.
15.3.2 Body of Data – Drug Product
The ICH M4Q technical guideline and ACTR also provide details on the information
to be included in the drug product sections of an application dossier. For drug
product intermediates and diluents, separate drug product sections should be
submitted.
Pharmaceutical Development (CTD section 3.2.P.2)
Detailed descriptions and discussions, with relevant data, which relate to the
development, and hence quality, of the drug product should be provided in the
relevant dossier sections. Examples include, but are not limited to:
• polymorphism, solubility or particle size of the drug substance and its effect on
the product’s quality;
• a description and the results of the formulation development;
• the rationale for the choice of dissolution method and a discussion of its
discriminatory nature, with data;
• compatibility of the container closure system with the product or preservative
efficacy test results; and
• optimisation of the manufacturing process, with data.
Process Validation (CTD section 3.2.P.3.5)
The description, documentation and complete results of the validation studies on
the manufacturing process should be provided in the dossier in this section.
Particular care should be taken to ensure that the documents include critical

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 70 of 168
processes for the manufacturing process: for example, blend uniformity validation
for oral dosage forms and terminal sterilisation or aseptic filling for sterile products.
Applicants should refer to the ASEAN Guidelines on Submission of Manufacturing
Process Validation Data for Drug Registration and the ASEAN Guideline on
Process Validation Q&A for the minimum data requirements for process validation.
Other relevant international guidelines may also be referred to as appropriate.
Where ranges of batch sizes are proposed, it should be demonstrated that
variations in batch size would not adversely alter the characteristics of the finished
product.
For drug product manufacturing sites that use parametric release (e.g. where a
terminally sterilised product is released based on the review of manufacturing
process data instead of sterility testing), a more detailed discussion with supporting
data on the process validation of the specific product in the proposed pack size or
fill volume should be provided. In addition, risk assessment for parametric release
should be based on prior knowledge, consistency of performance of the steriliser,
historical batch analysis data, risk of loading pattern/container/ contamination from
the environment to product sterility, re-processing plan, etc. A detailed discussion
on the control strategy should also be provided. This includes but is not limited to,
a tabulation of all validated critical process parameters and loading patterns, a
description of the process and requirements for the release/rejection of a batch,
bioburden monitoring and control program, the segregation of sterile products from
non-sterile products and the routine maintenance/re-validation program for the
steriliser.
Control of Excipients (CTD section 3.2.P.4)
This section refers to all excipients used in the drug product formulation, including
ingredients used in capsule shells and film coatings. The specifications and
analytical method(s) for each excipient should be described, including the validation
of any in-house test method(s), if applicable.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 71 of 168
Information on proprietary ingredients, such as flavourings, colourants, coatings,
perfumes and/or printing inks, should be as detailed as possible. Applicants are
advised not to use internal codes but instead to give commercial names for such
ingredients. In cases where the formula of the proprietary ingredient is confidential,
only the total quantity of the proprietary ingredient present in the final product needs
to be captured in PRISM. The formula of the proprietary ingredient should then be
provided by the proprietary ingredient manufacturer directly to HSA. A declaration
letter from the proprietary ingredient manufacturer should also be provided,
indicating that they will inform HSA (via email to [email protected])
and the applicant should there be any change in the formulation of the proprietary
ingredient.
A CoA for an excipient may be submitted in lieu of the excipient’s specifications. If
the standard claimed for an excipient is an officially recognised compendial
standard, it is sufficient to state that the excipient is tested according to the
requirements of that standard, rather than reproducing the specifications found in
the officially recognised compendial monograph.
For excipients derived from human plasma, applicants should refer to the Appendix
8 on the data requirements.
For excipients derived from animal sources, applicants should refer to Appendix 9
for more information. The checklist found in Annex 1 of Appendix 9 serves as a
guide to these documentary requirements for submission. Applicants should note
that the completed checklist in Annex 1 is to be submitted in CTD section 3.2.P.4.5
with the supporting documents submitted in ICH CTD section 3.2.A.2 or ACTD
section Q.A.2.
For milk and certain milk derivatives such as lactose, as these excipients are
generally considered non-infectious, a declaration from the supplier of the excipient
stating that the milk is from healthy cows fit for human consumption and that no
other potentially infectious ruminant-derived materials were used in the
manufacturing process would be sufficient. This declaration is to be submitted in
CTD section 3.2.P.4.5.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 72 of 168
Control of Drug Product (CTD section 3.2.P.5)
The drug product’s release and shelf-life specifications should be declared in
section 3.2.P.5.1.
For the parametric release of a terminally sterilised product, the release
specification and certificate of analysis should indicate that parametric release is
the method used for batch release. Additionally, sterility of the product is required
to be demonstrated in the stability studies even if approval for parametric release
has been granted.
Descriptions of all test methods with complete validation results of all in-house
methods should be submitted in sections 3.2.P.5.2 and 3.2.P.5.3.
Descriptions (including size, origin and use) and test results of all relevant batches
(e.g. pre-clinical, clinical, pilot and production batches) used to establish the
specification and evaluate the consistency in manufacturing should be provided.
Batch analysis data and/or CoAs from three batches of the drug product should be
provided in section 3.2.P.5.4.
The justification of the specifications (section 3.2.P.5.6) should be based on
scientific knowledge and data collected during product development.
Container Closure System (CTD section 3.2.P.7)
Technical information about each component of the container closure system(s)
used for the drug product should be included in the dossier. The technical
information to be included in the dossier includes, but is not limited to, schematic
diagrams, descriptions, specifications, analytical methods, CoAs and declarations
of compliance to international standards.
Stability Data of Drug Product (CTD section 3.2.P.8)
Since 01 April 2014, HSA has implemented the ASEAN Guideline on Stability Study
of Drug Product, a guideline on the conduct of stability studies for drug products for

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 73 of 168
the ASEAN region. Applicants should familiarise themselves with this guideline prior
to submission.
At the time of submission of the application, the minimum stability data required are
as follows:
• At least 12 months of data under long term storage conditions and 6 months of
data under accelerated storage conditions on at least three primary batches of
the drug product; and
• The primary batches should be at least of pilot scale, manufactured by the same
manufacturing process and packaged in the same container closure system as
that proposed for Singapore.
Where multiple drug product manufacturers are proposed for registration, if it can
be demonstrated that the submitted data is representative of the proposed sites, it
may be acceptable to extrapolate the stability data from one site to the other sites,
and stability data from each site may not be required at the point of submission. All
the following criteria must be met for the stability data to be considered
representative:
• The drug product is manufactured using the same formulation;
• The drug product is manufactured using the same manufacturing process,
including equipment type, process parameters and in-process tests. Scientific
justification should be provided to demonstrate equivalence between the sites
if differences exist.
• The drug product is controlled by the same set of specifications;
• The drug product is packaged in the same container closure system; and
• The drug product is of comparable quality to the drug product used in the
stability batches.
If any of the above criteria are not met, site-specific stability data are required to
support the application.
In addition, a commitment to conduct stability studies for one production batch of
drug product is required for each site that is not represented in the submitted
stability studies.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 74 of 168
Stability data from a site not proposed for registration may also be provided as
supporting data.
Where possible, batches of drug product should be manufactured using different
batches of drug substance. If multiple drug substance manufacturers are proposed
for any of the drug substances in the drug product, a commitment to conduct drug
product stability studies for one production batch using the drug substance from
each drug substance manufacturer that is not represented in the drug product
stability batches is required.
If multiple primary packaging sites for the same container closure system are
proposed for registration, transport validation of the bulk product to the other
proposed primary packaging site(s) is required, unless otherwise justified.
15.4 Non-clinical Documents
The non-clinical documents relate to Module 4 of the ICH CTD or Part III of the
ACTD.
Applicants should refer to the ICH CTD Guidelines M4S (Safety) technical
guidelines or the ACTD Part III: Non-clinical guidelines for detailed information on
the contents of non-clinical documents for the application dossier.
15.5 Clinical Documents
The clinical documents relate to Module 5 of the ICH CTD or Part IV of the ACTD.
Guidance on how to complete this Module/Part is provided in the ICH CTD
Guidelines M4E (Efficacy) technical guidelines, in particular the ICH E3 guidance
document on Structure and Contents of Clinical Study Reports, or the ACTD Part
IV: clinical Guidelines.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 75 of 168
Clinical studies should generally be conducted using the drug product formulation
submitted in the application and in the appropriate patient population for the
indication(s) and/or dosing regimen(s) as requested in the NDA.
Biopharmaceutic (e.g. bioavailability or bioequivalence) study reports are required
if the commercial formulation for the Singapore market differs from the clinical trial
formulation used in the pivotal studies.
The submission of risk management plans (RMPs) in support of all NDA-1
applications is mandatory. For NDA-2 or NDA-3 applications, HSA may also request
for RMPs to be submitted on a case-by-case basis when required, following the
evaluation of the safety concerns described in the product application. Guidance on
RMP submission requirements can be found here.
If the NDA is for a non-prescription medicine and is submitted via the abridged
evaluation route, the applicant may submit a written request for a waiver of clinical
data submission. Eligibility for a waiver is subject to the criteria defined in Appendix
6 Guideline on Submission for Non-Prescription Therapeutic Products. However,
HSA reserves the right to request for the complete clinical data set if it is deemed
necessary.
15.6 Documentary Requirements for Each Evaluation Route
15.6.1 Full Evaluation Route
Full information on the chemical/biological development, pharmaceutical/genetic
development, toxicological, pharmacological and clinical data needs to be
submitted in support of the application.
The technical documents required include:
• complete quality documents for both drug substance and drug product;
• complete pharmaco-toxicological or non-clinical documents; and
• complete clinical documents, i.e. all study reports from phase I to phase III,
including tables and appendices.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 76 of 168
15.6.2 Abridged Evaluation Route
All aspects of the product’s quality and direction(s) for use [including dosing
regimen(s), indication(s) and patient group(s)] should be the same as that approved
by the drug regulatory agency that issued the proof of approval.
The technical documents required include:
• complete quality documents for both the drug substance and drug product;
• a non-clinical overview; and
• a clinical overview, summaries of clinical efficacy and clinical safety, synopses
of relevant studies, a tabular listing of the clinical development programme and
study reports of the pivotal studies (the tables and appendices to the pivotal
study reports may be submitted upon request by HSA).
15.6.3 Verification Evaluation Route
The complete assessment report and other relevant supporting documents from the
chosen primary reference agency must be submitted, as tabulated below. The
assessment reports from the primary reference agency must be unredacted or
unedited, and should include details of imposed licensing conditions, final product
labelling, quality and clinical reviews, and other information in relation to the
product’s approval. Reports obtained from the public domain are deemed
unacceptable.
Applications submitted to HSA without the unredacted/ unedited reports from the
primary reference agency will not be accepted for evaluation via the verification
route and rejected at screening.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 77 of 168
Primary
reference
agency
Documentary requirements
EMA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
FDA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
Health Canada
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
MHRA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
Swissmedic
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 78 of 168
Primary
reference
agency
Documentary requirements
TGA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
#
If the drug substance section is submitted to the primary reference agency as a Drug
Master File (DMF), the complete assessment report for the DMF, including the assessment
on the Question & Answer (Q&A) documents between the DMF Holder & Agency and all
annexes should be provided. Assessment reports, approval letters and/or documents
pertaining to post-approval DMF updates should also be submitted, if applicable.
Administrative documents specific to the verification evaluation route that are
required at the time of submission include:
(a) Section 1.9 – Official approval letters, or equivalent documents, from the
relevant reference drug regulatory agencies that certify the registration status of
the drug product;
(b) Section 1.13 – Official letter declaring that the application submitted to HSA or
similar direction(s) of use, indication(s), dosing regimen(s) and/or patient
group(s) have not been rejected, withdrawn, approved via appeal process
11
, or
pending deferral
12
by any drug regulatory agency, with reasons in each case if
applicable;
(c) Section 1.14 – Official letter declaring that the Drug Master File provided is the
same as that submitted to the primary reference agency, if applicable; and
(d) Section 1.14 – Official letter declaring that all aspects of the product’s quality
intended for sale in Singapore are identical to that currently approved by the
primary reference drug regulatory agency. This includes, but is not limited to,
11
Approval via appeal process includes, but is not limited to, the following: approval following negative
opinion, approval following rejection, approval following non-approvable etc.
12
Deferral includes, but is not limited to, the following: non-approvable, approvable, conditional approval,
conditional marketing authorisation, notice of compliance with conditions etc.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 79 of 168
the formulation, site(s) of manufacture, release and shelf life specifications and
primary packaging.
The technical documents required include:
• Quality documents:
- From Sponsor:
▪ Complete documents for both drug substance and drug product (ICH
Module 3/ACTD Part II) as initially submitted to the primary reference
agency;
▪ Complete assessment reports including assessment on the Question
& Answer documents between the Sponsor and primary reference
agency, and other relevant supporting documents from the primary
reference agency;
▪ Questions and answers between the primary reference agency and
Sponsor – the answers should include the supporting documents used
in response to the questions;
▪ All post-approval variations (if applicable)
approved by the primary
reference agency up to the time of submission to HSA, including the
application letter for the variation, supporting documents for the
variation, assessment report for the variation, questions and answers
between the primary reference agency and Sponsor and the approval
letter for the variation from the primary reference agency; and
▪ Relevant documents required by HSA which have not been submitted
to the primary reference agency, e.g. stability studies in accordance to
ASEAN Stability Guidelines.
- From DMF Holder, if applicable:
▪ The initial open and closed parts of the DMF submitted to the primary
reference agency should be provided to HSA, together with a copy of
the Letter of Access;
▪ Complete DMF assessment report including assessment on the
Question & Answer documents between the DMF holder and the
primary reference agency, and other relevant supporting documents
from the primary reference agency;

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 80 of 168
▪ Questions and answers between the primary reference agency and
DMF Holder – the answers should include supporting documents used
in response to the questions; and
▪ All post-approval DMF updates (if applicable) approved by the primary
reference agency up to the time of submission to HSA, including the
application letter for the DMF update, supporting documents for the
DMF update, assessment report for the DMF updates, questions and
answers between the primary reference agency and Sponsor, and the
approval letter for the DMF update from the primary reference agency.
• Non-clinical overview; and
• Clinical documents, assessment report from the primary reference agency,
including assessment on the Question and Answer documents between the
Sponsor and Agency, and other relevant supporting documents from the primary
reference agency
All of the data submitted to HSA must be the same as the data package submitted
to the reference drug regulatory agencies. Differences between the dossier
submitted to HSA and data reviewed by the reference drug regulatory agencies will
not only delay the processing of the application, but may also lead to re-routing of
the dossier to the abridged evaluation route if significant undisclosed differences
are discovered.
In the event that the chosen primary reference agency does not bear the most
stringent indication(s), dosing regimen(s), patient group(s) and/or direction(s) of use
among those approved by the reference drug regulatory agencies, a supplemental
clinical assessment report from the reference drug regulatory agency that approved
the most stringent indication(s), dosing regimen(s), patient group(s) and/or
direction(s) of use is required. Reports from the public domain are acceptable. The
proposed PI/PIL should be identical to that bearing the most stringent indication(s),
dosing regimen(s), patient group(s) and/or direction(s) of use (with the exception of
country-specific information).

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP
Page 81 of 168
CHAPTER D GENERIC DRUG APPLICATION SUBMISSION
This chapter applies to applications to register generic products.
A generic drug application applies to a therapeutic product that contains one or
more chemical entities, and that is essentially the same with a current registered
product with respect to its qualitative and quantitative composition of active
ingredients, pharmaceutical dosage form and clinical indication.
Follow-on biologic products (also known as biosimilar products) are not eligible for
a GDA and are required to be submitted via a NDA.
16 APPLICATION TYPES
There are two application types for a generic drug application:
GDA Generic Drug Application
GDA-1:
For the first strength of a generic chemical product.
GDA-2:
For subsequent strength(s) of the generic chemical product that has been
registered or submitted as GDA-1. The product name and dosage form
should be the same as that for the GDA-1.
In cases where multiple strengths of a generic product are submitted together, the
strength of the product used in the BE study is considered as a GDA-1. The
remaining strength(s) should be submitted as GDA-2.
16.1 Generic Product
A generic product must have the same qualitative and quantitative composition in
active ingredients and be of the same pharmaceutical form as a currently registered
product in Singapore (known as the ‘Singapore reference product’). A generic
product must demonstrate bioequivalence to the Singapore reference product via
appropriate bioequivalence studies.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 82 of 168
The generic product must fulfil the following criteria:
(a) the generic product is the same pharmaceutical dosage form as the Singapore
reference product. However, different conventional oral immediate-release
dosage forms (i.e. tablets and capsules) are considered to be the same
pharmaceutical form;
(b) the route of administration of the generic product is the same as the Singapore
reference product;
(c) the conditions of use for the generic product fall within the directions for use
(including indication(s), dosing regimen(s) and patient group(s)) for the
Singapore reference product; and
(d) the generic product is bioequivalent with the Singapore reference product.
16.2 Singapore Reference Product
The Singapore reference product must be a currently registered product that has
been granted market authorisation based on the evaluation of the product’s quality,
efficacy and safety – i.e. a dossier with chemical, biological, pharmaceutical,
pharmacological-toxicological and clinical data. If such a reference product is not
registered in Singapore, then an alternate registered comparator product may be
used if adequately justified (e.g. a registered generic therapeutic product widely
used by local hospitals) by the applicant and agreed upon by HSA.
The generic product should contain the same active ingredient(s) and strength(s)
and be the same pharmaceutical dosage form as the Singapore reference product.
For generic products containing a different salt, ester, ether, isomer, mixture of
isomer, complex or derivative of the active ingredient compared to the Singapore
reference product, applicants are required to submit data to demonstrate that the
different form does not differ from the active ingredient in the Singapore reference
product in terms of safety and/or efficacy.
Applicants are advised to search Register of Therapeutic Products to identify the
Singapore reference product.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 83 of 168
Applicants submitting GDAs should also refer to Appendix 10 and Common
questions related to generic drug applications for further details on product
interchangeability and biowaiver requests.
17 EVALUATION ROUTES
There are three evaluation routes for a GDA: abridged, verification and verification-
CECA evaluation routes. The eligibility criteria are different for each evaluation
route. Applicants should be familiar with the criteria for each evaluation route
because each route has different documentary requirements.
Figure 4 is a schematic diagram illustrating the evaluation routes for GDAs:
17.1 Abridged Evaluation Route
Abridged evaluation applies to a product that has been approved by at least one
drug regulatory agency at the time of submission.
17.2 Verification Evaluation Route
Therapeutic products that have been approved by at least one of HSA’s reference
drug regulatory agencies may be eligible for submission via the verification
evaluation route. HSA’s reference drug regulatory agencies are:
• EMA via the Centralised Procedure
Approved by
at least one
of HSA’s
reference
agencies
and meets
verification
criteria?
GDA
ABRIDGED
ROUTE
VERIFICATION
ROUTE
Product is
manufactured
in India and
meets CECA
scheme
criteria?
VERIFICATION
-CECA ROUTE
NO
YES
NO
YES
Figure 4 Schematic Diagram of Evaluation Routes for GDAs

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 84 of 168
• FDA
• Health Canada
• MHRA via
- the national procedure, or
- as the Reference Member State (RMS) via the Mutual Recognition
Procedure or Decentralised Procedure on or prior to 31 January 2020
• Swissmedic
• TGA
However, approval by these reference drug regulatory agencies does not oblige
HSA to approve the application.
The pre-requisite requirements for the verification route include:
(a) The application must be submitted to HSA within two years from the date of
approval by the chosen reference drug regulatory agency;
(b) A declaration letter issued by the product owner/applicant must be provided
stating that all aspects of the product’s quality, including but not limited to the
formulation, manufacturing site(s), release and shelf life specifications and
primary packaging, are identical to that currently approved by the chosen
reference drug regulatory agency at the time of submission. However, a different
container closure system type (e.g. Alu/Alu blister vs. HDPE bottle) may be
proposed to meet ASEAN stability requirements;
(c) If a Drug Master File is submitted, then a separate declaration letter issued by
the applicant must also be provided to state that the DMF submitted to HSA is
identical to that submitted to the chosen reference drug regulatory agency;
(d) The product and its intended use – i.e. indication(s), dosing regimen(s) and
patient group(s) – have not been rejected, withdrawn, or approved via appeal
process or are not pending deferral by a drug regulatory agency for efficacy
and/or safety reasons.
The chosen reference drug regulatory agency is defined as the reference drug
regulatory agency for which the qualifying supporting documents (as outlined in this
guidance) will be submitted.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 85 of 168
18 DOCUMENTARY REQUIREMENTS
Table 7 outlines the CTD Modules/Parts required for GDAs submitted under each
evaluation route:
Table 7 Dossier Submission Requirements for a GDA
Documents
Location in
Module/Part required for
ICH
CTD
ACTD
Abridged
GDA
Verification
GDA
Verification
-CECA GDA
Administrative
Documents and
Product
Information
Module
1
Part I
Yes
Yes
(Refer to
section
18.5.2)
Yes
(Refer to
section
18.5.2)
Common
Technical
Document
Overview and
Summaries
Module
2
Incorporat
ed into
Part II
QOS
QOS
QOS
Quality
documents
Module
3
Part II
Yes
Yes
(Refer to
section
18.5.2)
Yes
(Refer to
section
18.5.2)
Non-clinical
documents
Module
4
Part III
No
No
No
Clinical
documents
Module
5
Part IV
BE studies
or biowaiver
justification
may be
inserted in
this section
Yes
(same
dataset as
that
submitted to
RA)
Yes
(same
dataset as
that
submitted to
RA)
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 86 of 168
18.1 Administrative Documents
The administrative documents relate to Module 1 of the ICH CTD or Part I of the
ACTD and are applicable to the evaluation routes for GDAs. The following sections
are to be submitted:
Cover Letter (to attach under CTD/PRISM section 1.2 - Introduction)
To include a cover letter stating the product name, and the number of CD/DVDs
submitted in the application dossier.
Applicants should ensure that the application dossier is complete. The omission of
any documents within the dossier or any deviation from the guidelines must be
appropriately justified.
Applicants should also give a concise summary of the application – for example, an
overview of the bioequivalence study and the Singapore reference product used in
support of the application.
Comprehensive Table of Contents (CTD/PRISM section 1.1)
The comprehensive table of contents is a complete list of all documents provided
in the application dossier listed by Module/Part. The location of each document
should be identified by the Module/Part number.
Labelling, Package Insert and Patient Information Leaflet (CTD/PRISM section 1.4)
All proposed labels are to be submitted for registration. Applicants are required to
provide the artwork/drafts of the proposed Singapore product labels, PI and/or PIL
for the product. The clinical information in the proposed PI/PIL should be consistent
with that currently approved for the Singapore reference product.
The submission of the proposed PI or PIL is dependent on the forensic classification
of the product to be registered, as described in Table 8:
NOTE: Applicants must complete the relevant checklists found in Appendix 2A or
Appendix 3A and attach the completed checklist under PRISM section 1.2

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 87 of 168
Table 8 Submission of Proposed PI or PIL According to Forensic
Classification in Singapore
Forensic Classification in
Singapore
POM
P
GSL
Package Insert (PI), also known as
prescribing information, SPC, or
product monograph
Required
Optional
Optional
Patient Information Leaflet (PIL), also
known as consumer medicine
information (CMI)
Optional,
unless
warranted
Required
Required
One PI and/or PIL should be registered for each product application. If multiple
manufacturing sites are proposed for registration, information for all sites should be
included in one PI and/or PIL. If there are different strengths or dosage forms, one
common PI/PIL for all strengths or dosage forms is encouraged. If separate PI/PILs
are to be registered for different strengths or dosage forms, the content should be
consistent across the PI/PILs, except for strength/dosage form-specific information.
All artwork and drafts should be legible. The draft artwork of the outer carton and
inner/blister labels should be consistent with the format, design and colour that are
to be printed. Separate labels must be submitted for each different pack size of the
drug product.
The product labels, PI and/or PIL must be in English. If non-English text is included
in the labelling, applicants must provide an official statement to declare that the non-
English text is complete, accurate and unbiased information and is consistent with
the English text.
Appendix 7 of this guidance contains specific details on the product labelling
requirements for Singapore.
Approved SPC/PI/PIL (CTD/PRISM section 1.5)

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 88 of 168
In this section, the applicant should submit the approved SPC, PI and/or PIL from
the drug regulatory agency that issued the proof of approval.
For applications submitted under the verification and verification-CECA evaluation
routes, the SPC/PI/PIL approved by the chosen reference drug regulatory agency
should be submitted.
The country from which the submitted SPC, PI and/or PIL originates should be
appropriately indicated (e.g. in the document file name).
Assessment Report from Reference Agencies (CTD/PRISM section 1.6)
This section refers only to applications submitted under the verification or
verification-CECA evaluation routes. Assessment reports and supporting
documents issued by the chosen reference drug regulatory agency and inserted
into this section must be unredacted and unedited. Applicants should refer to
section 18.5.2 Verification and Verification-CECA Evaluation Routes for specific
details on the required documents.
Description of Batch Numbering System (CTD/PRISM section 1.7)
Detailed information on the system of assigning unique codes to different
production batches of the product should be provided to allow for batch
identification. Where applicable, examples of the batch numbering system should
be included to illustrate how the batch number enables identification.
Proof of Approval (CTD/PRISM sections 1.8, 1.9)
Proof of approval is not required for GDAs undergoing abridged evaluation for
finished products manufactured (up to primary packaging) in Singapore.
For an abridged evaluation of an imported GDA, proof of approval from a competent
drug regulatory agency is required. For a verification or verification-CECA
evaluation of a GDA, proof of approval from the chosen reference drug regulatory
agency is required.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 89 of 168
A competent drug regulatory agency refers to a national regulatory authority
participating in the World Health Organization’s Certification Scheme on the Quality
of Pharmaceutical Products Moving in International Commerce, and listed as such
on the World Health Organization’s website.
Proof of approval must come in the form of:
• a Certificate of Pharmaceutical Product (CPP) that is valid at the time of
submission; or
• an official approval letter that certifies the product’s registration status in the
country at the point of submission to HSA); and
• the SPC, PI and/or PIL approved by the drug regulatory agency that issued the
proof of approval.
If the SPC is in a non-English language, applicants should refer to section 6.2.2
Language and Translation of this guidance document for more information on
acceptable translations.
Note that all aspects of the product’s quality should be the same as those approved
by the drug regulatory agency that issued the proof of approval.
If information such as the product formula, manufacturing sites, etc. are present in
the CPP, the information should be consistent with that proposed for the Singapore
market.
Electronic CPPs issued by the regulatory authority are acceptable.
NOTE: CPPs that indicate that the product is not licensed for marketing in the exporting
country (including scenario where the product is licensed for “solely for export only”) are
not acceptable proof of approval.
Approval letters should either be an original copy or a certified true copy and in
English. Applicants should refer to sections 6.2.2 Language and Translation and
6.2.3 Certifying Non-Original Documents for more details.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 90 of 168
Reference to drug regulatory authority websites in the form of website screenshot
and URL (for the website) for confirmation of the approval status of the products in
that regulatory authority are acceptable, provided that the product’s identity and
product’s ownership can be confirmed from that website, and the information stated
on the website is in English.
HSA reserves the right to request for a Certificate of Pharmaceutical Product (CPP),
if deemed appropriate.
If the brand name (trade name) of the product registered in the country which issued
the proof of approval is different from that proposed in Singapore, the applicant is
required to submit a declaration letter from the product owner to declare that both
products marketed under the different brand names are identical in all aspects of
quality, safety and efficacy except for the brand name.
Authorisation Letters (CTD/PRISM section 1.10)
All submitted authorisation letter(s) should be on the authorising company’s (i.e.
product owner’s) letterhead, dated and signed by the designated authorised person
in the company.
If the product owner is not the local applicant, manufacturer and/or batch releaser;
or the product owner’s address is different from that of the local applicant firm,
manufacturer and/or batch releaser, then the following authorisation letter(s) must
be submitted:
(a) from Product Owner to the Applicant (Company) (1.10.1) – this letter authorises
the local applicant to apply for and be the product registrant for a specific
therapeutic product (product name to be stated as in PRISM) and be responsible
for all matters pertaining to the registration of this product in Singapore.
(b) from Product Owner to Manufacturer (1.10.2) – this letter authorises the
specified manufacturer to produce, pack and/or label the drug product intended
for Singapore. If there are multiple drug product manufacturers, then the
applicant may opt to submit one authorisation letter which clearly states all of
the manufacturers (names and addresses) and their responsibilities relating to

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 91 of 168
the drug product (such as the manufacturing operation of each manufacturer in
relation to the product being submitted).
(c) from Product Owner to Batch Releaser (1.10.3) – this letter authorises the
specified company to batch release the drug product. If there are multiple sites
responsible for the batch release of the product, then the applicant may opt to
submit one authorisation letter which clearly states all of the batch releasers
(names and addresses) and their responsibilities.
The applicant may also issue an authorisation letter to authorise the specified
secondary packager located in Singapore to pack and/or label the drug product
intended for Singapore.
Applicants are to ensure that all names and addresses in the authorisation letter(s)
are consistent with the information provided in PRISM and the dossier. For
manufacturers and batch releasers, the actual site address of the named company
should be stated in the letter(s) – i.e. do not state the office address. Any
discrepancy found will delay the registration process.
All authorisation letters should also state specific product details, including the
product name, dosage form and strength as stated in the PRISM application form.
Applicants also have the option to combine the authorisation letters as stated above
into one document, provided that all names, addresses and responsibilities are
clearly stated.
GMP Certification/Proof of GMP Compliance (CTD/PRISM section 1.11)
a) Local Manufacturers
(i) Drug substance manufacturer
A valid Active Ingredient Manufacturer’s Licence (AIML) is required at the point
of application submission. If the site is also involved in the manufacturing of the

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 92 of 168
finished product, the Therapeutic Product Manufacturer’s Licence (TPML) may
be submitted.
(ii) Finished product manufacturer
A valid TPML is required at the point of product application submission.
The valid and active TPML number of the manufacturer must be stated in the
product application, and the submission of additional documentary evidence for
the manufacturer is not required.
b) Overseas Manufacturers and Batch Releasers
Documentary evidence must be provided to certify that the manufacturer(s)
complies with current applicable GMP standards.
The proof of GMP compliance has to be submitted for all the following sites
mentioned in the product submission:
(i) Drug substance manufacturer
13
An applicant may submit any of the following types of GMP compliance evidence
to support the application:
• Valid PIC/S GMP certificate with the drug substance (DS) of interest stated.
If the DS of interest is not specified on the GMP certificate, a Written
Confirmation
14
for the DS of interest from the PIC/S authority which issued
the GMP certificate is to be supplemented;
• GMP inspection report, with the DS of interest included in the scope, together
with the close-out letter (where applicable) for PIC/S authorities which do not
issue GMP certificates;
13
With effect from 1 October 2024, it is mandatory to provide evidence of GMP compliance for chemical drug
substance manufacturers.
14
Written Confirmation using the European Union (EU) template or any other official document from the PIC/S
authority is acceptable.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 93 of 168
• Valid Active Pharmaceutical Ingredient (API) Registration Certificate
covering the DS of interest listed on EudraGMDP, the database of the
European Community of manufacturing authorisations and of certificates of
good manufacturing practice;
• Certificate of Pharmaceutical Product (CPP) – Active Pharmaceutical
Ingredient (API) issued by FDA for the DS of interest;
• Other evidence such as a manufacturing licence issued by a PIC/S authority
covering the DS of interest and demonstrating that the site complies with
GMP requirements.
For applications supported by a valid CEP for the DS of interest, HSA leverages the
GMP compliance assessment under the EDQM Inspection Program for the site(s)
specified in the CEP, and submission of proof of GMP compliance for the drug
substance manufacturer is optional.
Proof of GMP compliance is required for micronisation and sterilisation sites of the
drug substance if these operations are performed by a different manufacturer. The
GMP compliance evidence should indicate the specific manufacturing operation,
i.e., micronisation or sterilisation.
(ii) Finished product manufacturer performing bulk production (including
solvent/diluent and drug product intermediate), primary and secondary
packaging activities and batch releaser
An applicant may submit any of the of the following documents as supporting
evidence:
• GMP certificate issued by a drug regulatory agency;
• CPP that bears the manufacturer’s name(s) and address(es) and states that the
certifying authority conducts periodic inspection of the manufacturing plant in
which the manufacturing activity and dosage form is produced; or
• Reference to Eudra GMP.
Applicants making reference to the Eudra GMP as proof of GMP compliance should
provide a screen capture of the Eudra GMP for the specific finished product

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 94 of 168
manufacturing site, as well as the URL to the website. Applicants should note that
the names and addresses of all manufacturers should be consistent throughout the
application – i.e. GMP certificate, Letter of Authorisation, CTD section S.2.1 and
P.3.1 and PRISM.
Certain accreditation documents/certificates issued by other drug regulatory
agencies (for example, PMDA Accreditation Certificate of Foreign Drug
Manufacturer, FDA Establishment Licence, Health Canada Establishment Licence)
are not acceptable proof of GMP compliance.
Proof of GMP compliance must be valid at the time of submission to HSA and must
be in English. Applicants should refer to section 6.2.2 Language and Translation.
If the submitted proof of GMP compliance is no longer valid or has less than 1
month’s validity at the time of acceptance of the application for evaluation, HSA
reserves the right to request for a commitment letter from the applicant to submit an
updated and valid proof of GMP compliance by a specified date post-acceptance.
It should be noted that diluents used for reconstituting the drug product and are
packaged together with the drug product will be considered as part of the final drug
product. Thus, manufacturer(s) of the supplied diluent(s) must follow the same
requirements applicable to the drug product, e.g. provide proof of GMP compliance.
For products manufactured in the USA or Canada, if the applicant is unable to
obtain any proof of GMP compliance (in the form of a CPP or GMP certificate) from
either FDA/Health Canada or other drug regulatory agencies, the applicant is
required to submit the latest Establishment Inspection Report (EIR) issued by FDA
or Inspection Exit Notice issued by Health Canada, and any other relevant
NOTE: For applications submitted via the verification-CECA evaluation route, a valid
GMP certificate and the latest inspection report as issued by the chosen reference drug
regulatory agency must be submitted.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 95 of 168
supporting documents
15
as proof of GMP compliance. The applicant is also required
to provide the following information if not found in the inspection reports:
• the last audited date by FDA/Health Canada;
• the approved dosage forms;
• any licensing conditions/restriction;
• the scope of inspection; and/or
• objective evidence and the date of a satisfactory close-out of the latest
inspection conducted by FDA/Health Canada.
For products manufactured in Switzerland, if the applicant is unable to obtain any
proof of GMP compliance (in the form of a CPP or GMP certificate) from either
Swissmedic or other drug regulatory agencies, the Manufacturer’s Licence issued
by Swissmedic is an acceptable documentary GMP evidence.
c) GMP Conformity Assessment for Overseas Finished Product
Manufacturers by HSA
If the drug product is manufactured by a new overseas drug product manufacturing
site not previously registered with HSA before 1
st
April 2004, a GMP Conformity
Assessment will be conducted by HSA. Thus, when applicable, applicants must
also submit the application form to request for GMP Evidence Evaluation or for an
Overseas GMP Audit with the required documents to the Therapeutic Products
Branch (as part of the product registration application) as stipulated in the Guidance
Notes on GMP Conformity Assessment of an Overseas Manufacturer. The relevant
GMP Conformity Assessment application form should be completed and submitted
as a supporting document in the product registration application. Hardcopy
submission is not required.
In general, a GMP Conformity Assessment is applicable to each specific
manufacturer, dosage form, manufacturing activities and applicant company. This
also includes contract manufacturers who perform certain manufacturing activities,
15
Any other supporting document which declares GMP compliance of the manufacturing site in the US and
signed by an official of the FDA.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 96 of 168
such as terminal sterilisation of the drug product, mixing of excipient with drug
substance (drug product intermediate), etc.
In general, applicant companies with active product registration that included the
finished product manufacturer(s) performing the same manufacturing activities
(excluding parametric release) for the same dosage form would not be required to
undergo another GMP Conformity Assessment by HSA when they submit a new
product application which included the same finished product manufacturer(s).
For drug product manufacturing sites that use parametric release (e.g. where a
terminally sterilised product is released based on the review of manufacturing
process data instead of sterility testing), a GMP Conformity Assessment is required
for overseas drug product manufacturers. Eligibility criteria for such applications for
overseas manufacturing sites are:
a) Country of origin must be a PIC/S country
b) Parametric release is approved by the local authority
If the above criteria are met, then approval letter and recent documentary evidence
of approval status (e.g. GMP Certificate) for parametric release issued by the local
authority should be provided. The manufacturing site and the product proposed for
parametric release should be clearly stated on these documents.
For local manufacturing site that would like to apply for parametric release,
applicants are advised to contact HSA prior to submission as pre-approval
inspection is required.
HSA reserves the right to request for a GMP Conformity Assessment if deemed
necessary, or to request for additional or updated documents as evidence of GMP
compliance during the course of the registration process. HSA also reserves the
right to conduct an audit of any overseas manufacturer irrespective of the
documentary GMP evidence that is approved by HSA or any other PIC/S member
authorities, if deemed appropriate.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 97 of 168
If in doubt whether a GMP Conformity Assessment by HSA is required for the
manufacturing sites included in the submission, applicants are encouraged to
complete and submit applications to request for GMP Conformity Assessment with
the product registration application, and HSA will advise accordingly whether the
GMP Conformity Assessment would be required.
Please note that GMP Conformity Assessment application forms submitted without
a valid pending product registration application would not be considered.
Patent Declaration (CTD/PRISM 1.12)
A signed and dated patent declaration form is required for each GDA. Applicants
should refer to section 3 for information on patent linkage and Appendix 1 – Form
1 for the Patent Declaration Form.
Guiding notes on filling the Patent Declaration form are provided below:
(a) Section 1 ‘Applicant Particulars’ state the name and address of the local
company.
(b) Section 2 ‘Product Particulars’ state the product name, name and strength of
active ingredient and dosage form. All product particulars should be consistent
with that stated in the product labels and other relevant documents as submitted
in PRISM.
(c) Section 3 ‘Application Category’ declare the patent category (A1, A2, A3, or B)
that the application falls under.
(d) Section 4 ‘Information for Category A1 Applications’ applicable if category A1
is selected in Section 3.
(e) Section 5 ‘Information for Category A2 Applications’ applicable if category A2
is selected in Section 3. Check the box which is relevant and provide details of
the restraining patent in force.
(f) Section 6 ‘Information for Category A3 Applications’ applicable if category A3
is selected in Section 3. Provide details of the restraining patent in force.
(g) Section 7 ‘Information for Category B Applications’ applicable if category B is
selected in Section 3. Check the box which is relevant and provide details of the
restraining patent in force.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 98 of 168
(h) Section 8 ‘Declaration’ the patent declaration must be signed by the person
authorised to make the declaration on behalf of the company named in Section
1. The authorised person is ordinarily an officer of the company such as
Company Director or Company Secretary as registered with ACRA, or
equivalent. Evidence of such authorisation is to be submitted together with the
declaration.
Evidence of authorisation for Section 8 can be in the form of:
• An ACRA printout
16
(BizFile) listing the Company Directors/Secretary;
• A resolution of board of directors;
• A resolution of a general meeting of the company; or
• An extract of the relevant portion of the company’s articles of association.
Declaration forms must bear the signatures of the authorised person in the
company.
The patent declaration form needs to be submitted twice – at the time of submitting
the application for registration and prior to the issuance of the regulatory decision
for registration (upon request by HSA), if the dossier was deemed satisfactory with
respect to the product’s safety, efficacy and quality aspects.
Declaration on Rejection, Withdrawal and Deferral (CTD/PRISM section 1.13)
The document required for this section is a declaration letter issued by the product
owner or applicant that states that the application submitted to HSA and the
directions of use including indication(s), dosing regimen(s) and patient population(s)
• have not been rejected or withdrawn;
• have not been approved via an appeal process; and
• are not pending deferral
16
The required information on the company’s business profile should be obtained directly from ACRA’s
website (BizFile).
NOTE: The applicant should ensure that the information provided in the patent
declaration form and the evidence of authorisation are current at the point of application
submission.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 99 of 168
by any drug regulatory agency. If any of the above conditions apply to the
application, details and reasons must be provided to HSA.
Declaration for GDA Verification and Verification-CECA (CTD/PRISM section 1.14)
This section applies only to the verification and verification-CECA evaluation routes.
A declaration letter issued by the product owner/applicant must be provided to state
that all aspects of the product’s quality are identical to that currently approved by
the chosen reference drug regulatory agency at the time of submission. Quality
aspects include, but are not limited to, formulation, manufacturing site(s), release
and shelf life specifications, and primary packaging.
If a Drug Master File is submitted, then a separate declaration letter issued by the
applicant must also be provided to state that the DMF submitted to HSA is identical
to that submitted to the chosen reference drug regulatory agency.
Registration Status in Other Countries (CTD/PRISM section 1.15)
The registration status of the product in other countries should be entered into
PRISM section 4.9 – refer to Appendix 17 of this document for further details.
In the event that the PRISM text space does not allow the input of the full details of
the indication(s) and/or reason(s), a brief description may be entered. The full
details should then be attached in softcopy (PDF) in PRISM section 7 (Supporting
Attachments). The document should be in the format shown in Table 6 in section
15.1 of this guidance document.
Confirmation of Reference Agency’s Approval of Chemistry & Manufacturing
Control (CMC) Aspects (CTD/ PRISM section 1.16)
For applications submitted under the abridged evaluation route and for which
approval was obtained from at least one of HSA’s reference agencies not more than
5 years before the date of submission to HSA, a copy of the completed Dossier
Clarification Supplement should be submitted in PRISM (refer to Appendix 18
Confirmation of Quality Dossiers with Reference Agency’s Approval for more
information).
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 100 of 168
18.2 CTD Overview and Summaries
The ICH or ASEAN Quality Overall Summary is to be inserted into Module 2 of the
ICH CTD or into Part II, section B of the ACTD. This document can be submitted
either in Word or PDF format.
18.3 Quality Documents
The quality documents relate to Module 3 of the ICH CTD or Part II of the ACTD. In
addition to the ICH or ACTD technical content requirements, the following
explanatory notes pertain to requirements specific to Singapore:
18.3.1 Body of Data – Drug Substance
The ICH M4Q technical guideline and ASEAN Common Technical Requirements
(ACTR) provide details on the information to be included in the drug substance
sections of an application dossier.
All of the drug substance sections of the CTD – i.e. S1 to S7, are required in the
application. Alternatively, applicants may submit either a Drug Master File (DMF) or
Certificate of Suitability of Monographs of the European Pharmacopoeia (CEP) in
lieu of the entire CTD drug substance sections.
Drug Master File (DMF)
A Drug Master File (DMF) is a compilation of information on facilities, processes or
components used in the manufacturing, processing, packaging and storing of a
drug that is submitted to the HSA. A DMF contains confidential information and is
submitted solely at the discretion of the DMF holder.
NOTE: If a drug product contains more than one drug substance, the information within
Module 3.2.S (ICH CTD) or Part 2.S (ACTD) must be provided in its entirety for each
drug substance.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 101 of 168
A DMF is submitted in support of a therapeutic product application and will be
reviewed only in connection with the review of an application. As such, a separate
approval will not be issued for a DMF.
Appendix 11 describes the DMF process and documentary requirements for DMF
submission.
Plasma Master File (PMF)
A Plasma Master File (PMF) contains information on the collection and control of
source materials, and is required where a human plasma-derived product is used
as an excipient. The PMF may be submitted either as a stand-alone document or
as part of the application dossier. Appendix 8 describes the PMF data requirements
for submission.
If the PMF is a stand-alone document, it should be filed separately from the
application dossier for pre-marketing evaluation. The applicant may cross-reference
a PMF of a human plasma-derived product that is used in currently registered
products, where applicable.
Certificates of Suitability (CEP)
A Certificate of Suitability is a document issued by the European Directorate for the
Quality of Medicines and Healthcare (EDQM) that certifies the quality of a drug
substance in compliance to the Ph. Eur. A CEP may be submitted in lieu of the CTD
S Section or a DMF.
If reference is made to a CEP, the applicant must submit a copy of the valid CEP,
including all annexes, and a copy of the Letter of Access from the CEP holder to
authorise the applicant to refer to the CEP in support of their application.
The following additional requirements apply for CEP-based submissions:
(a) If Ph. Eur. standard is claimed for the drug substance, the relevant CTDs should
be submitted:
(i) S.2.1;

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 102 of 168
(ii) S.4.1 and S.4.4 from both the drug substance and drug product
manufacturers; and
(iii) S.6 and S.7 should be provided if the re-test period/shelf life is not stated
on the CEP.
(b) If other standards are claimed for the drug substance, the relevant CTDs should
be submitted:
(i) S.2.1;
(ii) S.4.1 to S.4.5 from both drug substance and drug product manufacturers;
and
(iii) S.6 and S.7 should be provided if re-test period/shelf life is not stated on
the CEP.
If there is a CEP for animal-derived material used in the drug product, the applicant
may submit the CEP together with the documents stipulated in Annex 1- section 1.1
of Appendix 9 Guideline on the Registration of Human Medicinal Products
Containing Materials of Animal Origin.
It is the applicant’s responsibility to submit the latest CEP updates, with annexes,
as soon as they are available from EDQM.
Control of Drug Substance (CTD section 3.2.S.4)
As the drug product manufacturer is responsible for the quality control of the drug
substance that is used in the drug product, information on the control of the drug
substance, i.e., 3.2.S.4.1 to 3.2.S.4.5, must be provided from both the drug
substance and drug product manufacturers.
Batch analysis data should be provided by the drug substance and drug product
manufacturers, and if available, from the same drug substance batches. Analytical
results should be provided from a minimum of two batches from each proposed
drug substance manufacturer and should be sufficient to support the
specification(s) as well as to demonstrate consistency in manufacturing. The
NOTE: HSA reserves the right to request for any additional information about the CEP-
certified drug substance if it is deemed appropriate.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 103 of 168
batches submitted should preferably be of production scale or at least pilot scale
and should include the batch(es) used in the bioequivalence or biowaiver studies
(where applicable).
Stability Data of Drug Substance (CTD section 3.2.S.7)
At the time of submission, the minimum stability data required are as follows:
• At least 12 months of long term data and 6 months of accelerated data from at
least three primary batches of the drug substance; and
• The batches should be at least of pilot scale and manufactured by a method that
simulates the final commercial process.
Where multiple drug substance manufacturers are proposed for registration, if it can
be demonstrated that the submitted data is representative of the proposed sites, it
may be acceptable to extrapolate the stability data from one site to the other sites,
and stability data from each site may not be required at the point of submission. All
the following criteria must be met for the stability data to be considered
representative:
• The drug substance is manufactured using the same synthetic route and
process. Scientific justification should be provided to demonstrate equivalence
between the sites if differences exist;
• The drug substance is controlled by the same set of specifications;
• The drug substance is packaged in the same container closure system; and
• The drug substance is of comparable quality to the drug substance used in the
stability batches.
If any of the above criteria are not met, site-specific stability data are required to
support the application.
In addition, a commitment to conduct stability studies for one production batch of
drug substance is required for each site that is not represented in the submitted
stability studies.
Stability data from a site not proposed for registration may also be provided as
supporting data.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 104 of 168
18.3.2 Body of Data – Drug Product
The ICH M4Q technical guideline and ACTR also provide details on the information
to be included in the drug product sections of an application dossier. For drug
product intermediates and diluents, separate drug product sections should be
submitted.
Pharmaceutical Development (CTD section 3.2.P.2)
Detailed descriptions and discussions, with relevant data, which relate to the
development, and hence quality, of the drug product should be provided in the
relevant dossier sections. Examples include, but are not limited to:
• polymorphism, solubility or particle size of the drug substance and its effect on
the product’s quality;
• a description and the results of the formulation development;
• the rationale for the choice of dissolution method and a discussion of its
discriminatory nature, with data;
• compatibility of the container closure system with the product or preservative
efficacy test results; and
• optimisation of the manufacturing process, with data.
Process Validation (CTD section 3.2.P.3.5)
The description, documentation and complete results of the validation studies on
the manufacturing process should be provided in the dossier in this section.
Particular care should be taken to ensure that the documents include critical
processes for the manufacturing process: for example, blend uniformity validation
for oral dosage forms and terminal sterilisation or aseptic filling for sterile products.
Applicants should refer to the ASEAN Guidelines on Submission of Manufacturing
Process Validation Data for Drug Registration and the ASEAN Guideline on
Process Validation Q&A for the minimum data requirements for process validation.
Other relevant international guidelines may also be referred to as appropriate.
Where ranges of batch sizes are proposed, it should be demonstrated that
variations in batch size would not adversely alter the characteristics of the finished
product.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 105 of 168
For drug product manufacturing sites that use parametric release (e.g. where a
terminally sterilised product is released based on the review of manufacturing
process data instead of sterility testing), a more detailed discussion with supporting
data on the process validation of the specific product in the proposed pack size or
fill volume should be provided. In addition, risk assessment for parametric release
should be based on prior knowledge, consistency of performance of the steriliser,
historical batch analysis data, risk of loading pattern/ container/ contamination from
the environment to product sterility, re-processing plan and etc. A detailed
discussion on the control strategy should also be provided. This includes but is not
limited to, a tabulation of all validated critical process parameters and loading
patterns, a description of the process and requirements for the release/rejection of
a batch, bioburden monitoring and control program, the segregation of sterile
products from non-sterile products and the routine maintenance/re-validation
program for the steriliser.
Control of Excipients (CTD section 3.2.P.4)
This section refers to all excipients used in the drug product formulation, including
ingredients used in capsule shells and film coatings. The specifications and
analytical method(s) for each excipient should be described, including the validation
of any in-house test method(s) if applicable.
Information on proprietary ingredients such as flavourings, colourants, coatings,
perfumes and/or printing inks should be as detailed as possible. Applicants are
advised not to use internal codes but instead to give commercial names for such
ingredients. In cases where the formula of the proprietary ingredient is confidential,
only the total quantity of the proprietary ingredient present in the final product needs
to be captured in PRISM. The formula of the proprietary ingredient should then be
provided by the proprietary ingredient manufacturer directly to HSA. A declaration
letter from the proprietary ingredient manufacturer should also be provided,
indicating that they will inform HSA (via email to HSA[email protected])
should there be any change in the formulation of the proprietary ingredient.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 106 of 168
A CoA for an excipient may be submitted in lieu of the excipient’s specifications. If
the standard claimed for an excipient is an officially recognised compendial
standard, it is sufficient to state that the excipient is tested according to the
requirements of that standard, rather than reproducing the specifications found in
the officially recognised compendial monograph.
For excipients derived from human plasma, applicants should refer to the Appendix
8 on the data requirements.
For excipients derived from animal sources, applicants should refer to Appendix 9
for more information. The checklist found in Annex 1 of Appendix 9 serves as a
guide to these documentary requirements for submission. Applicants should note
that the completed checklist in Annex 1 is to be submitted in CTD section 3.2.P.4.5
with the supporting documents submitted in ICH CTD section 3.2.A.2 or ACTD
section Q.A.2.
For milk and certain milk derivatives such as lactose, as these excipients are
generally considered non-infectious, a declaration from the supplier of the excipient
stating that the milk is from healthy cows fit for human consumption and that no
other potentially infectious ruminant-derived materials were used in the
manufacturing process would be sufficient. This declaration is to be submitted in
CTD section 3.2.P.4.5.
Control of Drug Product (CTD section 3.2.P.5)
The drug product’s release and shelf-life specifications should be declared in
section 3.2.P.5.1.
For the parametric release of a terminally sterilised product, the release
specification and certificate of analysis should indicate that parametric release is
the method used for batch release. Additionally, sterility of the product is required
to be demonstrated in the stability studies even if approval for parametric release
has been granted.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 107 of 168
Descriptions of all test methods with complete validation results of all in-house
methods should be included in sections 3.2.P.5.2 and 3.2.P.5.3.
Descriptions (including size, origin and use) and test results of all relevant batches
(e.g. pre-clinical, clinical, pilot and production batches) used to establish the
specification and evaluate the consistency in manufacturing should be provided.
Batch analysis data and/or CoAs from three batches of the drug product should be
provided in section 3.2.P.5.4.
The justification of the specifications (section 3.2.P.5.6) should be based on
scientific knowledge and data collected during product development.
Container Closure System (CTD section 3.2.P.7)
Technical information about each component of the container closure system(s)
used for the drug product should be included in the dossier. The technical
information to be included in the dossier includes, but is not limited to, schematic
diagrams, descriptions, specifications, analytical methods, CoAs and declarations
of compliance to international standards.
Stability Data of Drug Product (CTD section 3.2.P.8)
Since 01 April 2014, HSA has implemented the ASEAN Guideline on Stability Study
of Drug Product, a guideline on the conduct of stability studies for drug products for
the ASEAN region. Applicants should familiarise themselves with this guideline prior
to submission.
At the time of submission of the application, the minimum stability data required are
as follows:
• For critical dosage forms or unstable drug substances, at least 12 months of
data under long term storage conditions and 6 months of data under accelerated
storage conditions on at least three primary batches of the drug product
• For conventional dosage forms and stable drug substances, at least 6 months
of data under long term storage conditions and 6 months of data under

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 108 of 168
accelerated storage conditions on at least two primary batches of the drug
product
• The primary batches should be at least of pilot scale, manufactured by the same
manufacturing process and packaged in the same container closure system as
that proposed for Singapore.
Where multiple drug product manufacturers are proposed for registration, if it can
be demonstrated that the submitted data is representative of the proposed sites, it
may be acceptable to extrapolate the stability data from one site to the other sites,
and stability data from each site may not be required at the point of submission. All
the following criteria must be met for the stability data to be considered
representative:
• The drug product is manufactured using the same formulation;
• The drug product is manufactured using the same manufacturing process,
including equipment type, process parameters and in-process tests. Scientific
justification should be provided to demonstrate equivalence between the sites if
differences exist.
• The drug product is controlled by the same set of specifications;
• The drug product is packaged in the same container closure system; and
• The drug product is of comparable quality to the drug product used in the stability
batches.
If any of the above criteria are not met, site-specific stability data are required to
support the application.
In addition, a commitment to conduct stability studies for one production batch of
drug product is required for each site that is not represented in the submitted
stability studies.
Stability data from a site not proposed for registration may also be provided as
supporting data.
Where possible, batches of drug product should be manufactured using different
batches of drug substance. If multiple drug substance manufacturers are proposed

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 109 of 168
for any of the drug substances in the drug product, a commitment to conduct drug
product stability studies for one production batch using the drug substance from
each drug substance manufacturer that is not represented in the drug product
stability batches is required.
If multiple primary packaging sites for the same container closure system are
proposed for registration, transport validation of the bulk product to the other
proposed primary packaging site(s) is required, unless otherwise justified.
Product Interchangeability – Bioequivalence (CTD section 3.2.P.9.1)
Since 01 April 2004, in vivo bioequivalence (BE) data are required for Prescription
Only Medicines (POM) in oral solid dosage forms.
GDA-2 applications will also require BE data if the application is for a POM in an
oral solid dosage form, even if the first strength (GDA-1) application was submitted
to HSA before 01 April 2004.
Applicants should be familiar with Appendix 10 Product Interchangeability and
Biowaiver Request for Chemical Generic Drug Applications.
Applicants should ensure that the submitted BE study is complete, including all
appendices and data, according to the relevant guidelines. Examples of information
to be included in the report are:
(a) Signature of the Principal Investigator to attest the authenticity of the report;
(b) Audit certificate(s);
(c) BE site inspection report from a national regulatory agency or WHO, if available;
(d) Approval letter(s) from the Institutional Review Board/Independent Ethics
Committee and the appropriate drug regulatory agency;
(e) Information about the reference and test products, such as the product name,
strength, dosage form, batch number, manufacturing site, batch size of the test
product, etc.;
(f) Certificates of Analysis of the reference and test products used in the BE study,
including the batch size of the test product and manufacturing/expiry date of
both products (where applicable);

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 110 of 168
(g) Bioanalytical study report and description of the bioanalytical method validation;
and
(h) A signed statement confirming that the test product used in the BE study is the
same formulation and is manufactured by the same process as that submitted
for registration.
Applicants should also provide a copy of the product labels (e.g. outer carton,
product insert) of the reference product used in the BE study for verification
purposes.
It is highly recommended that the generic or test product used in the BE study be
the same as the drug product submitted for registration in Singapore. If this cannot
be fulfilled, then applicants should refer to Appendix 10 Product Interchangeability
and Biowaiver Request for Chemical Generic Drug Applications for more
information regarding eligibility and documentary requirements.
In instances when the reference product used in the BE study is not the Singapore
reference product, if the criteria listed in section 2 of Appendix 10 are fulfilled, then
the following additional documents must be submitted in support of the application:
(a) A comparative table that lists the qualitative composition of both the BE and
Singapore reference products;
(b) Comparative dissolution profiles between the BE and Singapore reference
products; and
(c) Comparative dissolution profiles between the BE test and Singapore reference
products.
For Biopharmaceutics Classification System (BCS)-based biowaiver applications,
justifications and relevant supporting documents should also be included under
section 3.2.P.9.
Product Interchangeability – Comparative Dissolution Profile (CTD section
3.2.P.9.2)
Comparative dissolution profile data between the generic product and the
Singapore reference product should be submitted in support of the following GDAs:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 111 of 168
• POMs (immediate and modified release oral solid dosage forms); and
• P or GSL medicines (modified release oral solid dosage forms only).
For POMs supported by BE study data, the following additional comparative
dissolution profile data should be submitted (applicable only when the BE reference
product is not the Singapore reference product):
(a) Between the reference and test products used in the BE study; and
(b) Between the BE and Singapore reference products.
Applicants should also provide a copy of the product labels (e.g. outer carton,
product insert) of the BE and Singapore reference products used in the comparative
dissolution profile testing for verification purposes.
When a generic product is to be marketed in several strengths, applicants should
refer to Section 3 of Appendix 10 Product Interchangeability and Biowaiver Request
for Chemical Generic Drug Applications for more information on comparative
dissolution profile testing requirements.
For BCS-based biowaiver applications, justifications and relevant supporting
documents should also be included under section 3.2.P.9.
HSA reserves the right to request for any additional information required to
determine the product interchangeability of the generic product to the Singapore
reference product.
18.4 Non-clinical and Clinical Documents
Generally, non-clinical (animal) and clinical (human) data are not required to be
included in a GDA. Instead, the data demonstrating the generic product’s
interchangeability with the Singapore reference product, e.g. in vivo BE and
comparative dissolution studies, are required for submission.
Documentary requirements for establishing product interchangeability (including
BCS-based biowaiver applications) can be found in Appendix 10 Product
Interchangeability and Biowaiver Request for Chemical Generic Drug Applications.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 112 of 168
Where required, HSA may request for RMPs to be submitted on a case-by-case
basis for GDAs, following the evaluation of the safety profile of the product
described in the product application. Guidance on RMP submission requirements
can be found here.
18.5 Documentary Requirements for Each Evaluation Route
18.5.1 Abridged Evaluation Route
All aspects of the product’s quality which include, but are not limited to, the
formulation, site(s) of manufacture, release and shelf life specifications and primary
packaging should be the same as that approved by the drug regulatory agency that
issued the proof of approval.
The technical documents required include:
• complete quality documents for both the drug substance and drug product; and
• BE studies or justifications for applying a biowaiver, where applicable.
18.5.2 Verification and Verification-CECA Evaluation Routes
The complete assessment report and other relevant supporting documents from the
chosen reference drug regulatory agency must be submitted, as tabulated below.
The assessment reports must be unredacted or unedited, and should include
details of imposed licensing conditions, final product labelling, quality and clinical
reviews, and other information in relation to the product’s approval. Reports
obtained from the public domain are deemed unacceptable.
Applications submitted to HSA without the unredacted/ unedited reports from the
chosen reference agency will not be accepted for evaluation via the verification
route and rejected at screening.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 113 of 168
Reference
agency
Documentary requirements
EMA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
FDA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
Health Canada
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
MHRA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
Swissmedic
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 114 of 168
Reference
agency
Documentary requirements
TGA
• Complete Clinical and Quality
#
assessment reports,
including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
#
If the drug substance section is submitted to the chosen reference agency as a Drug
Master File (DMF), the complete assessment report of the DMF, including assessment on
the Question & Answer documents between the DMF Holder & Agency and all annexes
should be provided. Assessment reports, approval letters and/or documents pertaining to
post-approval DMF updates should also be submitted, if applicable.
Administrative documents specific to the verification and verification-CECA
evaluation routes that are required at the time of submission include:
(a) Section 1.4.3/1.4.4 – the proposed PI or PIL should be aligned to the currently-
registered Singapore reference product PI or PIL;
(b) Section 1.9 – Official approval letter from the chosen reference drug regulatory
agency that certify the registration status of the drug product;
(c) Section 1.13 – Official letter issued by the applicant or product owner declaring
that the application submitted to HSA or similar direction(s) of use, indication(s),
dosing regimen(s) and/or patient group(s) have not been rejected, withdrawn,
approved via appeal process
17
, or pending deferral
18
by any drug regulatory
agency, with reasons in each case if applicable;
(d) Section 1.14 – Official letter issued by the applicant or product owner declaring
that the Drug Master File provided is identical to that submitted to the chosen
reference drug regulatory agency, if applicable; and
(e) Section 1.14 – Official letter from the applicant or product owner declaring that
all aspects of the product’s quality intended for sale in Singapore are identical
to that currently approved by the chosen reference drug regulatory agency. This
17
Approval via appeal process includes, but is not limited to, the following: approval following negative
opinion, approval following rejection, approval following non-approvable etc.
18
Deferral includes, but is not limited to, the following: non-approvable, approvable, conditional approval,
conditional marketing authorisation, notice of compliance with conditions etc.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 115 of 168
includes, but is not limited to, the formulation, site(s) of manufacture, release
and shelf life specifications and primary packaging.
Specifically for the verification-CECA evaluation route, a valid GMP certificate and
the latest GMP inspection report as issued by the reference drug regulatory agency
must be submitted.
The technical documents required include:
• Quality documents:
- From Sponsor:
▪ Complete documents for both drug substance and drug product (ICH
Module 3/ACTD Part II) as initially submitted to the chosen reference
drug regulatory agency;
▪ Complete assessment reports including assessment on the Question
& Answer documents between the Sponsor and chosen reference drug
regulatory agency, and other relevant supporting documents from the
chosen reference drug regulatory agency;
▪ Questions and answers between the chosen reference drug regulatory
agency and Sponsor – the answers should include supporting
documents used in response to the questions;
▪ All post-approval variations (if applicable)
approved by the chosen
reference drug regulatory agency up to the time of submission to HSA,
including the application letter for the variation, supporting documents
for the variation, assessment report for the variation, questions and
answers between the chosen reference drug regulatory agency and
Sponsor, and the approval letter for the variation from the chosen
reference drug regulatory agency; and
▪ Relevant documents required by HSA which have not been submitted
to the chosen reference drug regulatory agency, e.g. stability studies
in accordance to ASEAN Stability Guidelines, comparative dissolution
studies, etc.
- From DMF Holder, if applicable:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 116 of 168
▪ The initial open and closed parts of the DMF submitted to the chosen
reference drug regulatory agency should be provided to HSA, together
with a copy of the Letter of Access;
▪ Complete DMF assessment report including assessment on the
Question & Answer documents between the DMF holder and the
chosen reference drug regulatory agency, and other relevant
supporting documents from the chosen reference drug regulatory
agency;
▪ Questions and answers between the chosen reference drug regulatory
agency and DMF Holder – the answers should include supporting
documents used in response to the questions; and
▪ All post-approval DMF updates (if applicable) approved by the chosen
reference drug regulatory agency up to the time of submission to HSA,
including the application letter for the DMF update, supporting
documents for the DMF update, assessment report for the DMF
updates, questions and answers between the chosen reference drug
regulatory agency and Sponsor, and the approval letter for the DMF
update from the reference drug regulatory agency.
• All clinical documents, such as BE studies or justification for biowaiver, as
initially submitted to the chosen reference drug regulatory agency with all
questions and answers, including supporting documents, between the reference
drug regulatory agency and Sponsor; and
• Any additional documents to demonstrate product interchangeability with the
Singapore reference product as described in section 18.3.2 Body of Data – Drug
Product, where applicable.
Applicants are reminded that generic products applied through the verification and
verification-CECA evaluation routes must still demonstrate product
interchangeability to the Singapore reference product.
Data submitted to HSA must be the same as the data package submitted to the
reference regulatory agencies. Differences between the dossier submitted to HSA
and data reviewed by the reference drug regulatory agencies will not only delay the

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 117 of 168
processing of the application, but may also lead to re-routing of the dossier to the
abridged evaluation route if significant undisclosed differences are discovered.
In addition, the BE test product must be manufactured at the same drug substance
and drug product manufacturing sites by the same manufacturing processes as
submitted in the GDA application dossier.
18.6 Documentary Requirements for Second Brand Registration of
Chemical Therapeutic Products
18.6.1 Definition
A second brand product refers to a chemical drug product which is identical to a
registered (original) drug product in all aspects of quality, safety and efficacy at the
time of its submission for market authorisation and is submitted by the same product
registrant of the original drug product.
18.6.2 Documentary Requirements
18.6.2.1 Administrative
A complete set of administrative documents as per section 18.1 Administrative
documents has to be submitted. In addition, a declaration that the second brand
product is identical to the original drug product in terms of quality, safety and
efficacy is required.
18.6.2.2 Quality
A complete set of quality documents as per sections 18.2 CTD overview and
summaries and 18.3 Quality documents has to be submitted. In addition, a
comparative table of each CTD section between the second brand and original drug
products is required and all differences between these two dossiers should be
stated. The impact of these differences (if any) should generally be justified by the
approval or submission of minor variation application - MIV (to state application
number) to the original product dossier (see Chapter H – Minor Variation (MIV)
Application Submission).

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 118 of 168
During the evaluation stage, if MIV has not been submitted for these differences to
the original drug product dossier, then a MIV-2 application will be requested to
update the original product dossier. A stop-clock will be imposed on the second
brand product application until the update is completed. The product registrant is
advised to file a MIV for the update of these differences prior to submission to avoid
any delay in the review process of the second brand product.
Submission of BE studies are generally not required for second brand product
applications if the original product was granted marketing authorisation based on
the evaluation of the product’s quality, safety and efficacy. However if the original
product was registered as a generic drug product before 01 April 2004, prior to the
implementation of BE requirements, the current BE requirements will apply to the
second brand product.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 119 of 168
CHAPTER E BIOSIMILAR PRODUCT APPLICATION
SUBMISSION
This chapter applies to new drug applications for biosimilar products.
A biosimilar product is a biological therapeutic product demonstrated to be similar,
in physicochemical characteristics, biological activity, safety and efficacy to an
existing registered biological product.
This chapter serves to provide guidance on the regulatory considerations of a
biosimilar product, as well as the procedures and requirements for registration of a
biosimilar product. Applicants are encouraged to refer to the relevant international
guidelines e.g. EMA CHMP and WHO on biosimilar products. Alternative proposals
to the recommended approach and requirements should be discussed with HSA
and agreed upon in advance. HSA may consider such alternative proposals if
substantiated by adequate scientific evidence and justifications, and may request
for information or specify conditions not described in this document if deemed
necessary to adequately assess the safety, efficacy and quality of the product.
19 APPLICATION TYPES
Biosimilar products are eligible for the NDA-2 and NDA-3 application types. When
selecting the Product Type in PRISM section 3.2, select ‘Biological Drug’.
NDA
New Drug Application
NDA-1:
Not applicable to biosimilar products.
NDA-2:
For the first strength of a biosimilar product with the same dosage form,
route of administration and presentation as the Singapore reference
biological product (SRBP).

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 120 of 168
NDA-3:
For subsequent strength(s) of a biosimilar product that has been registered
or has been submitted as an NDA-2. The product name, dosage form,
presentation, indication, dosing regimen and patient population should be
the same as that for the NDA-2.
19.1 Biosimilar Product
A biosimilar product is intended to be similar in terms of quality, safety and efficacy
to a registered biological product.
Due to the complexity of biological molecules which pose challenges in
characterisation to demonstrate the similarity of the products, the registration of a
biosimilar product should be based demonstration of similarity to the SRBP in
quality, non-clinical and clinical parameters via comparability exercise.
Demonstration of similarity of a biosimilar product to the SRBP in terms of quality is
a prerequisite for determining the non-clinical and clinical data set required for
registration. Significant differences between the biosimilar product and the SRBP
detected during the comparability exercise would be an indication that the products
are not similar and more extensive non-clinical and clinical data may be required to
support the application for registration. If relevant differences are found in the
quality, non-clinical, or clinical data, the product is unlikely to qualify as a biosimilar
product.
Comparability exercises to demonstrate similarity are more likely to be applied to
highly purified products, which can be thoroughly characterised (such as some
biotechnology-derived therapeutic products). Vaccines, blood or plasma-derived
products are not eligible for registration via the biosimilar pathway.
19.2 Singapore Reference Biological Product
The SRBP must be a currently registered therapeutic biological product registered
in Singapore. A biosimilar product cannot be used as a reference product. The
conditions of use for the biosimilar product must fall within the directions for use

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 121 of 168
including indication(s), dosing regimen(s) and patient group(s) of the SRBP. A
biological product with no suitable SRBP will not qualify for registration as a
biosimilar product in Singapore.
The active ingredient of a biosimilar product must be similar, in molecular and
biological terms, to the active ingredient of the SRBP. The pharmaceutical form,
strength, and the route of administration of the biosimilar product should be the
same as that of the SRBP. Any deviation from or differences between the biosimilar
product and the SRBP will have to be justified by appropriate studies. Applicants
are advised to search Register of Therapeutic Products to identify the SRBP.
The SRBP should be used throughout the comparability assessment for quality,
safety and efficacy studies during the development of a biosimilar product in order
to allow for scientifically relevant and meaningful comparisons between the
biosimilar and the SRBP.
The comparability exercise for a biosimilar product is designed to show that the
biosimilar product has highly similar quality attributes when compared to the SRBP.
If the comparative studies are performed with a reference product from a non-
Singapore registered manufacturing source, the manufacturer needs to
demonstrate that the reference product used is comparable to the SRBP and hence
suitable to support the application for marketing authorisation of a biosimilar product
by providing an additional bridging study. The type of bridging data needed will
typically include data from analytical studies (e.g. structural and functional data) that
compare the proposed biosimilar product, the SRBP and the reference product
used in the comparability studies, and may also include clinical PK and/or PD
bridging studies data for all three products. All comparisons should meet the target
acceptance criteria for analytical and PK/PD similarity. A final determination
regarding the adequacy of the scientific justification and bridging data will be made
during the evaluation of the application.
20 EVALUATION ROUTES

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 122 of 168
A biosimilar product is eligible for the abridged or verification evaluation route. The
product must have been approved by at least one of the following reference drug
regulatory agencies: EMA
19
, FDA, Health Canada, MHRA
20
, Swissmedic and TGA.
20.1 Abridged Evaluation Route
Abridged evaluation applies to a product that has been approved by at least one
competent drug regulatory agency at the time of submission.
A competent drug regulatory agency refers to a national regulatory authority
participating in the World Health Organization’s Certification Scheme on the Quality
of Pharmaceutical Products Moving in International Commerce, and listed as such
on the World Health Organization’s website.
20.2 Verification Evaluation Route
Therapeutic products with similar indication(s), dosing regimen(s), patient group(s),
and/or direction(s) for use that have been approved by at least two of HSA’s
reference drug regulatory agencies may be eligible for submission via the
verification evaluation route. HSA’s reference drug regulatory agencies are:
• EMA via the Centralised Procedure
• FDA
• Health Canada
• MHRA via
- the national procedure, or
- as the Reference Member State (RMS) via the Mutual Recognition
Procedure or Decentralised Procedure on or prior to 31 January 2020
• Swissmedic
• TGA
However, approval by these reference drug regulatory agencies does not oblige
HSA to approve the application. HSA may also re-categorise applications to other
19
For products approved via the Centralised Procedure
20
For products approved via the national procedure or where MHRA acted as the RMS for the MRP or
Decentralised Procedures on or prior to 31 January 2020

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 123 of 168
evaluation routes if the applications did not meet the eligibility criteria and/or
submission requirements.
The applicant must confirm one of the reference drug regulatory agencies as the
primary reference agency. The chosen primary reference agency is defined as the
reference drug regulatory agency from which the qualifying supporting documents
(as outlined in this guidance) will be submitted.
The pre-requisite requirements for the verification route include:
• The product has received full marketing approval by the reference agencies
following a complete independent scientific assessment (i.e. the approval is not
granted on the basis of less comprehensive data than normally would require or
subject to post-approval conditions that require submission of additional data to
confirm the product’s benefit-risk profile);
• The application must be submitted to HSA within three years from the date of
approval by the chosen primary reference agency;
• A declaration letter issued by the product owner/applicant must be provided
stating that all aspects of the drug product’s quality, including but not limited to
the formulation, manufacturing site(s), release and shelf life specifications and
primary packaging, are identical to that currently approved by the chosen
primary reference agency at the time of submission. However, a different
container closure system type (e.g. Alu/Alu blister vs. HDPE bottle) may be
proposed to meet ASEAN stability requirements;
• The product does not need independent assessment by HSA to contextualise
the benefit-risk profile due to local disease epidemiology, medical practice
and/or public health considerations. Examples of products that may require such
contextualised assessment are anti-infectives, vaccines etc; and
• The product and its intended use – i.e. indication(s), dosing regimen(s) and
patient group(s) – have not been rejected, withdrawn, or approved via appeal
process or are not pending deferral by a drug regulatory agency for safety and/or
efficacy reasons.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 124 of 168
20.2.1 NDA-3 Applications
For the NDA-3 application type, the verification evaluation route may be applied to
the registration of subsequent strengths of a currently-registered product in
Singapore. To qualify for the verification evaluation route for an NDA-3 application:
• if the product has been evaluated and approved by at least one of HSA’s
reference drug regulatory agencies, then the NDA-3 must be submitted within
two years from the date of approval by that reference drug regulatory agency;
or
• if the product has been evaluated and approved by at least two of HSA’s
reference drug regulatory agencies, then the NDA-3 must be submitted within
three years from the date of approval by the chosen primary reference agency.
All other eligibility criteria for the verification evaluation route as stated in section
20.2 above will apply to NDA-3 applications except for the following:
• The proposed indication(s), dosing regimen(s), patient group(s), and/or
direction(s) for use must be identical to the corresponding approved product;
and
• The proposed PI/PIL should also be consistent with that currently approved for
the corresponding NDA-2 product.
21 DOCUMENTARY REQUIREMENTS
Table 9 outlines the CTD Modules/Parts required for NDAs submitted for
registration of a biosimilar product.
Table 9 Dossier Submission Requirements for Biosimilar Products
Documents
Location in
Module/Part required for
ICH CTD
ACTD
Biosimilar product (all
evaluation routes)
Administrative
Documents
Module 1
Part I
Yes

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 125 of 168
Common Technical
Document Overview
and Summaries
Module 2
Incorporate
d in Parts II,
III and IV
Yes
Quality documents
Module 3
Part II
Complete quality module
including comparability
studies
Non-clinical
documents
Module 4
Part III
Complete non-clinical
module* including
comparability studies
Clinical documents
Module 5
Part IV
Complete clinical module*
including comparability
studies
*The non-clinical and clinical dataset may be reduced based on criteria under section 21.4
21.1 Administrative Documents
The administrative documents of the application dossier for biosimilar products is
the same as that described in section 15.1 Administrative Documents in Chapter C
New Drug Application Submission.
21.2 CTD Overviews and Summaries
The CTD overviews and summaries are the same as that described in section 15.2
in Chapter C New Drug Application Submission.
21.3 Quality Documents
The complete quality dossier as per Module 3 of the ICH CTD or Part 2 of the ACTD
for a new biological product must be submitted.
The SRBP used in the biosimilar product comparability exercise at the quality level
must be clearly identified (e.g. brand name, pharmaceutical form, cell substrate,
formulation, strength, manufacturing site of the reference medicinal product,
number of batches, lot number, age of batches used).

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 126 of 168
Comparability data between the biosimilar product and the SRBP (in terms of
quality) must be included in the quality dossier. The extent of the comparability
studies and the assessment criteria should take into consideration:
• the complexity of the molecular structure;
• the capability of the methods used to demonstrate comparability; and
• their impact on quality, safety and efficacy.
For the development of a biosimilar product, the quality target product profile
(QTPP) should be established based on the data obtained from extensive
characterisation of the SRBP in order to relate the biosimilar product to the SRBP
in terms of molecular characteristics and quality attributes. This QTPP should be
considered as a development tool through which some target ranges may evolve
during development, as further information on the SRBP becomes available.
For robust comparability analysis, a representative quality profile of the SRBP
should be generated from multiple different batches of the SRBP when establishing
the QTPP for the biosimilar product. Quantitative ranges should be established for
the biosimilar comparability exercise based primarily on the measured quality
attribute ranges of the SRBP and should not be wider than the range of variability
of the representative SRBP batches, unless otherwise justified.
An extensive comparability exercise is essential to demonstrate that the biosimilar
product has a highly similar quality profile when compared to the SRBP. The
manufacturer must carefully design the comparability exercise based upon full
knowledge of the molecular structure and its relevance to the mode of action. The
result is a series of physicochemical tests, along or in combination with such
biological tests as in vitro and in vivo bioassays, and receptor binding studies.
These analyses should include side-by-side comparative studies to demonstrate
the similarities and differences between the biosimilar product and the SRBP.
Where comparability testing cannot establish similarity or where differences arise,
the differences detected in the quality attributes will have to be appropriately
justified with respect to their potential impact on safety and efficacy.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 127 of 168
Extensive characterisation studies should be applied to the biosimilar and SRBP in
parallel, to demonstrate with a high level of assurance that the quality of the
biosimilar product is comparable to the SRBP. Methods used in the characterisation
studies form an integral part of the quality data package. The selected methods
should be appropriately qualified for the purpose of comparability and demonstrate
that the methods are of acceptable sensitivity and capable to detect slight
differences in all aspects pertinent to the evaluation of quality (e.g. ability to detect
relevant variants with high sensitivity).
For process changes that may occur during the development of the biosimilar
product, comparability exercise(s) for such process changes should be clearly
identified and addressed separately from the comparability exercise performed
against the SRBP. It is strongly recommended to generate the required quality,
safety and efficacy data using the test product manufactured with the final
manufacturing process (representing the quality profile of the batches to be
commercialised) for the demonstration of biosimilarity against the SRBP.
21.4 Non-clinical and Clinical Documents
Non-clinical and clinical data generated with the biosimilar product are required.
The amount of non-clinical and clinical data required will depend on:
• the product or class of products;
• the extent of characterisation which is possible to undertake when using state-
of-the-art analytical methods;
• observed or potential differences between the biosimilar product and the SRBP;
and
• the clinical experience with the product class.
A case-by-case approach is needed for each class of products.
Guidance on risk management plan submission can be found here..
A statement that the product is a biosimilar medicinal product should be included in
the package insert.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 128 of 168
21.4.1 Non-clinical Documentation
Before initiating clinical development, non-clinical studies should be performed.
These studies should be comparative in nature and should be designed to detect
differences in response between the biosimilar product and the SRBP. Available
product specific guidelines and relevant international guidelines should be referred
to in the design of an appropriate non-clinical study programme.
The requirements for the non-clinical documentation include:
• In vitro studies: Assays such as receptor-binding studies or cell-based assays
should normally be undertaken to establish comparability in reactivity and the
likely causative factor(s) if comparability cannot be established; and
• Animal studies should be performed to investigate the pharmacodynamic
effects/ activities relevant to the clinical application, non-clinical toxicity as
determined in at least one repeat dose toxicity study, including toxicokinetic
measurements, and specific safety concerns.
Generally, other toxicological studies to investigated safety pharmacology,
reproduction toxicology, mutagenicity and carcinogenicity are not required for
biosimilar products, unless the observed differences between the biosimilar product
and SRBP warrants further study.
21.4.2 Clinical Documentation
The requirements for clinical data will depend on the existing knowledge of the
SRBP and the claimed therapeutic indication(s). In addition to international
guidelines on biosimilar products, relevant disease specific guidelines should be
referred to in the design of an appropriate clinical study programme.
The clinical data for the comparability study should be generated with the test
product produced with the final manufacturing process and therefore representing
the quality profile of the batches to be commercialised. Any deviation from this is to
be justified and supported by adequate bridging data. The type of bridging data

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 129 of 168
needed will typically include comparability data from analytical studies (e.g.
structural and functional data) and may also include clinical PK and/or PD
comparability data.
The clinical comparability exercise should begin with PK and PD studies followed
by clinical efficacy and safety studies. It is a prerequisite to perform comparative PK
studies designed to demonstrate clinical comparability between the biosimilar
product and the SRBP with respect to the key PK parameters. PD parameters are
to be studied whenever feasible and the PD markers should be selected based on
their clinical relevance.
Generally, comparative clinical studies are required for the demonstration of clinical
comparability. In certain cases, comparative PK/PD studies between the biosimilar
product and the SRBP may be sufficient to demonstrate clinical comparability,
provided that all the following conditions are met:
• The PK profile of the SRBP is well characterised;
• There is sufficient knowledge of the PD properties of the SRBP, including the
binding to its target receptor(s) and intrinsic activity. There may be instances
where the mechanism of action of the biological product is disease-specific. A
relevant PD endpoint can be used when it is an established surrogate of efficacy
or when it can be linked to the mechanism of action of the product; and
• The relationship between dose/exposure and response/efficacy of the SRBP is
sufficiently characterised.
For comparative clinical studies to demonstrate clinical comparability between the
biosimilar product and the SRBP, clinical comparability margins should be pre-
specified and adequately justified. The most sensitive clinical model should be used
to detect potential differences between the biosimilar product and the SRBP.
In cases where the SRBP has more than one indication, the efficacy and safety of
the biosimilar product has to be justified, or demonstrated separately for each of the
claimed indications. In certain instances, it may be possible to extrapolate
therapeutic similarity shown in one indication to other indications of the SRBP.
Justification of extrapolation to other indications will depend on various factors,

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 130 of 168
which may include the sensitivity of the clinical study population, clinical experience,
available literature data, mechanisms of action, target receptors, pattern of
molecular signalling upon binding to the receptor, PK in different patient
populations, PD parameters, patient-related factors, etc. Possible safety issues in
different subpopulations should also be addressed.
Immunogenicity
Immunogenic responses may develop in patients who are treated with biological
products, including biosimilars. The development of antibodies in some instances
is a benign effect causing few, if any, undesirable symptoms in patients receiving
therapy. On the other hand, the induced antibodies may be associated with
undesirable consequences, which manifest themselves as mild to severe
anaphylactoid reactions. Efficacy may also be diminished by the presence of
neutralising antibodies or binding antibodies, which may affect PK. Therefore, the
immunogenicity of a biosimilar product must be investigated.
Animal studies may not be able to predict immunogenicity of a biological product,
particularly the more complex proteins as immunogenic response is species-
dependent. The assessment of immunogenicity requires an optimal antibody testing
strategy, characterisation of the observed immune response, as well as evaluation
of the correlation between antibodies and PK or PD, and the impact of antibodies
on clinical safety and efficacy. It is also important to consider the risk of
immunogenicity in different therapeutic indications separately.
The extent of independent testing needed depends on various factors such as the
indication, whether the product is to be administered chronically, the overall
assessment of the product's immunogenic potential, and whether there is the
possibility of generating a cross-reaction with an important endogenous molecule.
21.5 Documentary Requirements for Each Evaluation Route
21.5.1 Abridged Evaluation Route
The technical documents required include:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 131 of 168
• complete quality documents for both drug substance and drug product including
comparability studies;
• non-clinical documents including comparability studies; and
• clinical documents including comparability studies
21.5.2 Verification Evaluation Route
The complete assessment report and other relevant supporting documents from the
chosen primary reference agency must be submitted, as tabulated below. The
assessment reports from the primary reference agency must be unredacted or
unedited, and should include details of imposed licensing conditions, final product
labelling, quality, non-clinical and clinical reviews, and other information in relation
to the product’s approval. Reports obtained from the public domain are deemed
unacceptable.
Applications submitted to HSA without the unredacted/ unedited reports from the
primary reference agency will not be accepted for evaluation via the verification
route and rejected at screening.
Primary
reference
agency
Documentary requirements
EMA
• Complete Clinical, Non-clinical and Quality assessment
reports, including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
FDA
• Complete Clinical, Non-clinical and Quality assessment
reports, including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 132 of 168
Primary
reference
agency
Documentary requirements
Health Canada
• Complete Clinical, Non-clinical and Quality assessment
reports, including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
MHRA
• Complete Clinical, Non-clinical and Quality assessment
reports, including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
Swissmedic
• Complete Clinical, Non-clinical and Quality assessment
reports, including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
TGA
• Complete Clinical, Non-clinical and Quality assessment
reports, including assessment on the Question & Answer
documents between the Sponsor & Agency and all
annexes
• Assessment reports and/or documents pertaining to post-
approval variations, if applicable
Administrative documents specific to the verification evaluation route that are
required at the time of submission include:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 133 of 168
(e) Section 1.9 – Official approval letters, or equivalent documents, from the
relevant reference drug regulatory agencies that certify the registration status of
the drug product;
(f) Section 1.13 – Official letter declaring that the application submitted to HSA or
similar direction(s) of use, indication(s), dosing regimen(s) and/or patient
group(s) have not been rejected, withdrawn, approved via appeal process
21
, or
pending deferral
22
by any drug regulatory agency, with reasons in each case if
applicable; and
(g) Section 1.14 – Official letter declaring that all aspects of the product’s quality
intended for sale in Singapore are identical to that currently approved by the
primary reference drug regulatory agency. This includes, but is not limited to,
the formulation, site(s) of manufacture, release and shelf life specifications and
primary packaging.
The technical documents required include:
• Quality documents:
- From Sponsor:
▪ Complete documents for both drug substance and drug product (ICH
Module 3/ACTD Part II) as initially submitted to the primary reference
agency;
▪ Complete assessment reports including assessment on the Question
& Answer documents between the Sponsor and primary reference
agency, and other relevant supporting documents from the primary
reference agency;
▪ Questions and answers between the primary reference agency and
Sponsor – the answers should include the supporting documents used
in response to the questions;
▪ All post-approval variations (if applicable)
approved by the primary
reference agency up to the time of submission to HSA, including the
application letter for the variation, supporting documents for the
variation, assessment report for the variation, questions and answers
21
Approval via appeal process includes, but is not limited to, the following: approval following negative
opinion, approval following rejection, approval following non-approvable etc.
22
Deferral includes, but is not limited to, the following: non-approvable, approvable, conditional approval,
conditional marketing authorisation, notice of compliance with conditions etc.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 134 of 168
between the primary reference agency and Sponsor and the approval
letter for the variation from the primary reference agency; and
▪ Relevant documents required by HSA which have not been submitted
to the primary reference agency, e.g. stability studies in accordance to
ASEAN Stability Guidelines.
• Non-clinical documents, assessment report from the primary refence agency,
including assessment on the Question and Answer documents between the
Sponsor and Agency, and other relevant supporting documents from the primary
reference agency; and
• Clinical documents, assessment report from the primary reference agency,
including assessment on the Question and Answer documents between the
Sponsor and Agency, and other relevant supporting documents from the primary
reference agency.
All of the data submitted to HSA must be the same as the data package submitted
to the reference drug regulatory agencies. Differences between the dossier
submitted to HSA and data reviewed by the reference drug regulatory agencies will
not only delay the processing of the application, but may also lead to re-routing of
the dossier to the abridged evaluation route if significant undisclosed differences
are discovered.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP
Page 135 of 168
CHAPTER F POST-APPROVAL PROCESS
Changes to a product registration throughout its life cycle must be submitted to HSA
via a variation application. These include administrative/editorial, quality and
clinical/non-clinical changes. In general, once the application has been
approved/processed, the changes should be implemented by the next importation,
or when logistically feasible.
22 APPLICATION TYPES
There are two types of variation applications – major variation applications (MAV)
and minor variation applications (MIV).
MAV
Major Variation application for an existing registered
product
MAV-1:
Any variation to the approved indication(s), route(s) of
administration, dosing regimen(s), patient group(s),
and/or inclusion of clinical information extending the
usage of the product (e.g. clinical trial information
related to an unapproved indication, dosing regimen
and/or patient population; additional bacterial strains
with clinical (in vivo) data to expand the indication(s) for
antimicrobial products; additional viral
serotypes/genotypes to expand the indication(s) for
antiviral products, etc.).
MAV-2:
A change in the current approved forensic classification,
also known as reclassification.
MIV
Minor Variation application for an existing registered
product

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 136 of 168
MIV-1
A minor variation that
• Is specified under Part A: Checklist on Dossier
Requirements for MIV-1 variations of Appendix 13
(Chemicals) or Appendix 14 (Biologics).
• Requires prior approval before the change(s) can
be implemented.
MIV-2 (Notification)
A minor variation that
• Is specified under Part B: Checklist on Dossier
Requirements for MIV-2 (Notification) Variation of
Appendix 13 (Chemicals) or Appendix 14
(Biologics);
• May be implemented within 40 days upon
application submission if there are no objections
raised by HSA.
MIV-2 (Do-and-Tell)
A minor variation that
• Is specified under Part C: Checklist on Dossier
Requirements for MIV-2 (Do-and-Tell) Variation of
Appendix 13 (Chemicals) or Appendix 14
(Biologics);
• Does not require prior approval, but must be
submitted to HSA within 6 months following
implementation of the specified changes. [Refer to
Chapter H, 27.2.2 for more information]
HSA may re-categorise the application type if appropriate (e.g. MIV to MAV-1, MIV-
2 to MIV-1, or vice versa). Applicants will be notified if they are required to withdraw
and resubmit the application according to the correct category.
Please refer to Chapters G and H for more information on MAV and MIV
respectively.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 137 of 168
23 VARIATION APPLICATION PROCESS
Figure 5 is a schematic diagram illustrating the variation approval process:
For information on the variation application processing time, refer to Appendix 5
Target Processing Timeline of this guidance document.
23.1 Pre-Submission Consultation Mechanisms
There is a range of mechanisms that enable companies to self-help, which includes
the use of guidelines, flow charts, frequently asked questions (FAQ) and self-help
tools as alternatives to pre-submission meeting.
In the event that applicants are still unable to determine the type of variation, the
MIV Enquiry Form may be submitted.
Figure 5 Schematic Diagram of the Variation Application Process.
PRE-SUBMISSION
PREPARATION
APPLICATION
SUBMISSION
APPLICATION SCREENING
(For MAV-1 and MAV-2 only)
APPLICATION
EVALUATION
REGULATORY DECISION
(for MAV and MIV-1 only)
NON-ACCEPTANCE /
WITHDRAWAL
ACCEPTANCE
NON-APPROVAL /
WITHDRAWAL

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 138 of 168
For more information on TPB’s pre-submission consultation mechanisms, refer to
the website: Pre-submission Consultation Mechanisms
23.1.1 Pre-Submission Notification
A pre-submission meeting is not compulsory for making an application to HSA. (see
section 24.1 Evaluation Routes for more information on MAV-1 evaluation routes).
Nonetheless, the applicant is required to notify HSA at least two months prior to the
intended submission date for applications submitted via the full evaluation route.
The notification should include information on the product name (if available), active
ingredient(s), summaries of the clinical data (e.g. Overviews), planned submissions
in other countries, and planned date of submission to HSA.
23.2 Application Submission
The submission of an application comprises two key steps – (i) online submission
of the application form via PRISM and (ii) submission of the technical dossier.
23.2.1 PRISM Application Form
Applicants should refer to Appendix 17 Guideline on PRISM Submission for further
details.
23.2.2 Variation Application Dossier
The technical dossier accompanying the application should be submitted within 2
working days of the PRISM application submission to prevent delays in the
processing of the application. The date of receipt of the actual technical dossier
by HSA will be taken as the submission date where the processing time starts.
The dossier submitted for variation applications should be in the same CTD format
as that used for the original new product application.
Application checklists for both ICH CTD and ACTD dossiers are provided in
Appendix 2B and 3B, respectively, to guide applicants on the submission
requirements and to ensure completeness of the dossier. Each MAV application

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 139 of 168
must be accompanied by a checklist duly completed by the applicant and
attached in PRISM.
23.2.2.1 Submission Requirements
The complete application dossier – i.e. Modules 1 to 5 of the ICH CTD or Parts I to
IV of the ACTD – must be submitted in an electronic format.
Refer to section 6.2.1 on the Submission Requirements of the complete application
dossier.
For submission requirements for MIV applications, please refer to Appendix 13 and
Appendix 14 for registered chemical products and registered biological products,
respectively.
23.2.2.2 Language and Translation
All documents submitted in support of an application to HSA must be in English.
For documents in original language which is not English, a certified translation or a
verified translation may be acceptable.
Translation
type
Type of
Documents
Requirements
Certified
Translation
▪ Official
certificates
issued by
the drug
regulatory
agency of a
country
▪ Proof of
approval
issued by
the drug
regulatory
Notarisation & Authentication
(10) Notarisation
▪ These documents must be notarised by a
notary public in country where document is
issued.
▪ Details of particulars to be included by
notary:
(i) The name of the notary;
(ii) A statement that the notary is duly
admitted to practice in the place of issue
of the certificate;

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 140 of 168
agency of a
country
(iii) The names of the signatories and the
capacity in which they have executed the
document, whether on their own behalf or
in an official or representative capacity;
(iv) A statement authenticating the
signatures of the parties and, where
appropriate, indicating that evidence has
been produced to the notary proving the
capacity in which they have executed the
document;
(v) The place and date of issue of the
notarial certificate; and
(vi) The signature and seal of the notary.
(10) Authentication
▪ These documents must be authenticated
(i.e. the origin of the document is attested
to) by one of the following government
bodies:
(i) The Ministry of Foreign Affairs of the
country in which the document was
issued; or
(ii) The Singapore Embassy/Consulate in
the country where the document was
issued.
Applicants are advised to consult the Singapore
Embassy/Consulate in the country where the
document originated regarding the local
requirements for document legalisation, as
these may deviate from the process as outlined
in the preceding paragraph.
Verified
Translation
▪ Technical
documents
Verification Document

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 141 of 168
(e.g.
package
insert,
submission
dataset)
▪ A verification document must be provided
by the translator of the document into the
English language.
▪ The verification document must state that
the translation into English is accurate.
▪ Details of particulars to be included in
verification document:
(i) the name of translator;
(ii) a statement that he/she is well versed in
English and the relevant foreign
language; and
(iii) a reference to the document being
translated.
Refer to the sample verification document for
translator enclosed in Appendix 4.
With Singapore acceding to the Apostillle Convention on 16 September 2021, for
certified translated document issued by a country which acceded to the Apostille
Convention, an apostille certificate can be submitted in lieu of a
notarised/authenticated certified translation.
23.2.2.3 Certifying non-original documents
If the softcopy official document (e.g. CPP, GMP certificate, etc.) submitted to HSA
in PRISM is not a scan of the original document, the document must be certified
prior to submission. A certified true copy certifies that the photocopy presented is a
true and accurate copy of the original document. Acceptable certification of
documents to support drug product applications to HSA can be done by the
Company Director or Company Secretary as registered with ACRA or above, or by
an independent authority such as a lawyer, notary public, Commissioner for
Oaths/Declarations/Affidavits, Justice of Peace, the original issuer of the document
or Embassy/Consulate. A notarised and authenticated copy is the same as a
certified true copy.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 142 of 168
A certified true copy of approval letters requires certification by the drug regulatory
agency that issued the approval letter, notary public or Singapore
Embassy/Consulate in the country where the approval letter was issued.
Certification of approval letters is not required in the event the approval letter is
available on the drug regulatory agency’s website. In this instance, applicants
should provide the internet address (URL) for validation by HSA.
23.3 Application Screening
MAV-1 and MAV-2 applications will be screened to ensure the correctness of the
application type and the completeness of the dossier. The date of receipt of the
application dossier (i.e. the technical dossier [e.g. in a CD/DVD] including the
application checklist) will be taken as the submission date and the start of the
screening timeline.
If an application is identified to be more appropriately submitted under a different
application type, the applicant will be informed of this change and the necessary
actions to effect this change via an Input Request. More information on the change
in application type is described in section 23.6.2.1 Changes to Application
Types and Re-routing of Evaluation During Screening.
An MAV application submitted without the clinical dossier or an application
submitted via the verification route without assessment reports will not be screened,
An Input Request will be issued to the applicant to withdraw the application.
Applicants are also advised to ensure that the dossier is compiled according to the
required format. Failure to adhere to the required CTD format will lead to the non-
acceptance of the dossier without screening.
If the dossier submitted is considered to be incomplete, a screening query stating
the deficiencies will be issued via PRISM (Input Request) to the applicant. The stop-
clock starts when an Input Request is sent and ends upon receipt of a complete
and satisfactory response to the query. For MAV applications, the total number of
Input Requests sent during screening is capped at two. Applicants will be given 20

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 143 of 168
working days to respond to each Input Request, starting from the date the Input
Request is sent.
The application will only be accepted when all deficiencies have been adequately
addressed and HSA is satisfied that the dossier is complete for evaluation. An
acceptance notice will then be issued via PRISM and the date of acceptance of the
application will be taken as the start of the evaluation timeline.
If the applicant fails to address the deficiencies raised during screening, the dossier
is considered incomplete for evaluation. An Input Request will be issued to the
applicant to withdraw the application. If the application is subsequently re-
submitted, it will be processed as a new application.
For MIV-1 applications, applicants will receive an “Acceptance” notification sent
within 3 working days after submission of an MIV-1 application via PRISM. For
applications submitted under an incorrect application type and evaluation route (e.g.
MAV-1 changes submitted as a MIV-1, or abridged application submitted as
verification application), applicants will be requested to withdraw the application
during evaluation.
23.4 Application Evaluation and Regulatory Decision
Once the application is accepted, the evaluation stage begins. Evaluation queries
may be issued via Input Request to the applicant if clarification or additional
information is required.
The stop-clock starts whenever HSA issues a query and ends upon the receipt of a
complete and satisfactory response from the applicant.
In situations where the applicant is unable to provide a complete response within
the specified timeframe, the applicant should notify HSA as soon as possible after
NOTE: The screening process only checks for completeness of the application dossier
for evaluation. The acceptance of the dossier for evaluation does not denote the
adequacy of the data for regulatory approval.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 144 of 168
receiving HSA’s queries. The application will be considered withdrawn if the
applicant fails to observe the specified response deadline.
Applicants are reminded that the submission of additional supporting data not
requested by HSA following the acceptance of the application will not be
considered, unless prior arrangement with HSA is made for the submission
concerned. During the evaluation process, HSA may assess that the application is
more suitably evaluated via an alternative route, in which case the application will
be re-routed to the appropriate route. Any re-routing of the application will be
discussed with the applicant.
HSA may engage external evaluators, experts and advisory committees in the
evaluation process, when necessary. These experts include scientists and
clinicians from both local and overseas institutions. All external evaluators and
experts are bound by agreement to protect the information made available to them.
The identity of the external evaluators is kept confidential.
For MAV-1 applications (full and abridged evaluation routes), applicants can check
on the progress of the evaluation and may view the evaluation stage via
Track@PRISM. Table 10 describes the stages of the evaluation process for MAV-
1 applications.
Table 10 Variation Applications Applicable for Notification of Stages During
Evaluation
Stages of
Notification to
Applicant
1
st
Stage
2
nd
Stage
3
rd
Stage
4
th
Stage
Application
Type
Evaluation
Route
Evaluation Status
Acceptance
for
Evaluation
Active
Evaluation
in Progress
Evaluation at
Midway
Completed
Evaluation
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 145 of 168
MAV-1
Full or
Abridged
Application
is accepted
for
evaluation
and has
entered the
evaluation
queue.
This marks
the start of
the
evaluation
timeline.
Application
is under
active
evaluation.
Applicants
can expect
to receive
the first set
of
evaluation
queries (if
any) from
us towards
the end of
this stage.*
Application is
approximately
midway
through the
evaluation.
Applicants are
expected to
submit the
response to
evaluation
queries.
Evaluation is
completed for
the application.
Application is
now
undergoing the
regulatory
decision phase,
after which a
regulatory
decision
#
will
be issued.
Applicants can
still expect
further queries
from HSA
during this
stage.
* For applications without any evaluation queries, recommended changes to product labels
will be communicated to the applicant during the regulatory decision phase.
#
The issuance of a regulatory decision would mark the end of the evaluation timeline for a
product application.
The following screenshots illustrate the change in stages of a pending application:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 146 of 168
Applicants are also notified via system-generated emails whenever an evaluation
stage change occurs.
After the application is accepted for evaluation, applicants can expect to receive the
first evaluation Input Request by:
Type of Applications
Evaluation Route
No. of working days
MAV-1
Full
160
MAV-1
Abridged
120
Note: excluding any stop-clock time between acceptance and issuance of first evaluation Input
Request.
Upon approval/notification of a variation application, applicants will be informed via
system-generated email and the product registration information in PRISM will be
updated to reflect the changes (if applicable). Applicants may refer to
Active Evaluation
The evaluation stage
is seen here.
Enter PRISM application to
view stage of evaluation.
Choose these options from
the drop-down lists.
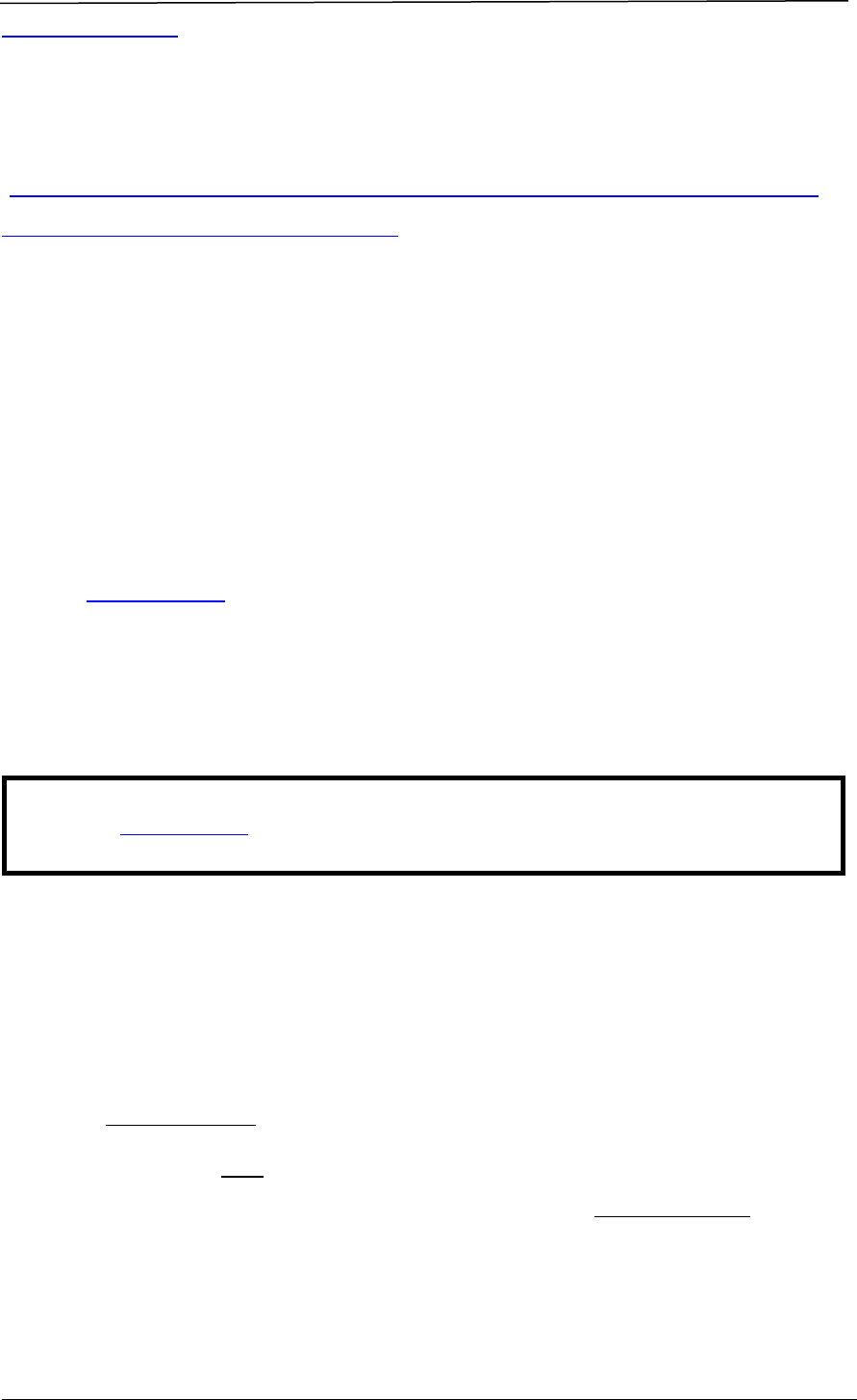
GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 147 of 168
Enquire@PRISM to view the latest product registration information (including
registration conditions and post-approval commitments) of their products.
For submission of documents to fulfil registration conditions, please use this form
(Submission of Documents to Fulfil Therapeutic Product Registration Conditions -
https://go.gov.sg/fulfil-tp-reg-conditions).
23.5 Target Processing Timelines
Please refer to Appendix 5 for information on target processing timelines for the
different application types and evaluation routes.
23.6 Fees
As the fees may be subject to revision from time to time, applicants are advised to
visit the HSA website for updated information on fees.
Payment can be made via GIRO or other electronic payment modes such as eNets
or eCredit card.
Regardless of payment mode selection, the collection of both screening and
evaluation fee for applications submitted via the full evaluation route occurs upon
issuance of the screening outcome.
23.6.1 Screening Fee
The screening fee is only applicable for MAV-1 applications and is payable at the
time of online submission via PRISM. The screening fee is non-refundable once the
application is submitted via PRISM.
For payment via GIRO, the screening fee will be debited upon the successful
submission of an online application.
NOTE: Applicants are strongly encouraged to apply for eGIRO for the convenience of
payment (apply eGIRO). subsequent payment for retention fee for the registered
products.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 148 of 168
For payment via other electronic payment modes (i.e. eNets or eCredit card), the
screening fee must be paid before the application is considered successfully
submitted online.
A screening fee is not applicable for other types of variation applications.
23.6.2 Evaluation Fee
There are two different evaluation fees for MAV-1 applications:
(a) Evaluation fees for a single-strength product or the first product in a series of
products of different strengths; and
(b) Evaluation fees for each subsequent product in a series of products of different
strengths.
An evaluation fee for a MAV-1 application is payable upon the acceptance of the
dossier for evaluation and is non-refundable once the application is accepted. For
payments via GIRO, the evaluation fee will be debited upon the acceptance of the
application.
For payments via other electronic payment modes (i.e. eNets or eCredit card), the
evaluation fee will be collected together with the screening fee. In the event that the
application is not accepted for evaluation, the fee collected will be refunded to the
applicant’s mode of payment.
Applicants may opt for the progressive payment scheme for payment of evaluation
fee. This is an opt-in scheme eligible for applicants who make payment via GIRO
and is only applicable to the application types listed in Table 11:
Table 11 Variation Applications Applicable for Progressive Payment Scheme
Percentage of Evaluation Fee Payable at Each Stage
Evaluation Status

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 149 of 168
Application
Type
Evaluation
Route
Acceptance
for
Evaluation
Active
Evaluation in
Progress
Evaluation
at Midway
Completed
Evaluation
MAV-1
Full or
Abridged
30%
40%
20%
10%
Once the application is submitted, the selected payment scheme (full or
progressive) cannot be amended. Applicants who wish to change their selected
payment scheme will have to withdraw and re-submit the application(s); and any
upfront payment made (e.g. screening fee) are non-refundable.
For applications under the progressive payment scheme, in the event that the
application is withdrawn during the evaluation stage, any fees that had been
charged, but not debited from the GIRO account would remain payable. Any paid
fee is non-refundable.
23.6.2.1 Changes to Application Types and Re-routing of Evaluation During
Screening
If an application type or evaluation route is incorrectly selected, applicants will be
informed via an Input Request. Such changes may result in a different evaluation
fee upon acceptance of the application.
In the situation where the applicant decides not to pursue the application due to the
said changes, the screening fee is not refundable.
For applications which require withdrawal and resubmission, the screening fee is
not refundable.
23.6.2.2 Change of Application between Different Application Types
This refers to a change in the application type between MAV-1, MAV-2, MIV-1 or
MIV-2.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 150 of 168
The applicant will be required to withdraw and resubmit the application if the
applicant intends to pursue the application.
23.6.2.3 Change of Evaluation Route
This refers to a change in evaluation route (e.g. Full to Abridged, Verification to
Abridged, Abridged to Verification, etc.).
The applicant will be required to withdraw and resubmit the application if the
applicant intends to pursue the application.
23.6.3 Application Fee
An application fee for a MIV-1 application is payable upon the submission of the
application in PRISM and is non-refundable.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 151 of 168
CHAPTER G MAJOR VARIATION (MAV) APPLICATION
SUBMISSION
This chapter applies to major variation applications for currently registered
products.
24 MAV-1 APPLICATIONS
An MAV-1 application applies to variations to any of the following:
(a) approved indication(s);
(b) approved route(s) of administration;
(c) approved dosing regimen(s);
(d) approved patient group(s); and/or
(e) inclusion of clinical information extending the usage of the product – for
example, clinical trial information related to an unapproved indication, dosing
regimen and/or patient population; additional bacterial strains with clinical (in
vivo) data to expand the indication(s) for antimicrobial products; additional viral
serotypes/genotypes to expand the indication(s) for antiviral products, etc.
For each product registration, applicants may submit up to a maximum of three
concurrent MAV-1 applications at any one time.
24.1 Evaluation Routes
There are three evaluation routes for an MAV-1 – full, abridged and verification. The
eligibility criteria and documentary requirements are different for each evaluation
route.
Approved by
HSA’s reference
agencies and
meets
verification
criteria?
Is the major
variation
approved by
any drug
regulatory
agency?
NO
FULL ROUTE
ABRIDGED
ROUTE
YES
NO
YES
VERIFICATION
ROUTE
MAV-1
Figure 5 Schematic Diagram of Evaluation Routes for MAV-1s

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 152 of 168
24.1.1 Full Evaluation Route
Full evaluation will apply to a major variation that has not been approved by any
drug regulatory agency at the time of submission.
For a submission under the full evaluation route, the applicant is required to notify
HSA at least two months prior to the intended submission date of the application
dossier. The notification should include information on the product name (if
available), active ingredient(s), summaries of the quality, non-clinical and clinical
data (e.g. Overviews), planned submissions in other countries, and the planned
date of submission to HSA.
24.1.2 Abridged Evaluation Route
Abridged evaluation applies to a major variation that has been evaluated and
approved by at least one competent drug regulatory agency. The proposed
variation – i.e. the proposed indication(s), route(s) of administration, dosing
regimen(s), patient group(s) and/or clinical information – should be the same as that
approved by the regulatory agency that issued the proof of approval.
A competent drug regulatory agency refers to a national regulatory authority
participating in the World Health Organization’s Certification Scheme on the Quality
of Pharmaceutical Products Moving in International Commerce, and listed as such
on the World Health Organization’s website.
24.1.3 Verification Evaluation Route
A major variation that has been approved by at least two of HSA’s reference drug
regulatory agencies may be eligible for submission via the verification evaluation
route. HSA’s reference drug regulatory agencies are:
• EMA via the Centralised Procedure
• FDA
• Health Canada
• MHRA via
- the national procedure, or

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 153 of 168
- as the Reference Member State (RMS) via the Mutual Recognition
Procedure or Decentralised Procedure on or prior to 31 January 2020
• Swissmedic
• TGA
However, approval by these reference drug regulatory agencies does not oblige
HSA to approve the application. HSA may also re-categorise applications to other
evaluation routes if the applications did not meet the eligibility criteria and/or
submission requirements.
The applicant must confirm one of the reference drug regulatory agencies as the
primary reference agency. The chosen primary reference agency is defined as the
reference drug regulatory agency from which the qualifying supporting documents
(as outlined in this guidance) will be submitted and which approved the strictest
indication(s), route(s) of administration, dosing regimen(s), patient groups(s) and/or
direction(s) for use among the reference drug regulatory agencies which approved
the variation.
The pre-requisite requirements for the verification route include:
• The product has received full marketing approval by the reference agencies
following a complete independent scientific assessment (i.e. the approval is not
granted on the basis of less comprehensive data than normally would require or
subject to post-approval conditions that require submission of additional data to
confirm the product’s benefit-risk profile);
• The application must be submitted within three years from the date of approval
by the chosen primary reference agency;
• The product does not need independent assessment by HSA to contextualise
the benefit-risk profile due to local disease epidemiology, medical practice and/or
public health considerations. Examples of products that may require such
contextualised assessment are anti-infectives, vaccines etc.; and
• The product and its intended use – i.e. indication(s), route(s) of administration,
dosing regimen(s) and patient group(s) – have not been rejected, withdrawn,
approved via appeal process or pending deferral by a drug regulatory agency for
safety and/or efficacy reasons.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 154 of 168
24.2 Documentary Requirements
Table 12 outlines the CTD Modules/Parts required for MAV-1s submitted under
each evaluation route:
Table 2 Dossier Submission Requirements for MAV-1s
Location in
Module/Part required for
ICH
CTD
ACTD
Full
MAV-
1
Abridged
MAV-1
Verification
MAV-1
Administrative
Documents and
Product
Information
Module
1
Part I
Yes
Yes
Yes
Common
Technical
Document
Overview and
Summaries
Module
2
Incorporated
into Parts II,
III and IV
Yes
Yes
Yes
Quality
documents
Module
3
Part II
No
No
No
Non-clinical
documents
Module
4
Part III
No
§
No
#
No
#
Clinical
documents
Module
5
Part IV
Yes
Study
report(s) of
pivotal
studies and
synopses of
all studies
(phase I-IV)
relevant to
requested
indication,
Study
report(s) of
pivotal
studies and
synopses of
all studies
(phase I-IV)
relevant to
requested
indication,

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 155 of 168
dosing
and/or
patient
group
dosing
and/or
patient
group
§
If the proposed MAV-1 is related to non-clinical data, non-clinical summary and non-
clinical overview as well as relevant study reports is required.
#
Non-clinical overview only, if applicable.
24.2.1 Administrative Documents
The three evaluation routes for an MAV-1 share the same documentary
requirements for CTD Module 1/Part I. The documents required are:
(a) Section 1.1 – Comprehensive Table of Contents;
(b) Section 1.2 – Cover Letter – including the Table of Amendment Details of PRISM
section 0.5;
(c) Section 1.4 – Labelling, Package Insert and Patient Information Leaflet –
(i) Both the proposed and currently approved Singapore product labels and
PI/PIL are required.
(ii) For the proposed labelling/PI/PIL, a pristine and an annotated version
(which highlights the changes made to the currently approved labelling) are
required.
(iii) Annotations should be made on the current approved labelling materials
based on the actual text to be added.
(iv) Current approved text proposed for deletion should be struck through,
whereas newly added and proposed text should be underlined or
highlighted.
(v) Current approved text that is not intended to be deleted should not be
annotated.
NOTE: Applicants must complete the relevant checklists found in Appendix 2B or
Appendix 3B and attach the completed checklist under PRISM section 1.2

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 156 of 168
(vi) Proposed changes to all labels must be clearly annotated, and the final
approved changes would be as annotated in the final label submitted in
PRISM.
(d) Section 1.5 – Approved SPC/PI/PIL from the drug regulatory agency that issued
the proof of approval and from each of HSA’s reference drug regulatory
agencies (where applicable);
(e) Section 1.6 – Assessment Report from Reference Agencies – only for
verification route (see section 24.2.5.3 Verification Evaluation Route);
(f) Sections 1.8, 1.9 – Proof of Approval – for an MAV-1, the official approval
letter(s) must contain information on the requested Singapore variation. For the
verification evaluation route, the approval letters issued by at least two reference
drug regulatory agencies, including the chosen primary reference agency,
should be submitted;
(g) Section 1.13 – Declaration on rejection, withdrawal and deferral; and
(h) Section 1.15 – Registration Status in Other Countries.
24.2.2 CTD Overviews and Summaries
The following documents are to be submitted:
• a non-clinical overview, if applicable; and
• a clinical overview and summaries of clinical efficacy and clinical safety.
24.2.3 Quality Documents
Quality documents (Module 3/Part II) are not required for MAV-1 applications.
24.2.4 Non-clinical and Clinical Documents
Each evaluation route will have different non-clinical and clinical documentary
requirements. Refer to section 24.2.5 Specific Documentary Requirements for Each
Evaluation Route below for more information.
For MAV applications, HSA may request for RMPs to be submitted on a case-by-
case basis following the evaluation of the safety concerns described in the product
application, where necessary. For such instances, please refer to the guidance on
RMP submission requirements found here.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 157 of 168
If the MAV-1 is for a non-prescription medicine and is submitted via the abridged
evaluation route, the applicant may submit a written request for a waiver of clinical
data submission. Eligibility for a waiver is subject to the criteria defined in Appendix
6 Guideline on Submission for Non-Prescription Therapeutic Products. However,
HSA may request for the complete clinical data set if it is deemed appropriate.
24.2.5 Documentary Requirements for Each Evaluation Route
24.2.5.1 Full Evaluation Route
The technical documents required include:
• complete non-clinical documents, if applicable; and
• complete clinical documents; i.e. all study reports from phase I to phase III,
including tables and appendices.
24.2.5.2 Abridged Evaluation Route
The technical documents required include:
• a non-clinical overview, if applicable; and
• a clinical overview, summaries of clinical efficacy and clinical safety, synopses
of relevant studies, a tabular listing of the clinical development programme and
study reports of the pivotal studies (the tables and appendices to the pivotal
study reports may be submitted upon request by HSA).
24.2.5.3 Verification Evaluation Route
The complete assessment report and other relevant supporting documents from the
chosen primary reference agency must be submitted, as tabulated below. The
assessment reports from the primary reference agency must be unredacted or
unedited, and should include details of imposed licensing conditions, final product
labelling, clinical reviews, and other information in relation to the product’s approval.
Reports obtained from the public domain are deemed unacceptable.
Applications submitted to HSA without the unredacted/ unedited reports from the
primary reference agency will not be accepted for evaluation via the verification
route and rejected at screening.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 158 of 168
Primary
reference
agency
Documentary requirements
EMA
• Complete Clinical assessment reports, including
assessment on the Question & Answer documents
between the Sponsor & Agency and all annexes
FDA
• Complete Clinical assessment reports, including
assessment on the Question & Answer documents
between the Sponsor & Agency and all annexes
Health Canada
• Complete Clinical assessment reports, including
assessment on the Question & Answer documents
between the Sponsor & Agency and all annexes
MHRA
• Complete Clinical assessment reports, including
assessment on the Question & Answer documents
between the Sponsor & Agency and all annexes
Swissmedic
• Complete Clinical assessment reports, including
assessment on the Question & Answer documents
between the Sponsor & Agency and all annexes
TGA
• Complete Clinical assessment reports, including
assessment on the Question & Answer documents
between the Sponsor & Agency and all annexes
The technical documents required include:
• a non-clinical overview, if applicable; and
• Clinical documents, assessment report from the primary reference agency,
including assessment on the Question and Answer documents between the
Sponsor and Agency, and other relevant supporting documents from the primary
reference agency
All the data submitted to HSA must be the same as the data package submitted to
the reference drug regulatory agencies. Differences between the dossier submitted
to HSA and data reviewed by the reference drug regulatory agencies will not only

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 159 of 168
delay the processing of the application but may also lead to the re-routing of the
dossier to the abridged evaluation route if significant undisclosed differences are
discovered.
25 MAV-2 APPLICATIONS
An MAV-2 application applies to variations involving a change in forensic
classification of a registered product, otherwise known as “reclassification”.
Examples of reclassification include from POM to P or from P to GSL. Applicants
are advised to refer to section 1.2 Forensic classification for more information.
Reclassification may also be undertaken when experience gained shows that there
is a need to supervise the use of a product – i.e. from GSL to P or POM.
More information on reclassified medicines may be found on the HSA website.
25.1 Evaluation Routes
Only the abridged evaluation route applies for MAV-2 applications.
25.2 Eligibility Criteria
A change of forensic classification of a POM or P drug product to a less stringent
classification may be considered if the following criteria are met:
(a) The use of the product has been sufficiently extensive;
(b) The product has been marketed for a period of time sufficient to establish a post-
marketing adverse event profile;
(c) The product’s safety profile gives no cause for concern during the marketing
period; and
(d) The product is presented in an appropriate pack size with consumer-friendly
labelling (PIL/outer carton).
Applicants who wish to submit a request for the reclassification of a therapeutic
product should provide justifications based on the following information:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 160 of 168
(a) The forensic classification and approved indication(s) and dosing regimen(s) of
the product in Australia, Canada, Switzerland, UK and/or US, where applicable
(b) The period of product registration in Singapore, as well as in Australia, Canada,
Switzerland, UK and US, where applicable, with specific information on its
forensic classification (i.e. POM, P and/or GSL) and duration of sale in that
classification;
(c) The period of actual product sale in Singapore;
(d) The rationale for requesting a change in the forensic classification;
(e) Patient exposure to the product and its safety profile based on worldwide
spontaneous adverse drug reaction reports, data from post-marketing
surveillance studies, clinical trials, published literature and locally reported
adverse drug reactions; and
(f) Potential problems and hazards arising from the inappropriate use of the
product.
25.3 Documentary Requirements
One set of documents, as outlined in the checklists in Appendix 2B or Appendix 3B,
should be submitted in softcopy.
The documentary requirements for an MAV-2 submission include:
(a) Section 1.1 – Comprehensive Table of Contents;
(b) Section 1.2 – Cover Letter– including the justification for reclassification, as
listed above, and the Table of Amendment Details of PRISM section 0.5;
(c) Section 1.4 – Product Labels – the proposed product labels/PIL should also be
submitted, if applicable;
(d) Section 1.5 – Approved SPC/PI/PIL;
(e) Section 1.8 – Proof of Approval – proof of the approved indication(s) and dosing
regimen(s) for the reclassified product in Australia, Canada, Switzerland, UK
and/or US;
(f) Section 1.15 – Registration Status in Other Countries; and,
(g) Module 2/Part IV – Summary of Clinical Safety – the summary should include
the following:

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 161 of 168
(i) The forensic classification of the product in Australia, Canada, Switzerland,
UK and US, with specific information on its forensic classification and
duration of sale in that classification;
(ii) The experience of patient exposure to the product – e.g. sales volume,
patient-years;
(iii) A summary of the product safety profile based on worldwide and local
spontaneous adverse drug reaction reports, post-marketing surveillance
data, clinical trials and published literature;
(iv) A list of the potential problems arising from using the product without
medical supervision; and
(v) An analysis of the hazards arising from therapeutic misuse or drug abuse,
whether deliberate or accidental e.g. consequence of delay in seeking
medical attention.
25.4 ‘Me-too’ Reclassification
A me-too MAV-2 application may be submitted if it is riding on a previous forensic
classification of an analogous product.
The documentary requirements include:
(a) Section 1.1 – Comprehensive Table of Contents;
(b) Section 1.2 – Cover Letter– including the justification for reclassification, and the
Table of Amendment Details of PRISM section 0.5;
(c) Section 1.4 – Product Labels – the proposed product labels/PIL should also be
submitted, if applicable; and
(d) Section 1.5 – Approved SPC/PI/PIL, if applicable.
The Summary of Clinical Safety in Module 2/Part IV is not required.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP
Page 162 of 168
CHAPTER H MINOR VARIATION (MIV) APPLICATION
SUBMISSION
This chapter applies to minor variation applications for currently registered
products.
26 APPLICATION TYPES
There are two types of minor variation applications – MIV-1 and MIV-2:
MIV-1:
A minor variation that
• Is specified under Part A: Checklist on Dossier
Requirements for MIV-1 variations of Appendix 13
(Chemicals) or Appendix 14 (Biologics);
• Requires prior approval before the change(s) can be
implemented.
MIV-2 (Notification)
A minor variation that
• Is specified under Part B: Checklist on Dossier
Requirements for MIV-2 (Notification) Variation of
Appendix 13 (Chemicals) or Appendix 14 (Biologics);
• May be implemented within 40 days upon application
submission if there are no objections raised by HSA.
MIV-2 (Do-and-Tell)
A minor variation that
• Is specified under Part C: Checklist on Dossier
Requirements for MIV-2 (Do-and-Tell) Variation of
Appendix 13 (Chemicals) or Appendix 14 (Biologics);
• Does not require prior approval, but must be
submitted to HSA within 6 months following
implementation of the specified changes.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 163 of 168
27 APPLICATION SUBMISSION
Applicants should be familiar with the guidelines and documentary requirements
described in Appendix 13 (chemical) and Appendix 14 (biologics) before submitting
minor variation applications. The appropriate variation may be selected with the aid
of the MIV self-guided tool . Changes that do not require notification to HSA are
listed in Section 4 of Appendices 13 and 14.
Any undisclosed variation(s) embedded in the submitted data, including any follow-
on changes, will not be considered. Evaluation will be based on the data relevant
to the proposed variation(s) unless HSA specifically requests for additional
information.
For applications where there are proposed changes to the product labels:
(i) Both the proposed and currently approved Singapore product labels and
PI/PIL are required.
(ii) For the proposed labelling/PI/PIL, a pristine and an annotated version
(which highlights the changes made to the currently approved labelling) are
required.
(iii) Annotations should be made on the current approved labelling materials
based on the actual text to be added.
(iv) Current approved text proposed for deletion should be struck through,
whereas newly added and proposed text should be underlined or
highlighted.
(v) Current approved text that is not intended to be deleted should not be
annotated.
(vi) Proposed changes to all labels must be clearly annotated, and the final
approved changes would be as annotated in the final label submitted in
PRISM.
Applicants are strongly encouraged to submit variation applications for multiple
strengths of the same therapeutic product at the same time. Applicants should also
indicate in the PRISM application form and cover letter if the proposed change(s)

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 164 of 168
affect multiple products and if there are other pending variation (MAV/MIV)
applications for the same therapeutic product.
Suggested guidance for further reading:
• Appendix 5 Target Processing Timeline
• Appendix 17 Guideline on PRISM Submission.
27.1 MIV-1 Applications
There are two submission routes – abridged or verification route.
An application may be submitted via the verification route if:
(i) The proposed variation(s) is identical to those approved by one of HSA’s
reference agencies; and
(ii) The application is accompanied by the proof of approval or approved product
labels of that reference agency.
Applications that do not fulfil the above requirements should be submitted via the
abridged route.
For each product registration, applicants may submit up to a maximum of five
concurrent MIV-1 applications at any one time.
27.1.1 Submitting multiple/consequential changes
MIV-1 changes can be grouped into a single MIV-1 application if the changes are
consequential. A consequential change refers to a change that is unavoidable and
a direct result of another change, e.g., consequential change of manufacturing
process due to a change of manufacturing site. Applicants should indicate the main
change as the primary change and any consequential change(s) as the secondary
change(s) in the PRISM application form.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 165 of 168
Non-consequential MIV-1 changes should be submitted in separate MIV-1
applications, e.g., MIV-1 changes in product labelling for clinical information should
not be grouped with any quality MIV-1 changes.
For MIV applications containing both MIV-1 and MIV-2 changes, the application
should be categorised as MIV-1.
27.2 MIV-2 Applications
Please note that at any one time, there can only be one MIV-2 application per
product registration. Multiple changes are allowed in a single MIV-2 application.
27.2.1 MIV-2 Notification
MIV-2 change(s) can be implemented if there is no objection from HSA within the
notification timeline of 40 working days, excluding stop-clock.
27.2.2 MIV-2 Do-and-Tell
Please refer to Appendix 13C and 14C for the list of Do-and-Tell changes.
Applicants have 2 submission options:
(i) 6-Monthly Notification
Consolidate all “Do-and-Tell” changes that have been implemented within a 6-
month timeframe of the scheduled submission periods of January (changes made
from July to December of the preceding year) and July (January to June of the
present year).
(ii) Flexible Notification
Submit a Do-and-Tell change anytime as a MIV-2 submission, or together with other
standard MIV-2 changes provided that the change was implemented within the
preceding 6 months.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 166 of 168
If the same Do-and-Tell change was amended and re-implemented during the 6-
month timeframe, only the latest version of the change should be submitted. You
may also combine Do-and-Tell changes in an MIV-1 application provided that these
are consequential changes.
Please refer to the table below for examples:
Scenario
What you should do
1
You have 3 Do-and-Tell changes
implemented in March, May and
June within the same year.
Combine the changes in one MIV-2
Do-and-Tell application and submit
by the end of July of the same year.
2
You have implemented a Do-and-
Tell change in March, but omitted
this in your July submission
package.
You may submit the omitted change
as a standalone or combined MIV-2
application after the submission
period, latest by September(6 months
from implementation).
3
Your MIV-2 is still pending
notification by HSA in January
and you need to submit a Do-and-
Tell variation.
You may submit the Do-and-Tell
variation after the pending MIV-2
application has been processed
(within 6 months of implementation),
or make a written request to include
the Do-and-Tell change in the
pending MIV-2 application.
4
You have prepared one Do-and-
Tell MIV-2 submission package
scheduled for July. However you
need to also submit an urgent
MIV-2 variation at the same time.
You may combine both changes in
the same MIV-2 application.

GUIDANCE ON THERAPEUTIC PRODUCT REGISTRATION IN SINGAPORE AUGUST 2024
HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Page 167 of 168
5
You have submitted a MIV-2 Do-
and-Tell application which is
pending processing. However,
you need to submit an urgent
MIV-2 change.
You may withdraw the pending MIV-2
Do-and-Tell application and resubmit
a fresh MIV-2 application which
incorporates both the additional MIV-
2 change and the previously
submitted Do-and-Tell change.


