
1
LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
To cite: LeeM- T, LeeJW,
LeeHJ, etal. Interstitial lung
disease following COVID- 19
vaccination: a disproportionality
analysis using the Global Scale
Pharmacovigilance Database
(VigiBase). BMJ Open Respir
Res 2023;10:e001992.
doi:10.1136/
bmjresp-2023-001992
► Additional supplemental
material is published online
only. To view, please visit the
journal online (http:// dx. doi.
org/ 10. 1136/ bmjresp- 2023-
001992).
Received 31 July 2023
Accepted 14 November 2023
For numbered affiliations see
end of article.
Correspondence to
Dr Sun- Young Jung;
jsyoung@ cau. ac. kr
Interstitial lung disease following
COVID- 19 vaccination: a
disproportionality analysis using the
Global Scale Pharmacovigilance
Database (VigiBase)
Min- Taek Lee,
1,2
Ju Won Lee,
1,2
Hyeon Ji Lee,
1,2
Jong- Min Lee,
1,2
Jae Chol Choi,
3,4
Kang- Mo Gu,
4,5
Sun- Young Jung
1
Interstitial lung disease
© Author(s) (or their
employer(s)) 2023. Re- use
permitted under CC BY- NC. No
commercial re- use. See rights
and permissions. Published by
BMJ.
ABSTRACT
Background and objective Despite several case reports,
population- based studies on interstitial lung disease (ILD)
following COVID- 19 vaccination are lacking. Given the
unprecedented safety issue of COVID- 19 vaccination,
it is important to assess the worldwide patterns of ILD
following COVID- 19 vaccination. This study aimed to
investigate the signals of COVID- 19 vaccine- associated ILD
compared with other vaccinations using disproportionality
analysis.
Methods We analysed the VigiBase database during the
period between 13 December 2020 and 26 January 2023.
We adopted the case/non- case approach to assess the
disproportionality signal of ILD for COVID- 19 vaccines via
1:10 matching by age and sex. We compared COVID- 19
vaccines with all other vaccines as the reference group.
Results Among 1 233 969 vaccine- related reports, 679
were reported for ILD. The majority of ILD cases were
related to tozinameran (376 reports, 55.4%), Vaxzevria
(129 reports, 19.0%) and elasomeran (78 reports, 11.5%).
The reporting OR of ILD following COVID- 19 vaccination
was 0.86 (95% CI 0.64 to 1.15) compared with all other
vaccines.
Conclusion No signicant signal of disproportionate
reporting of ILD was observed for COVID- 19 vaccines
compared with all other vaccines. Moreover, when
compared with the inuenza vaccines that are known
to cause ILD, no signal was observed. This study results
might help decision- making on the subsequent COVID- 19
vaccination strategy of ILD. Further large and prospective
studies are required for more conclusive evidence.
INTRODUCTION
As of September 2023, a total of 13.5 billion
doses of COVID- 19 vaccines have been
administered, which averted millions of death
worldwide.
1 2
The World Health Organization
(WHO) has recently declared the expira-
tion of COVID- 19 public health emergency
and revise the long- term COVID- 19 disease
management strategies.
3
It is expected that
the COVID- 19 vaccines will be included in
the regular immunisation schedule similar
to other seasonal influenza vaccines.
4 5
To
ensure a successful vaccination programme,
safety information is important, especially for
concerns that are not fully addressed. WHO
encourages countries to perform research
on vaccines with respect to unknown critical
information.
3
Since August 2021, cases of interstitial lung
disease (ILD) following COVID- 19 vaccina-
tion have been reported, although the under-
lying aetiology remained poorly understood.
ILD is a heterogeneous group of diseases
characterised by progressive inflammation
and injury to the interstitium and alveoli.
6 7
The incidence of ILD varies according to age,
sex, region and race and the prevalence is
WHAT IS ALREADY KNOWN ON THIS TOPIC
⇒ Since the rst case report of interstitial lung disease
(ILD) following COVID- 19 vaccination was published
on 9 June 2021, several ILD cases have been re-
ported. However, population- based studies on ILD
following COVID- 19 vaccination are lacking.
WHAT THIS STUDY ADDS
⇒ No signicant signal of disproportionate reporting of
ILD was observed for COVID- 19 vaccines compared
with other vaccines (reporting OR 0.86, 95% CI 0.64
to 1.15). These ndings were consistent across sev-
eral analyses conducted after considering potential
biases.
HOW THIS STUDY MIGHT AFFECT RESEARCH,
PRACTICE OR POLICY
⇒ This study may provide information that can be use-
ful for making decisions on subsequence COVID- 19
vaccine strategies. Moreover, further studies using
patient- level information such as disease history
and diagnostic test results are required for more
conclusive evidence.
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from

2
LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
Open access
approximately 6.3–76.0 cases per 100 000 people.
8 9
The
causes of ILD are not clearly known, but several poten-
tial risk factors have been suggested, including systemic
autoimmune disease and drug exposure.
7
The incidence
and prevalence of drug- induced ILD are not well known;
however, approximately 2.5%–5.0% of all prevalent ILD
cases are estimated to be drug induced.
10 11
Amiodarone
and methotrexate are known to cause drug- induced ILD,
and the use of these medications has been reported in
over 10% of cases with mortality.
10
According to previous
reports, vaccination, especially for influenza, is likely to
cause ILD.
12–14
Conversely, there have been some case
reports suggesting an association between COVID- 19
vaccination and the development and progression
of ILD.
15–22
It remains unknown whether COVID- 19
vaccination- associated ILD has distinct characteristics
compared with the disease induced by other vaccines,
such as the influenza vaccine.
Spontaneous reports are a useful source to assess
signals of rare but serious adverse events (AEs), including
COVID- 19 vaccine- induced ILD. Studies suggest a tempo-
rary increase in reporting rate after product approval
and safety alerts due to safety concerns. Therefore, it is
necessary to verify ILD cases as the COVID- 19 vaccination
rate increases. Given that the unprecedented safety issues
of COVID- 19 vaccines have been raised, it is important
to study the identifying characteristics of ILD following
COVID- 19 vaccination and investigate factors affecting
the risk of ILD or reporting rate. Therefore, this study
aimed to assess the disproportionality of reporting of
ILD associated with COVID- 19 vaccines using the case/
non- case approach by analysing the WHO global phar-
macovigilance database.
METHODS
Data source
We used VigiBase, the largest global pharmacovigilance
database with over 30 million reports of suspected AEs
of medicines since 1968.
23
It was developed and main-
tained by WHO- Uppsala Monitoring Centre (UMC).
The WHO- UMC receives individual case safety reports
(ICSRs) from over 150 countries participating in the
WHO programme for international drug monitoring.
23
VigiBase is composed of several medical and drug clas-
sification elements, such as the medical dictionary for
regulatory activities (MedDRA) and WHODrug. The AEs
analysed in our study were investigated using MedDRA
version 26.0 (released March 2023) with preferred terms
(PTs) and lowest level terms (LLTs), and drugs were
coded using WHODrug Global B3/C3- format 1 March
2023.
Variables
We extracted the ICSRs with vaccines as suspected drugs
between 13 December 2020 and 26 January 2023.
24
ILD was defined using MedDRA- standardised MedDRA
queries (SMQ) (SMQ code=20000042) narrow terms
to provide a clear definition and to account for speci-
ficity (cases highly likely to be of interest). There were
79 PTs and 132 LLTs in the ILD defined by MedDRA
SMQ (online supplemental table 1). The COVID- 19
vaccines tozinameran, elasomeran, Vaxzevria, Ad26.
COV2.S, Gam- COVID- Vac, NVX- CoV2373 and GBP510
were included in our study and were defined using drug
record numbers in WHODrug (online supplemental
table 2) and the Anatomical Therapeutic Chemical
(ATC) classification (J07BN). The other vaccines were
classified according to ATC classification (J07; vaccines).
We included physicians, pharmacists and other health
professionals as notifier types, and excluded reports with
missing values, including age and sex. The dates on which
the reports were entered into the VigiBase were arranged
by quarters. The geographical regions were divided
into five groups: Africa, the Americas, South- East Asia,
Europe, Eastern Mediterranean and Western Pacific.
Using ICSRs, we calculated the time- to- onset, which is the
time interval between vaccine administration and initi-
ation of the event. In VigiBase, ICSRs contain multiple
AEs with several different time- to- onset. Therefore, we
selected the vaccine- AE pairs with the most information,
including dechallenge action/outcome and rechallenge
action/outcome as representatives. Moreover, if vaccine–
AE pairs have an equal number of outcome informa-
tion, we chose the ICSR with the shortest time- to- onset.
Furthermore, we regarded time- to- onset as an outlier by
individual pairs (coded as missing values) if it was outside
the study period.
Statistical analysis
We performed a descriptive analysis of ILD cases and
non- cases. Continuous variables, including time- to- onset,
were presented as the mean±SD and were compared using
the Student’s t- test. The categorical variables, including
reported quarter, age groups, sex, type of report, type
of COVID- 19 vaccines, seriousness, region and type of
notifier, were reported as numbers (percentage) and
compared using the χ
2
test and Fisher’s exact test.
The association between COVID- 19 vaccines and
ILD was evaluated using case/non- case analysis.
25
The
case/non- case analysis is a disproportionality approach
performed in the pharmacovigilance databases devel-
oped during the early 1980s.
26
Briefly, it is similar to case–
control analysis but uses non- case instead of control. In
the spontaneous AE report database, the ICSRs indi-
cate reports of exposure to the drug of interest at least
once and any AE experienced any AE at least once.
25
In
our study, cases were defined as ICSRs of ILD while the
remaining ICSRs were considered non- cases. The primary
analysis compared the COVID- 19 vaccines with all other
vaccines. We compared ILD cases and non- cases by 1:10
matching according to age and sex as matching variables.
The logistic regression model was used for calculating
reporting ORs (RORs) and 95% CI.
27
We determined the
detection of a signal according to the three criteria: the
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from

LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
3
Open access
ROR is greater than 1, the lower bound 95% CI is greater
than 1 and the number of cases is greater than 3.
25
We performed subgroup analyses using stratification
by age groups, sex and region. We determined factors
reported in previous case reports that may affect the
occurrence of ILD. Individuals were categorised into
two groups based on (1) age (<65 and ≥ 65 years), (2)
sex (male and female) and (3) region (Western Pacific
region and the other regions).
Moreover, sensitivity analyses were performed to iden-
tify diverse AE definitions and the extent of contribution
of potential biases as follows. First, we designed sensitivity
analysis 1 to define ICSRs with vaccines as suspected,
concomitant and interaction drugs, as opposed to the
primary analysis performed with suspected drugs. Second,
we defined ILD using both MedDRA SMQ narrow and
broad terms (broad search), thereby including all
possible cases. Third, we excluded ICSRs that included
drugs known to cause ILD, such as those used to treat
cancer, rheumatic diseases, infection and cardiac diseases
(online supplemental table 2) as these can influence
the likelihood of detecting a signal between COVID- 19
vaccines (drug competition bias).
28
Fourth, since events
known as scientific and specific medical concerns about
COVID- 19 vaccines could affect other signal events
(competition bias), we excluded reports containing 14
AEs of special interest of COVID- 19 vaccine, including
myocarditis, pericarditis and thrombosis, as suggested by
Brighton collaboration.
29
Fifth, ICSRs reported as serious
AEs were restricted in sensitivity analysis 5. The Weber
effect could arise due to the market authorisation of
new COVID- 19 vaccines. Sixth, we included ICSRs with
a reporting date before 9 August 2021, which may have
influenced reporting in sensitivity analysis 6 (notoriety
bias).
15
Finally, in sensitivity analysis 7, we compared the
COVID- 19 vaccines with the influenza vaccines (ATC:
J07BB, influenza vaccines) as a positive control. This
choice was based on previous reports
12–14
indicating that
influenza vaccines have been known to cause ILD. Addi-
tionally, there is a report suggesting that the mechanism
of ILD following COVID- 19 vaccination may be similar to
the mechanism of ILD following influenza vaccination.
13
We have organised the overall analysis strategies in online
supplemental table 3.
Patient and public involvement
As this is a secondary database study, the database is
anonymised and served without identifiers of the study
participants. The patients were not involved in the
design, conduct or dissemination of this study.
RESULTS
A total of 1 233 969 reports with AEs following COVID- 19
vaccination were identified from 12 December 2020 to
26 January 2023. After 1:10 matching by age group and
sex, 7469 reports were determined to be ILD cases (679
ICSRs) and non- cases (6790 ICSRs) (figure 1). The
characteristics of ILD cases/non- cases of the primary
analysis, including reported quarter, age groups, sex, type
of report, type of vacines, seriousness, region and time-
to- onset, are shown in table 1. This study analysed six
COVID- 19 vaccines, including tozinameran, elasomeran,
Vaxzevria, Ad26.COV2.S., Gam- COVID- Vac and NVX-
CoV2373. GBP510 was not included in our study. Most
of the reports were received in the third quarter of 2021
(104 ICSRs, 17.1%) with ILD cases following COVID- 19
vaccination being first reported (table 1). A significant
proportion of ILD cases received tozinameran (376
ICSRs, 55.4%) and were Europeans (577 reports, 85.0%).
Serious AEs including death, life- threatening conditions
and hospitalisation/prolonged hospitalisation were
more likely in ILD cases (625 ICSRs, 92.1%) compared
with non- cases (2343 ICSRs, 34.5%). ILD cases had the
highest proportion of reports received by physicians (527
ICSR, 77.6%), followed by other health professionals
(102 ICSRs, 105.8%) and pharmacists (50 ICSRs, 7.4%).
The median time- to- onset was 7 (IQR 1–36) days for ILD
cases and 1 (IQR 0–15) day for non- cases (p=0.0077).
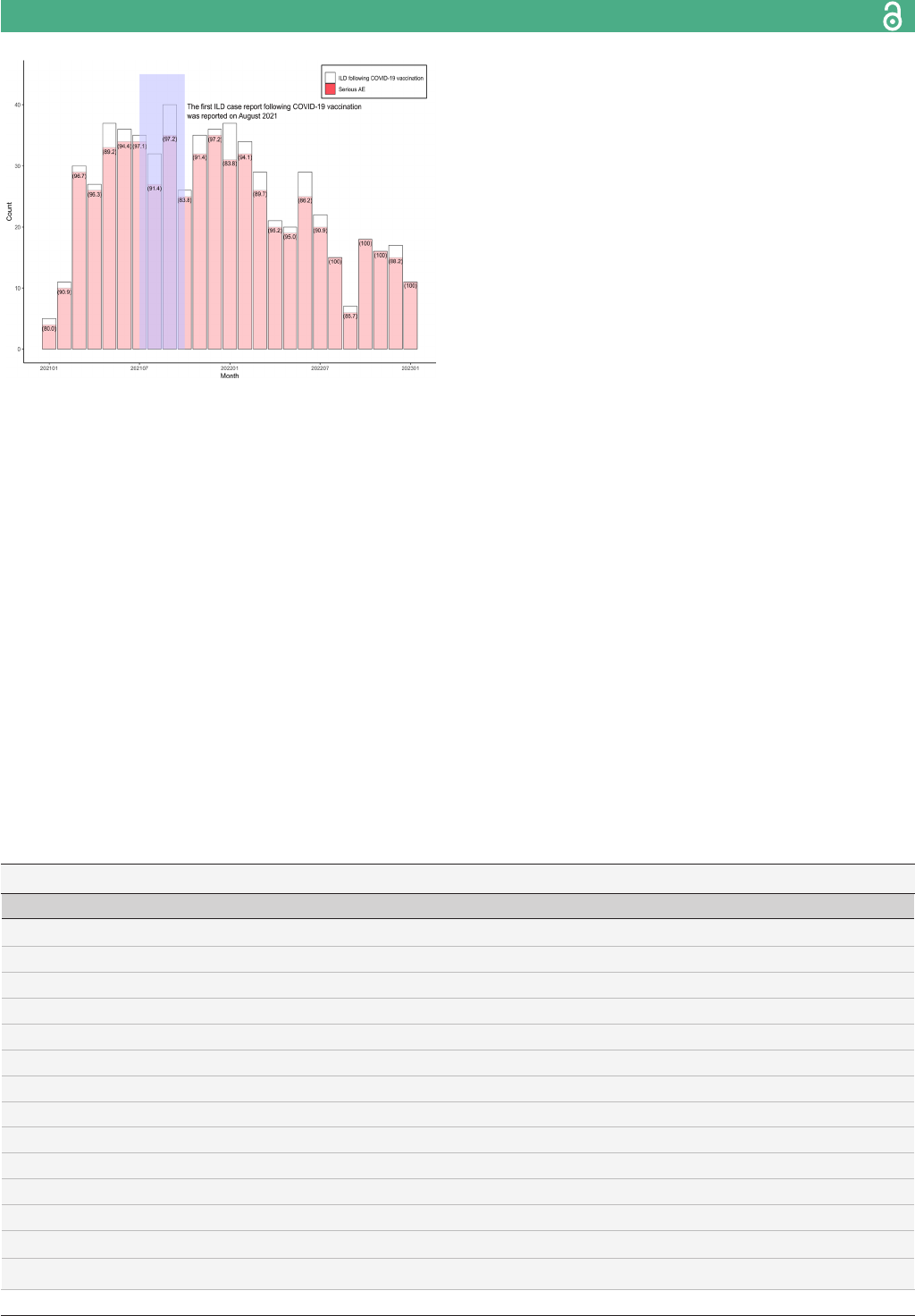
The number of monthly ILD cases following COVID- 19
vaccination is shown in figure 2. Most of the cases of
ILD following COVID- 19 vaccination (40 cases) were
Figure 1 Flow chart of primary analysis. Primary analysis
compared COVID- 19 vaccines with all other vaccines.
ICSR, individual case safety report; ILD, interstitial lung
disease; MedDRA, Medical Dictionary for Regulatory
Activities.
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from
4
LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
Open access
Table 1 Characteristics of interstitial lung disease (ILD) and non- ILD cases from VigiBase database: primary analysis
(compared COVID- 19 vaccines with all other vaccines)
ILD cases (N=679) Non- cases (N=6790)
P valueN (%) N (%)
Reported quarter (Q) 0.036
2020.4Q (13 December 2020–31 December 2020) 0 (0.0) 19 (0.3)
2021.1Q (1 January 2021–31 March 2021) 49 (7.2) 703 (10.4)
2021.2Q (1 April 2021–30 June 2021) 104 (15.3) 1193 (17.6)
2021.3Q (1 July 2021–30 September 2021) 116 (17.1) 1066 (15.7)
2021.4Q (1 October 2021–31 December 2021) 104 (15.3) 1002 (14.8)
2022.1Q (1 January 2022–31 March 2022 102 (15.0) 883 (13.0)
2022.2Q (1 April 2022–30 June 2022) 80 (11.8) 820 (12.1)
2022.3Q (1 July 2022–30 September 2022) 44 (6.5) 463 (6.8)
2022.4Q (1 October 2022–31 December 2022) 66 (9.7) 560 (8.3)
2023.1Q (1 January 2023–26 January 2023) 14 (2.1) 81 (1.2)
Age groups 1
0–27 days 2 (0.3) 20 (0.3)
28 days to 23 months 26 (3.8) 260 (3.8)
2–11 years 4 (0.6) 40 (0.6)
12–17 years 4 (0.6) 40 (0.6)
18–44 years 92 (13.6) 920 (13.6)
45–64 years 187 (27.5) 1870 (27.5)
65–74 years 144 (21.2) 1440 (21.2)
≥75 years 220 (32.4) 2200 (32.4)
Sex 1
Male 356 (52.4) 3560 (52.4)
Female 323 (47.6) 3230 (47.6)
Report type <0.0001
Spontaneous 618 (91.0) 6205 (91.4)
Report from study 54 (8.0) 317 (4.7)
Other 7 (1.0) 267 (3.9)
Not available to sender (unknown) 0 (0.0) 1 (0.0)
Vaccines type
COVID- 19 vaccines 626 (92.2) 6331 (93.2) 0.3039
Tozinameran 376 (55.4) 3324 (49.0) 0.0014
Elasomeran 78 (11.5) 642 (9.5) 0.0871
Vaxzevria 129 (19.0) 1492 (22.0) 0.073
Ad26.COV2.S 8 (1.2) 224 (3.3) 0.0024
Gam- COVID- Vac 0 (0.0) 2 (0.0) 0.6547
NVX- CoV2373 0 (0.0) 3 (0.0) 1.000
Inuenza vaccines 34 (5.0) 123 (1.8) <0.0001
Pneumococcal vaccines 11 (1.6) 105 (1.6) 0.8824
Drug known to cause ILD included in the ICSRs*
Cancer therapy 17 (2.5) 10 (0.2) <0.0001
Rheumatology therapy 27 (4.0) 25 (0.4) <0.0001
Anti- infection agent 3 (0.4) 3 (0.0) 0.0121
Cardiology drugs 62 (9.1) 241 (3.6) <0.0001
Continued
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from
LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
5
Open access
reported in September 2021, while the first report was
from January 2021. The number of reports decreased
steadily until the end of the study. Serious AE reports
accounted for 84.4% to 100% of all ILD cases following
COVID- 19 vaccination (figure 2).
We identified characteristics of ILD cases by COVID- 19
vaccines, influenza vaccines and other vaccines. The
COVID- 19 vaccines contained reports from European
and had the longest median time- to- onset (1 (IQR 1–36)
day) than the influenza vaccine (5 (IQR 2–23)) and others
(6 (IQR 1–21)) (online supplemental table 4). The most
frequently reported AEs with regard to PT or LLT were
pneumonitis (134 ICSRs, 19.7%), ILD (70 ICSRs, 10.3%)
and interstitial pneumonia (46 ICSRs, 6.8%) in ILD cases
(online supplemental table 5). AEs that included both
narrow and broad terms were aligned with the AEs from
narrow terms. Details of ILD cases are provided in online
supplemental tables 4 and 5.
Case/non-case analysis
The results of ILD cases/non- cases, including those of
primary, secondary and subgroup analyses, are shown in
table 2. The ROR of ILD following COVID- 19 vaccina-
tion was 0.86 (95% CI 0.64 to 1.15) compared with other
vaccines. The ROR of mRNA vaccines was 0.99 (95% CI
0.73 to 1.34), tozinameran was 0.98 (95% CI 0.73 to 1.33)
and elasomeran was 1.04 (95% CI 0.71 to 1.50). Moreover,
no signal of disproportionate reporting was observed in
viral vector COVID- 19 vaccines (viral vector COVID- 19
vaccines ROR 0.69 (95% CI 0.50 to 0.96); Vaxzevria ROR
0.75 (95% CI 0.53 to 1.05) and Ad26.COV2.S ROR 0.32
(95% CI 0.15 to 0.67)) (table 2).
In the subgroup analysis of primary analysis, we did
not find an increased reporting of ILD according to age
groups, sex and region (table 3, online supplemental
table 6). The ILD following COVID- 19 vaccination was
not associated with a disproportionality signal regardless
ILD cases (N=679) Non- cases (N=6790)
P valueN (%) N (%)
Serious <0.0001
Yes 625 (92.1) 2323 (34.5)
Seriousness <0.0001
Death 116 (17.1) 287 (4.2)
Life threatening 97 (14.3) 162 (2.4)
Caused/Prolonged hospitalisation 285 (42.0) 582 (8.6)
Disabling/incapacitating 13 (1.9) 91 (1.3)
Congenital anomaly/birth defect 0 (0.0) 3 (0.0)
Other 114 (16.8) 1218 (17.9)
Region <0.0001
African 4 (0.6) 364 (5.4)
Americas 54 (8.0) 632 (9.3)
South- East Asia 6 (0.9) 121 (1.8)
European 577 (85.0) 4398 (64.8)
Eastern Mediterranean 8 (1.2) 342 (5.0)
Western Pacic 30 (4.4) 933 (13.7)
Notier type <0.0001
Physician 527 (77.6) 3675 (54.1)
Pharmacist 50 (7.4) 1055 (15.5)
Other health professional 102 (15.0) 2060 (30.3)
Time to onset (case=574, non- case=6064) 0.0077
Mean±SD 32.7±64.7 26.6±57.3
Median (Q1–Q3) 7 (1–35) 1 (0–15)
*Cancer therapy: bleomycin; gemcitabine; epidermal growth factor receptor- targeted agent (erlotinib, getinib, panitumumab, cetuximab);
mammalian target of rapamycin- inhibitor (everolimus, temsirolimus, sirolimus); immune checkpoint inhibitor (nivolumab, pembrolizumab,
avelumab, durvalumab, ipilimumab), rheumatology drugs: methotrexate; leunomide, biological disease- modifying anti- rheumatic drugs
(tumour necrosis factor) agent (iniximab, etanercept, adalimumab), tocilizumab, rituximab), anti- infection agents (nitrofurantoin, daptomycin,
interferon), cardiology drugs (amiodarone, bepridil, statin (lovastatin, simvastatin, pravastatin, atorvastatin, uvastatin, rosuvastatin,
cerivastatin)).
ICSRs, individual case safety reports.
Table 1 Continued
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from
6
LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
Open access
of age groups (under 65 years; ROR 0.94 (95% CI 0.65
to 1.35), 65 years and older; ROR 0.70 (95% CI 0.41 to
1.17). The COVID- 19 vaccination emerged with no signal
in both males and females (males ROR 0.81 (95% CI 0.55
to 1.19), females ROR 0.93 (95% CI 0.59 to 1.46)). There
was no signal when stratifying Western Pacific region and
the other regions (ROR 0.42 (95% CI 0.16 to 1.14) and
ROR 0.88 (95% CI 0.65 to 1.21).
The results of the sensitivity analyses were similar to
those of the primary analysis (figure 3, online supple-
mental table 7). There was no disproportionality signal
when considering diverse AE definitions and potential
biases (figure 3, online supplemental table 7). The ROR
of influenza vaccines was 0.44 (95% CI 0.27 to 0.71). The
results of sensitivity analysis 7 compared with influenza
vaccines were consistent with the primary analysis (online
supplemental tables 8 and 9)
DISCUSSION
The present study aimed to identify the characteristics of
ILD following COVID- 19 vaccination and the dispropor-
tionality between COVID- 19 vaccines and ILD using the
global pharmacovigilance database. We identified 679
ILD cases from VigiBase defined using MedDRA SMQ
and performed disproportionality analysis. To the best of
our knowledge, this is the first study to investigate the
signals of disproportionate reporting of ILD associated
with COVID- 19 vaccines. Compared with other vaccines,
no significant signal of disproportional reporting of ILD
was observed for COVID- 19 vaccines. These findings were
consistent across several analyses conducted after consid-
ering potential biases. Moreover, the signal of dispropor-
tionality was not detected when compared with the influ-
enza vaccine which is known to induce ILD.
In our study, reports received from European
accounted for the majority of ILD cases (85.0%) following
COVID- 19 vaccination. In contrast to the present study,
most ILD cases following COVID- 19 vaccination have
been reported in South- East Asia, including South Korea
and Japan since Park et al reported the first ILD case
following mRNA COVID- 19 vaccination.
15 16 18–20
Kono et
al suggested that South- East Asian population should be
carefully monitored since it is at a high risk of COVID- 19
vaccine- related ILD.
30
Among 30 cases of ILD identified
following COVID- 19 vaccination in the Western Pacific,
which is classified as Asia by WHO, and the signal of ILD
was not detected when compared with other vaccines
(ROR 1.68, 95% CI 0.68 to 4.16) (table 2). However,
the result of subgroup analysis according to the region
should be interpreted with caution because of the small
number of cases and incomplete information on ICSRs.
Figure 2 The number of ILD cases following COVID- 19
vaccination during the study period. The brackets () present
the proportion of serious AE among ICSRs reported ILD
following COVID- 19 vaccination. AE, adverse event; ICSR,
individual case safety report; ILD, interstitial lung disease.
Table 2 Reporting OR (ROR) of COVID- 19 vaccines and all other vaccines (primary analysis)
Type of analysis ILD cases Non- cases ROR (95% CI)
Primary analysis (cases: 679, non- cases: 6790)
The other vaccines 53 (7.8) 459 (6.8) Reference
COVID- 19 vaccines 626 (92.2) 6331 (93.2) 0.86 (0.64 to 1.15)
mRNA COVID- 19 vaccines 448 (66.0) 3912 (57.6) 0.99 (0.73 to 1.34)
Tozinameran 373 (58.9) 3282 (54.4) 0.98 (0.73 to 1.33)
Elasomeran 73 (11.5) 611 (10.1) 1.04 (0.71 to 1.50)
Viral vector COVID- 19 vaccines 137 (20.2) 1718 (25.3) 0.69 (0.50 to 0.96)
Vaxzevria 126 (19.9) 1461 (24.2) 0.75 (0.53 to 1.05)
Gam- COVID- Vac 0 (0.0) 2 (0.0) NC
Ad26.COV2.S 8 (1.3) 220 (3.6) 0.32 (0.15 to 0.67)
Protein- based COVID- 19 vaccines 0 (0.0) 3 (0.0) NC
NVX- CoV2373 0 (0.0) 3 (0.1) NC
Others 41 (6.0) 698 (10.3) 0.51 (0.33 to 0.78)
ILD, interstitial lung disease; NC, not calculated.
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from

LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
7
Open access
A previous systematic review of drug- induced ILD iden-
tified male has been as a risk factor for drug- induced
ILD, especially in those treated with amiodarone, metho-
trexate, epidermal growth factor receptor tyrosine kinase
inhibitor (EGFR- TKI) and premetrexed.
31
Males were
predominant in previous case reports of ILD related
to COVID- 19 vaccination.
15 16 18–20
However, this study
observed no signal of disproportionate reporting regard-
less of sex (males (ROR 0.81, 95% CI 0.55 to 1.19), females
(ROR 0.93, 95% CI, 0.59 to 1.46)) (online supplemental
table 6). Further studies are required to identify the risk
according to demographic characteristics.
We analysed the data of spontaneous reporting systems
to assess signals of AE of COVID- 19 vaccination. The
spontaneous reporting systems have several biases due
to factors that could affect reporting, which results in
incorrect signal detection. These biases can be notoriety
bias, information bias, selection bias and competition
bias.
25 32 33
We implemented different minimisation strat-
egies against these biases. First, we designed a primary
analysis to address factors that could lead to information
bias by considering to be suspected, healthcare profes-
sionals and complete information on age groups and
sex. Moreover, in sensitivity analysis 2, we used MedDRA
SMQ with narrow and broad terms. This result was in
line with the primary analysis that showed no signal of
disproportionate reporting (ROR 0.77, 95% CI 0.59 to
1.00). Second, for competition bias, it is necessary to
eliminate factors associated with vaccines/AEs of interest
(sensitivity analyses 3, 4). Results derived from sensitivity
analysis considering competition biases showed that
Table 3 Reporting OR (ROR) of COVID- 19 vaccines and all other vaccines in subgroup analysis
Type of analysis ILD cases Non- cases ROR (95% CI)
Subgroup analysis
Age
Age <65 (case=315, non- case=3150)
The other vaccines 36 (11.4) 339 (10.8) Reference
COVID- 19 vaccines 268 (85.1) 2447 (77.7) 0.94 (0.65 to 1.35)
Age ≥65 (case=364, non- case=3640)
The other vaccines 17 (4.7) 120 (3.3) Reference
COVID- 19 vaccines 347 (95.3) 3520 (96.7) 0.70 (0.41 to 1.17)
Gender
Male (case=356, non- case=3560)
The other vaccines 31 (8.7) 254 (7.1) Reference
COVID- 19 vaccines 325 (91.3) 3306 (92.9) 0.81 (0.55 to 1.19)
Female (case=323, non- case=3230)
The other vaccines 22 (6.8) 205 (6.4) Reference
COVID- 19 vaccines 301 (93.2) 3025 (93.7) 0.93 (0.59 to 1.46)
Region
Western Pacic region (case=30, non- case=933)
The other vaccines 5 (16.7) 73 (7.8) Reference
COVID- 19 vaccines 25 (83.3) 860 (92.2) 0.42 (0.16 to 1.14)
The other regions (case=649, non- case=5857)
The other vaccines 48 (7.4) 386 (6.6) Reference
COVID- 19 vaccines 601 (92.6) 5471 (93.4) 0.88 (0.65 to 1.21)
ILD, interstitial lung disease.
Figure 3 Reporting OR (ROR) of sensitivity analysis. AE,
adverse event; AESI, adverse event of special event; ICSR,
individual case safety report; ILD, interstitial lung disease;
MedDRA, medical dictionary for regulatory activities; SMQ,
standardised MedDRA queries.
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from

8
LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
Open access
COVID- 19 vaccines had no disproportionality signal of
ILD compared with the other vaccines (ROR 1.02, 95%
CI 0.73 to 1.42 and ROR 0.96, 95% CI, 0.69 to 1.33,
respectively). Third, in pharmacovigilance, temporal
bias (the Weber effect or notoriety bias) refers to varia-
tion in the number of reports after a specific event, such
as safety alerts and market authorisation. The signal of
ILD was not observed when minimising temporal biases;
the RORs of sensitivity analyses 5 and 6 were 0.72 (95%
CI 0.52 to 0.98) and 0.75 (95% CI 0.38 to 1.48), respec-
tively. Fourth, we defined reference groups that received
influenza and other vaccines instead of all other drugs
to avoid selection bias. The analysis using influenza
vaccines as a positive control in the secondary analysis
showed that COVID- 19 vaccines emerged with no signal
when compared with influenza vaccines (ROR 0.44, 95%
CI 0.27 to 0.71). However, the risk–benefits of COVID- 19
vaccines should be carefully assessed.
The mechanisms of COVID- 19 vaccine- induced ILD
are unclear. To date, both cytotoxicity and immune-
mediated lung injury are considered as main mecha-
nisms that initiate drug- induced ILD. Although it is rare,
the event can be fatal and patients might require hospi-
talisation.
34
According to previous reports, the influenza
vaccination can induce ILD by increasing the levels of
inflammatory cytokines.
12
Several cases of COVID- 19
mRNA vaccine associated ILD have been reported.
15–21
Given the similar clinical characteristics with influenza
vaccine- induced ILD, including onset time, chest CT
findings and responsiveness to corticosteroids, it can
be speculated that ILD following COVID- 19 vaccina-
tion might also be due to immune- mediated pulmonary
injury. These studies suggest that COVID- 19 vaccination
induces immune- mediated injury to the lungs through
T- cells, which adopt a predominant type one phenotype
in susceptible patients.
17–21 35
However, further studies
with a large number of ILD patients who received the
COVID- 19 vaccines are needed.
12 15
This study has several limitations. First, selective
reporting of AEs might have been compromised in the
spontaneous reporting database although we strived to
minimise biases. During the pandemic, the number of
ICSRs following COVID- 19 vaccination increased rapidly,
which might have resulted in differential reporting
rates and influenced parameters. We applied 1:10 exact
matching to reduce the imbalance between case and
non- case and performed various analyses. The results
of our study did not provide exhaustivity of COVID- 19
vaccine- induced ILD although it suggests focusing on the
risk. Second, we analysed ICSRs without causality assess-
ment. However, VigiBase contains essential information
required for causality assessment, including age, sex,
primary reporter and time- to- onset. Third, concerns on
the validity of ILD in spontaneous reporting database
might be raised. To overcome this limitation, we restricted
physicians (77.6%), pharmacists (7.4%) and other health
professionals (14.8%) as notifier types and defined ILD
using MedDRA SMQs, which are validated by expert
discussion. Fourth, in the present study, the majority
of ILD cases following vaccination were predominantly
in the European population, which may introduce bias
due to population heterogeneity. Fifth, previous studies
have suggested that COVID- 19 infection can lead to the
occurrence or exacerbation of ILD, referred to as post-
COVID- 19 ILD. Notably, the VigiBase we used cannot
ascertain the COVID- 19 infection status. Therefore,
the study findings should be interpreted with caution.
Finally, this study did not assess the risk of specific molec-
ular components of vaccines. The excipients such as adju-
vants, stabilisers, preservatives and trace components can
cause AE following immunisation. Therefore, besides
vaccines, the safety of excipients should also be evalu-
ated. Despite these limitations, our study used a global
pharmacovigilance database with over 30 million ICSRs
and could offer additional hypotheses for AEs. In addi-
tion, the case/non- case approach allowed us to study rare
AEs and could represent the use of drugs in real world
settings.
25
Since there were no population- based studies
and previous case reports have included exacerbation of
pre- existing ILD with death, additional safety studies are
needed.
CONCLUSION
In conclusion, we identified no significant dispropor-
tionality signal of ILD associated with COVID- 19 vaccines
using global pharmacovigilance database. This finding is
consistent regardless of the subpopulation. Furthermore,
the disproportional analysis compared with the influenza
vaccines that are known to cause ILD emerged with no
signal. However, serious AE accounted for the majority
of ILD cases following COVID- 19 vaccination and events,
including hospitalisations, have been reported. We
suggest careful monitoring of COVID- 19 vaccine- induced
ILD. This study may provide information that can be
useful for making decisions on subsequence COVID- 19
vaccine strategies. However, further studies using patient-
level information such as disease history and diagnostic
test results are required for more conclusive evidence.
Author afliations
1
College of Pharmacy, Chung- Ang University, Seoul, Korea
2
Department of Global Innovative Drugs, The Graduate School of Chung- Ang
University, Seoul, Korea
3
Division of Pulmonary and Allergy Medicine, Department of Internal
Medicine, Chung- Ang University Gwangmyeong Hospital, Gwangmyeong- si,
Gyeonggi- do, Korea
4
Department of Internal Medicine, Chung- Ang University College of Medicine,
Seoul, South Korea
5
Division of Pulmonary and Allergy Medicine, Department of Internal
Medicine, Chung- Ang University Hospital, Seoul, Korea
Contributors M- TL and S- YJ participated in the conception and design of study.
M- TL and S- YJ participated in the data acquisition and data analysis. M- TL, JWL,
JCC, K- MG and S- YJ participated in the data interpretation. M- TL participated
in the draft of the manuscript. JWL, HJL, J- ML, JCC and K- MG helped to revise
the manuscript for intellectual content. All authors read and approved the nal
manuscript. S- YJ is reponsible for the overall content as guarantor.
Funding This research, and the journal’s Rapid Service Fee, was supported
by Government wide R&D Fund Project for Infectious Disease Research
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from

LeeM- T, etal. BMJ Open Respir Res 2023;10:e001992. doi:10.1136/bmjresp-2023-001992
9
Open access
(GFID) by Republic of Korea (grant number: HG18C0066). This research was
supported by Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education (grant number
2021R1A6A1A03044296).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in
the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not applicable.
Ethics approval This study was conducted in accordance with the Declaration
of Helsinki. The study protocol was approved for exemption from review by the
Institutional Review Board of Chung- Ang University (IRB number: 1041078- 201903-
HR- 071- 01), because this study analyaed a secondary database. Informed consent
from subjects was waived due to the database containing anonymised data that
cannot identify study subjects.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data may be obtained from a third party and are not
publicly available. The data analysed in this study are available from VigiBase upon
formal request to the Uppsala Monitoring Centre at the WHO Collaborating Centre
for International Drug Monitoring.
Supplemental material This content has been supplied by the author(s). It has
not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been
peer- reviewed. Any opinions or recommendations discussed are solely those
of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and
responsibility arising from any reliance placed on the content. Where the content
includes any translated material, BMJ does not warrant the accuracy and reliability
of the translations (including but not limited to local regulations, clinical guidelines,
terminology, drug names and drug dosages), and is not responsible for any error
and/or omissions arising from translation and adaptation or otherwise.
Open access This is an open access article distributed in accordance with the
Creative Commons Attribution Non Commercial (CC BY- NC 4.0) license, which
permits others to distribute, remix, adapt, build upon this work non- commercially,
and license their derivative works on different terms, provided the original work is
properly cited, appropriate credit is given, any changes made indicated, and the
use is non- commercial. See:http://creativecommons.org/licenses/by-nc/4.0/.
ORCID iD
Sun- YoungJung http://orcid.org/0000-0003-2032-112X
REFERENCES
1 WHO Coronavirus (COVID- 19) dashboard. n.d. Available: https://
covid19.who.int/2023
2 Savinkina A, Bilinski A, Fitzpatrick M, etal. Estimating deaths
averted and cost per life saved by scaling up mRNA COVID- 19
vaccination in low- income and lower- middle- income countries in
the COVID- 19 Omicron variant era: a modelling study. BMJ Open
2022;12:e061752.
3 World Health Organization. From emergency response to long- term
COVID- 19 disease management. 2023: 14.
4 FDA. FDA Brieng Document Future Vaccination Regimens
Addressing COVID- 19. 2023.
5 Kozlov M. Should COVID vaccines be given yearly? Proposal divides
US scientists. Nature 2023. 10.1038/d41586-023-00234-7 [Epub
ahead of print 27 Jan 2023].
6 Huapaya JA, Wilfong EM, Harden CT, etal. Risk factors for mortality
and mortality rates in interstitial lung disease patients in the intensive
care unit. Eur Respir Rev 2018;27:180061.
7 Aronson KI, Danoff SK, Russell A- M, etal. Patient- centered
outcomes research in interstitial lung disease: an ofcial American
Thoracic Society research statement. Am J Respir Crit Care Med
2021;204:e3–23.
8 Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet
2022;400:769–86.
9 Olson AL, Hartmann N, Patnaik P, etal. Healthcare resource
utilization and related costs in chronic brosing interstitial lung
diseases with a progressive phenotype: a US claims database
analysis. Adv Ther 2022;39:1794–809.
10 Schwaiblmair M, Behr W, Haeckel T, etal. Drug induced interstitial
lung disease. Open Respir Med J 2012;6:63–74.
11 Spagnolo P, Bonniaud P, Rossi G, etal. Drug- induced interstitial lung
disease. Eur Respir J 2022;60:2102776.
12 Watanabe S, Waseda Y, Takato H, etal. Inuenza vaccine- induced
interstitial lung disease. Eur Respir J 2013;41:474–7.
13 DeDent AM, Farrand E. Vaccine- induced interstitial lung disease: a
rare reaction to COVID- 19 vaccination. Thorax 2022;77:9–10.
14 Okusaki T, Fukuhara K. Exacerbation of connective tissue disease-
associated interstitial lung disease due to inuenza vaccination.
Respir Med Case Rep 2021;33:101463.
15 Park JY, Kim J- H, Lee IJ, etal. COVID- 19 vaccine- related interstitial
lung disease: a case study. Thorax 2022;77:102–4.
16 Kono A, Yoshioka R, Hawke P, etal. Correction to: a case of
severe interstitial lung disease after COVID- 19 vaccination. QJM
2022;115:hcac066.
17 Sgalla G, Magrì T, Lerede M, etal. COVID- 19 vaccine in patients with
exacerbation of idiopathic pulmonary brosis. Am J Respir Crit Care
Med 2022;206:219–21.
18 Matsuzaki S, Kamiya H, Inoshima I, etal. COVID- 19 mRNA vaccine-
induced pneumonitis. Intern Med 2022;61:81–6.
19 So C, Izumi S, Ishida A, etal. COVID- 19 mRNA vaccine- related
interstitial lung disease: two case reports and literature review.
Respirol Case Rep 2022;10:e0938.
20 Yoshifuji A, Ishioka K, Masuzawa Y, etal. COVID- 19 vaccine induced
interstitial lung disease. J Infect Chemother 2022;28:95–8.
21 Ehteshami- Afshar S, Raj R. COVID- 19 mRNA vaccines and
interstitial lung disease exacerbation: causation or just a temporal
association Am J Respir Crit Care Med 2022;206:919.
22 Yoo H, Kim SY, Park MS, etal. COVID- 19 vaccine- associated
Pneumonitis in the Republic of Korea: a nationwide multicenter
survey. J Korean Med Sci 2023;38:e106.
23 Vigibase WHO- UMC 2023. n.d. Available: https://who-umc.org/
vigibase/
24 Smadja DM, Yue Q- Y, Chocron R, etal. Vaccination against
COVID- 19: insight from arterial and venous thrombosis occurrence
using data from VigiBase. Eur Respir J 2021;58:2100956.
25 Faillie JL. Case- non- case studies: principle, methods, bias and
interpretation. Therapies 2019;74:225–32.
26 Montastruc J- L, Sommet A, Bagheri H, etal. Benets and strengths
of the disproportionality analysis for identication of adverse drug
reactions in a pharmacovigilance database. Br J Clin Pharmacol
2011;72:905–8.
27 Noguchi Y, Tachi T, Teramachi H. Detection algorithms and attentive
points of safety signal using spontaneous reporting systems as a
clinical data source. Brief Bioinform 2021;22:bbab347.
28 Pariente A, Didailler M, Avillach P, etal. A potential competition
bias in the detection of safety signals from spontaneous reporting
databases. Pharmacoepidemiol Drug Saf 2010;19:1166–71.
29 Law B, Sturkenboom MD. 2.3.1 tier 1 AESI: ICD- 9/10- CM and
Meddra codes. 2017.
30 Kono A, Hawke P, Yoshioka R. Response to: Multisystem
inammatory syndrome following COVID- 19 vaccination: ignored
and underdiagnosed. QJM 2022;115:698.
31 Skeoch S, Weatherley N, Swift AJ, etal. Drug- induced interstitial
lung disease: a systematic review. J Clin Med 2018;7:356.
32 Raschi E, Poluzzi E, Salvo F, etal. Pharmacovigilance of sodium-
glucose co- transporter- 2 inhibitors: what a clinician should know on
disproportionality analysis of spontaneous reporting systems. Nutr
Metab Cardiovasc Dis 2018;28:533–42.
33 Raschi E, Fusaroli M, Diemberger I, etal. Direct oral anticoagulants
and interstitial lung disease: emerging clues from pharmacovigilance.
Drug Saf 2020;43:1191–4.
34 Matsuno O. Drug- induced interstitial lung disease: mechanisms and
best diagnostic approaches. Respir Res 2012;13:39.
35 Keech C, Albert G, Cho I, etal. Phase 1–2 trial of a SARS- CoV-2
recombinant spike protein nanoparticle vaccine. N Engl J Med
2020;383:2320–32.
by copyright.
on September 9, 2024 by guest. Protectedhttp://bmjopenrespres.bmj.com/BMJ Open Resp Res: first published as 10.1136/bmjresp-2023-001992 on 11 December 2023. Downloaded from
