
The University of the State of New York
REGENTS HIGH SCHOOL EXAMINATION
PHYSICAL SETTING
CHEMISTRY
Tuesday, June 18, 2013 — 9:15 a.m. to 12:15 p.m., only
This is a test of your knowledge of chemistry. Use that knowledge to answer all
questions in this examination. Some questions may require the use of the 2011 Edition
Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all
parts of this examination according to the directions provided in this examination
booklet.
A separate answer sheet for Part A and Part B–1 has been provided to you. Follow
the instructions from the proctor for completing the student information on your
answer sheet. Record your answers to the Part A and Part B–1 multiple-choice
questions on this separate answer sheet. Record your answers for the questions in
Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on
the front of your answer booklet.
All answers in your answer booklet should be written in pen, except for graphs and
drawings, which should be done in pencil. You may use scrap paper to work out the
answers to the questions, but be sure to record all your answers on your separate
answer sheet or in your answer booklet as directed.
When you have completed the examination, you must sign the statement printed
on your separate answer sheet, indicating that you had no unlawful knowledge of the
questions or answers prior to the examination and that you have neither given nor
received assistance in answering any of the questions during the examination. Your
answer sheet and answer booklet cannot be accepted if you fail to sign this
declaration.
DO NOT OPEN THIS EXAMINATION BOOKLET UNTIL THE SIGNAL IS GIVEN.
The possession or use of any communications device is strictly prohibited when
taking this examination. If you have or use any communications device, no matter how
briefly, your examination will be invalidated and no score will be calculated for you.
Notice. . .
A four-function or scientific calculator and a copy of the 2011 Edition Reference Tables for
Physical Setting/Chemistry must be available for you to use while taking this examination.
P.S./CHEMISTRY
P.S./CHEMISTRY

Part A
Answer all questions in this part.
Directions (1–30): For each statement or question, record on your separate answer sheet the number of the
word or expression that, of those given, best completes the statement or answers the question. Some questions
may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.
P.S./Chem.–June ’13 [2]
1 According to the wave-mechanical model of the
atom, an orbital is a region of the most probable
location of
(1) an alpha particle (3) an electron
(2) a gamma ray (4) a proton
2 Which particles have approximately the same
mass?
(1) an electron and an alpha particle
(2) an electron and a proton
(3) a neutron and an alpha particle
(4) a neutron and a proton
3 During a flame test, a lithium salt produces a
characteristic red flame. This red color is produced
when electrons in excited lithium atoms
(1) are lost by the atoms
(2) are gained by the atoms
(3) return to lower energy states within the atoms
(4) move to higher energy states within the atoms
4 Compared to the energy and charge of the
electrons in the first shell of a Be atom, the
electrons in the second shell of this atom have
(1) less energy and the same charge
(2) less energy and a different charge
(3) more energy and the same charge
(4) more energy and a different charge
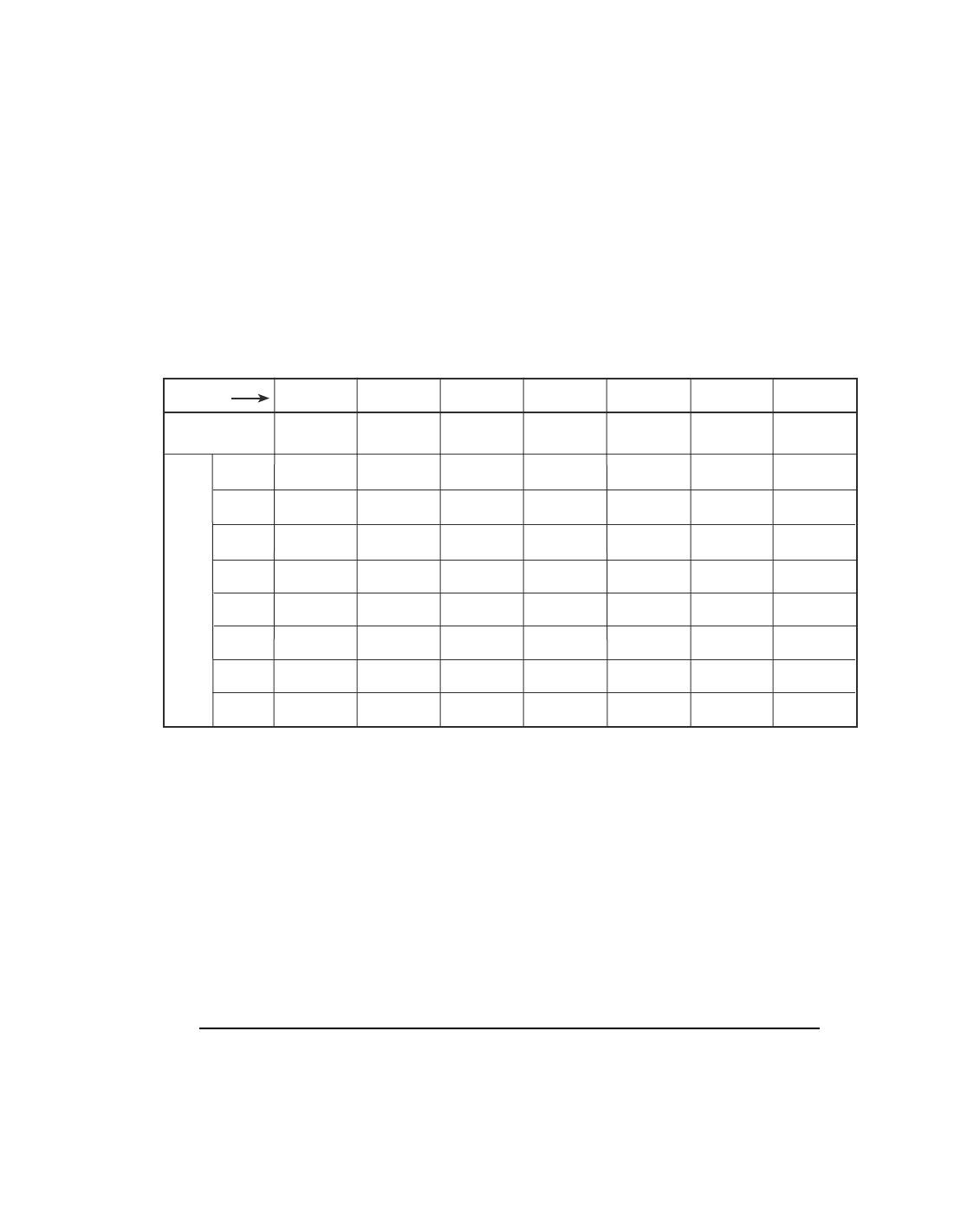
5 Which quantity can vary among atoms of the
same element?
(1) mass number
(2) atomic number
(3) number of protons
(4) number of electrons
6 Which substances have atoms of the same
element but different molecular structures?
(1) He(g) and Ne(g) (3) K(s) and Na(s)
(2) O
2
(g) and O
3
(g) (4) P
4
(s) and S
8
(s)
7 An atom that has 13 protons and 15 neutrons is
an isotope of the element
(1) nickel (3) aluminum
(2) silicon (4) phosphorus
8 Which elements have the most similar chemical
properties?
(1) Si, As, and Te (3) Mg, Sr, and Ba
(2) N
2
, O
2
, and F
2
(4) Ca, Cs, and Cu
9 Which list includes three types of chemical
formulas for organic compounds?
(1) covalent, metallic, isotopic
(2) covalent, metallic, molecular
(3) empirical, structural, isotopic
(4) empirical, structural, molecular
10 In a bond between an atom of carbon and an
atom of fluorine, the fluorine atom has a
(1) weaker attraction for electrons
(2) stronger attraction for electrons
(3) smaller number of first-shell electrons
(4) larger number of first-shell electrons
11 A sample of CO
2
(s) and a sample of CO
2
(g)
differ in their
(1) chemical compositions
(2) empirical formulas
(3) molecular structures
(4) physical properties

P.S./Chem.–June ’13 [3] [OVER]
12 Which statement defines the temperature of a
sample of matter?
(1) Temperature is a measure of the total
electromagnetic energy of the particles.
(2) Temperature is a measure of the total
thermal energy of the particles.
(3) Temperature is a measure of the average
potential energy of the particles.
(4) Temperature is a measure of the average
kinetic energy of the particles.
13 For a chemical reaction, the difference between
the potential energy of the products and the
potential energy of the reactants is equal to the
(1) heat of fusion
(2) heat of reaction
(3) activation energy of the forward reaction
(4) activation energy of the reverse reaction
14 Which equation represents sublimation?
(1) Hg(ℓ) → Hg(s) (3) NH
3
(g) → NH
3
(ℓ)
(2) H
2
O(s) → H
2
O(g) (4) CH
4
(ℓ) → CH
4
(g)
15 Which statement describes the particles of an
ideal gas, based on the kinetic molecular theory?
(1) The motion of the gas particles is orderly
and circular.
(2) The gas particles have no attractive forces
between them.
(3) The gas particles are larger than the dis-
tances separating them.
(4) As the gas particles collide, the total energy
of the system decreases.
16 Two grams of potassium chloride are completely
dissolved in a sample of water in a beaker. This
solution is classified as
(1) an element
(2) a compound
(3) a homogeneous mixture
(4) a heterogeneous mixture
17 Which compound has the strongest hydrogen
bonding between its molecules?
(1) HBr (3) HF
(2) HCl (4) HI
18 Powdered sulfur is yellow, and powdered iron is
gray. When powdered sulfur and powdered iron
are mixed at 20°C, the powdered iron
(1) becomes yellow (3) remains ionic
(2) becomes a liquid (4) remains magnetic
19 An effective collision between reactant particles
requires the particles to have the proper
(1) charge and mass
(2) charge and orientation
(3) energy and mass
(4) energy and orientation
20 Which term is defined as a measure of the
disorder of a system?
(1) heat (3) kinetic energy
(2) entropy (4) activation energy
21 Which process is used to determine the concen-
tration of an acid?
(1) chromatography (3) electrolysis
(2) distillation (4) titration
22 The compounds CH
3
OCH
3
and CH
3
CH
2
OH
have different functional groups. Therefore, these
compounds have different
(1) chemical properties
(2) gram-formula masses
(3) percent compositions by mass
(4) numbers of atoms per molecule
23 Which term identifies the half-reaction that
occurs at the anode of an operating electro-
chemical cell?
(1) oxidation (3) neutralization
(2) reduction (4) transmutation
24 During the operation of a voltaic cell, the cell
produces
(1) electrical energy spontaneously
(2) chemical energy spontaneously
(3) electrical energy nonspontaneously
(4) chemical energy nonspontaneously

P.S./Chem.–June ’13 [4]
25 In which type of chemical reaction are electrons
transferred?
(1) organic addition
(2) oxidation-reduction
(3) double replacement
(4) acid-base neutralization
26 A substance that dissolves in water and produces
hydronium ions as the only positive ions in the
solution is classified as
(1) an alcohol (3) a base
(2) an acid (4) a salt
27 According to one acid-base theory, a base is an
(1) H
⫹
acceptor (3) Na
⫹
acceptor
(2) H
⫹
donor (4) Na
⫹
donor
28 Which compound is an electrolyte?
(1) CCl
4
(3) C
6
H
12
O
6
(2) CH
3
OH (4) Ca(OH)
2
29 Which term identifies a type of nuclear reaction?
(1) fermentation (3) reduction
(2) deposition (4) fission
30 Which radioisotopes have the same decay mode
and have half-lives greater than 1 hour?
(1) Au-198 and N-16 (3) I-131 and P-32
(2) Ca-37 and Fe-53 (4) Tc-99 and U-233

P.S./Chem.–June ’13 [5] [OVER]
32 What is the overall charge of an ion that has
12 protons, 10 electrons, and 14 neutrons?
(1) 2⫺ (3) 4⫺
(2) 2⫹ (4) 4⫹
33 As the elements in Period 3 are considered in
order of increasing atomic number, there is a
general decrease in
(1) atomic mass
(2) atomic radius
(3) electronegativity
(4) first ionization energy
34 Which electron configuration represents the
electrons of a sulfur atom in an excited state?
(1) 2-6-6 (3) 2-8-4
(2) 2-7-7 (4) 2-8-6
35 Given the word equation:
sodium chlorate → sodium chloride ⫹ oxygen
Which type of chemical reaction is represented
by this equation?
(1) double replacement (3) decomposition
(2) single replacement (4) synthesis
Part B–1
Answer all questions in this part.
Directions (31–50): For each statement or question, record on your separate answer sheet the number of the
word or expression that, of those given, best completes the statement or answers the question. Some questions
may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.
31 The diagram below represents the bright-line spectra of four elements and a bright-line spectrum
produced by a mixture of three of these elements.
Which element is not present in the mixture?
(1) A (3) X
(2) D (4) Z
Bright-Line Spectra
Element A
Element D
Element X
Element Z
Mixture
Wavelength (nm)
400 500 600 700 750
400 500 600 700 750

P.S./Chem.–June ’13 [6]
36 Which compound has the highest percent com-
position by mass of strontium?
(1) SrCl
2
(3) SrO
(2) SrI
2
(4) SrS
37 Given the formula for hydrazine:
How many pairs of electrons are shared
between the two nitrogen atoms?
(1) 1 (3) 3
(2) 2 (4) 4
38 Which formulas represent one ionic compound
and one molecular compound?
(1) N
2
and SO
2
(3) BaCl
2
and N
2
O
4
(2) Cl
2
and H
2
S (4) NaOH and BaSO
4
39 Which Kelvin temperature is equal to 200.°C?
(1) ⫺73 K (3) 200. K
(2) 73 K (4) 473 K
40 A 10.0-gram sample of H
2
O(ℓ) at 23.0°C
absorbs 209 joules of heat. What is the final
temperature of the H
2
O(ℓ) sample?
(1) 5.0°C (3) 28.0°C
(2) 18.0°C (4) 50.0°C
41 Given the equation representing a system at
equilibrium:
AgCl(s) Ag
⫹
(aq) ⫹ Cl
⫺
(aq)
When the concentration of Cl
⫺
(aq) is increased,
the concentration of Ag
⫹
(aq)
(1) decreases, and the amount of AgCl(s)
increases
(2) decreases, and the amount of AgCl(s)
decreases
(3) increases, and the amount of AgCl(s)
increases
(4) increases, and the amount of AgCl(s)
decreases
42 Which particle diagram represents a sample of
matter that can not be broken down by chemical
means?
43 Which formula represents an unsaturated
hydrocarbon?
( 1 )
H
H
C
H
H
C
H
H
( 2 )
( 3 )
H
H
C
H
H
C
OH
H
H
H
C
C
H
O
H
( 4 )
H
C
C
H
HC
H
H
2
O
Key
= atom of one element
= atom of a different element
( 1 )
( 2 )
( 3 )
( 4 )
H
H
H
H
NN

P.S./Chem.–June ’13 [7] [OVER]
44 When the pH of a solution is changed from 4 to 3,
the hydronium ion concentration of the solution
(1) decreases by a factor of 10
(2) increases by a factor of 10
(3) decreases by a factor of 100
(4) increases by a factor of 100
45 Three samples of the same solution are tested,
each with a different indicator. All three indicators,
bromthymol blue, bromcresol green, and thymol
blue, appear blue if the pH of the solution is
(1) 4.7 (3) 7.8
(2) 6.0 (4) 9.9
46 A 10.0-milliliter sample of NaOH(aq) is neutral-
ized by 40.0 milliliters of 0.50 M HCl. What is
the molarity of the NaOH(aq)?
(1) 1.0 M (3) 0.25 M
(2) 2.0 M (4) 0.50 M
47 Radiation is spontaneously emitted from
hydrogen-3 nuclei, but radiation is not
spontaneously emitted from hydrogen-1 nuclei
or hydrogen-2 nuclei. Which hydrogen nuclei
are stable?
(1) nuclei of H-1 and H-2, only
(2) nuclei of H-1 and H-3, only
(3) nuclei of H-2 and H-3, only
(4) nuclei of H-1, H-2, and H-3
48 Given the equation representing a nuclear
reaction in which X represents a nuclide:
232
90
Th →
4
2
He ⫹ X
Which nuclide is represented by X?
(1)
236
92
Ra (3)
236
92
U
(2)
228
88
Ra (4)
228
88
U
49 After decaying for 48 hours,
1
__
16
of the original
mass of a radioisotope sample remains unchanged.
What is the half-life of this radioisotope?
(1) 3.0 h (3) 12 h
(2) 9.6 h (4) 24 h
50 Which balanced equation represents nuclear
fusion?
(1)
2
1
H ⫹
2
1
H →
4
2
He
(2) 2H
2
⫹ O
2
→ 2H
2
O
(3)
6
3
Li ⫹
1
0
n →
3
1
H ⫹
4
2
He
(4) CaO ⫹ CO
2
→ CaCO
3

Base your answers to questions 51 through 53 on the information below and on your knowledge of chemistry.
When magnesium is ignited in air, the magnesium reacts with oxygen and nitrogen.
The reaction between magnesium and nitrogen is represented by the unbalanced equation
below.
Mg(s) ⫹ N
2
(g) → Mg
3
N
2
(s)
51 Balance the equation in your answer booklet for the reaction between magnesium and
nitrogen, using the smallest whole-number coefficients. [
1]
52 In the ground state, which noble gas has atoms with the same electron configuration as
a magnesium ion? [
1]
53 Explain, in terms of electrons, why an atom of the metal in this reaction forms an ion
that has a smaller radius than its atom. [1]
Base your answers to questions 54 through 56 on the information below and on your knowledge of chemistry.
The balanced equation below represents a reaction.
O
2
(g) ⫹ energy → O(g) ⫹ O(g)
54 Identify the type of chemical bond in a molecule of the reactant. [
1]
55 In the space in your answer booklet, draw a Lewis electron-dot diagram of one oxygen
atom. [
1]
56 Explain, in terms of bonds, why energy is absorbed during this reaction. [1]
P.S./Chem.–June ’13 [8]
Part B–2
Answer all questions in this part.
Directions (51–65): Record your answers in the spaces provided in your answer booklet. Some questions
may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.

P.S./Chem.–June ’13 [9] [OVER]
Base your answers to questions 57 through 59 on the information below and on your knowledge of chemistry.
Starting as a solid at ⫺25°C, a sample of H
2
O is heated at a constant rate until the
sample is at 125°C. This heating occurs at standard pressure. The graph below represents
the relationship between temperature and heat added to the sample.
57 Describe what happens to both the potential energy and the average kinetic energy of
the molecules in the H
2
O sample during interval AB. [1]
58 Using the graph, determine the total amount of heat added to the sample during
interval CD. [
1]
59 Explain, in terms of heat of fusion and heat of vaporization, why the heat added during
interval DE is greater than the heat added during interval BC for this sample of water. [1]
50.
100.
Heat Added (kJ)
Heating Curve for H
2
O
4 12 20. 28 36 44 52 60.
125
75
25
0
–25
A
BC
D
E
F

Base your answers to questions 60 through 62 on the information below and on your knowledge of chemistry.
Cylinder A has a movable piston and contains hydrogen gas. An identical cylinder, B,
contains methane gas. The diagram below represents these cylinders and the conditions of
pressure, volume, and temperature of the gas in each cylinder.
60 Compare the total number of gas molecules in cylinder A to the total number of gas
molecules in cylinder B. [
1]
61 State a change in temperature and a change in pressure that will cause the gas in
cylinder A to behave more like an ideal gas. [
1]
62 In the space in your answer booklet, show a numerical setup for calculating the volume
of the gas in cylinder B at STP. [1]
Cylinder A
Hydrogen gas
P
= 1.2 atm
V
= 1.25 L
T
= 293 K
Cylinder B
Methane gas
P
= 1.2 atm
V
= 1.25 L
T
= 293 K
P.S./Chem.–June ’13 [10]

P.S./Chem.–June ’13 [11] [OVER]
Base your answers to questions 63 through 65 on the information below and on your knowledge of chemistry.
There are several isomers of C
6
H
14
. The formulas and boiling points for two of these
isomers are given in the table below.
63 Identify the homologous series to which these isomers belong. [
1]
64 Write the empirical formula for isomer 1. [
1]
65 Explain, in terms of intermolecular forces, why isomer 2 boils at a lower temperature
than isomer 1. [1]
H
H
C
H
CC
H
H
H
H
CC
H
H
C
H
H
H
H
H
Isomer
1
2
Formula Boiling Point at 1 atm (°C)
68.7
49.7
H
H
C
CC
H
C
H
H
C
H
H
H
H
H
H
CH
H
H
Base your answers to questions 66 through 69 on the information below and on your knowledge of chemistry.
Before atomic numbers were known, Mendeleev developed a classification system for
the 63 elements known in 1872, using oxide formulas and atomic masses. He used an R in
the oxide formulas to represent any element in each group. The atomic mass was listed in
parentheses after the symbol of each element. A modified version of Mendeleev’s classification
system is shown in the table below.
66 Identify one characteristic used by Mendeleev to develop his classification system of the
elements. [
1]
67 Based on Mendeleev’s oxide formula, what is the number of electrons lost by each atom
of the elements in Group III? [
1]
68 Based on Table J, identify the least active metal listed in Group I on Mendeleev’s table. [
1]
69 Explain, in terms of chemical reactivity, why the elements in Group 18 on the modern
Periodic Table were not identified by Mendeleev at that time. [1]
Oxide
formulas
H(1)
Li(7)
Na(23)
K(39)
Cu(63)
Rb(85)
Ag(108)
Cs(133)
I II III IV V VI VII
R
2
OROR
2
O
3
RO
2
R
2
O
5
RO
3
R
2
O
7
1
2
3
4
5
6
7
8
Group
Series
Be(9.4)
Mg(24)
Ca(40)
Zn(65)
Sr(87)
Cd(112)
Ba(137)
B(11)
Al(27.3)
Yt(88)
In(113)
Di(138)
C(12)
Si(28)
Ti(48)
Zr(90)
Sn(118)
Ce(140)
N(14)
P(31)
V(51)
As(75)
Nb(94)
Sb(122)
O(16)
S(32)
Cr(52)
Se(78)
Mo(96)
Te(125)
F(19)
Cl(35.5)
Mn(55)
Br(80)
I(127)
Modified Version of Mendeleev’s Table
P.S./Chem.–June ’13 [12]
Part C
Answer all questions in this part.
Directions (66–85): Record your answers in the spaces provided in your answer booklet. Some questions
may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry.
P.S./Chem.–June ’13 [13] [OVER]
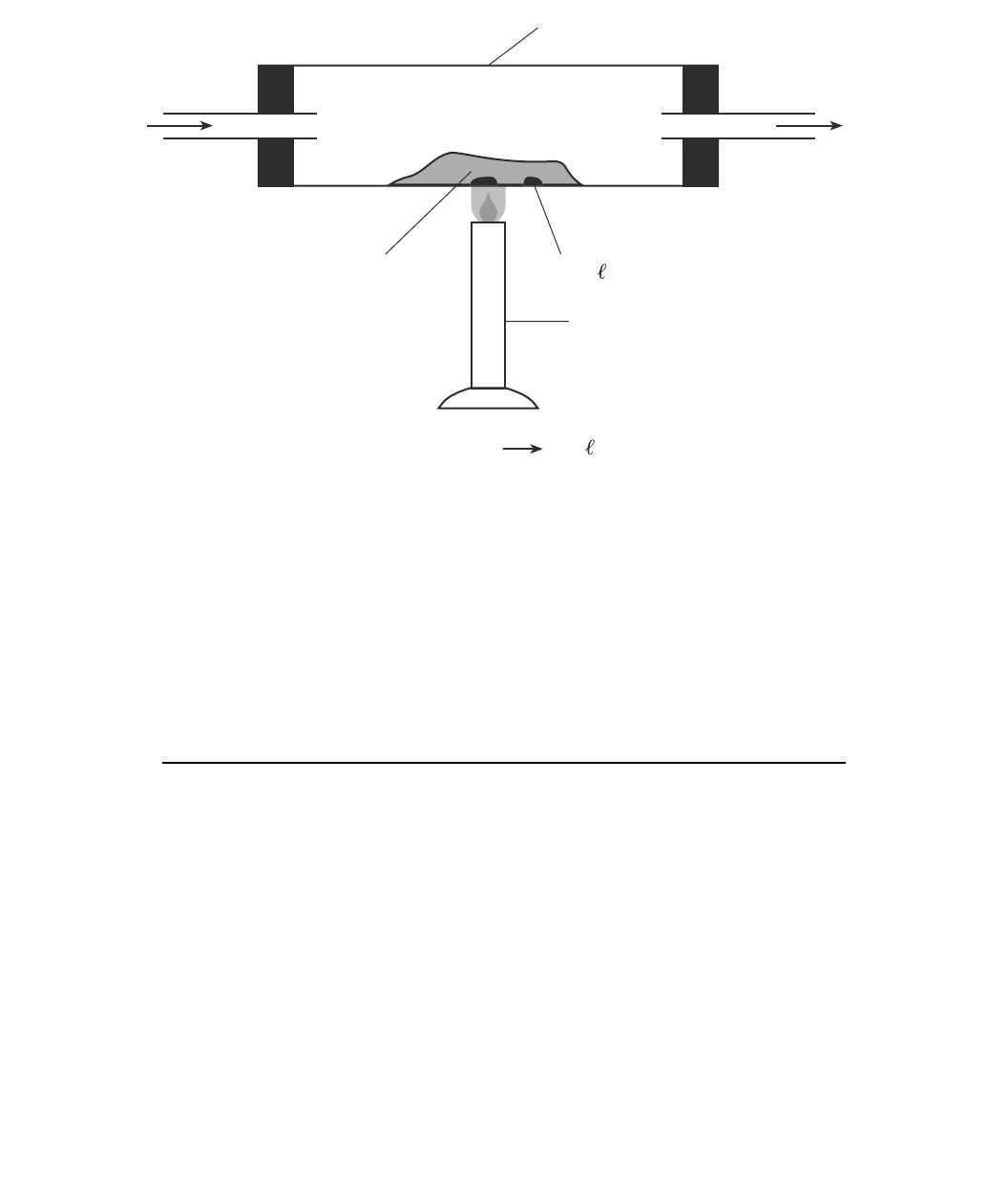
Base your answers to questions 70 through 73 on the information below and on your knowledge of chemistry.
In a laboratory apparatus, a sample of lead(II) oxide reacts with hydrogen gas at high
temperature. The products of this reaction are liquid lead and water vapor. As the reaction
proceeds, water vapor and excess hydrogen gas leave the glass tube. The diagram and
balanced equation below represent this reaction.
70 Determine the change in oxidation number for the hydrogen that reacts. [
1]
71 Write a balanced half-reaction equation for the reduction of the Pb
2⫹
ions in this
reaction. [
1]
72 Explain why the reaction that occurs in this glass tube can not reach equilibrium. [
1]
73 State one change in reaction conditions, other than adding a catalyst, that would cause
the rate of this reaction to increase. [1]
Glass tube
H
2
(g)
H
2
O(g)
and
H
2
(g)
PbO(s)
Laboratory burner
PbO(s) + H
2
(g) + heat Pb( ) + H
2
O(g)
Pb( )

Base your answers to questions 74 through 77 on the information below and on your knowledge of chemistry.
In the late 19th century, the Hall-Herroult process was invented as an inexpensive way
to produce aluminum. In this process, Al
2
O
3
(ℓ) extracted from bauxite is dissolved in
Na
3
AlF
6
(ℓ) in a graphite-lined tank, as shown in the diagram below. The products are
carbon dioxide and molten aluminum metal.
74 Compare the chemical properties of a 300.-kilogram sample of Al
2
O
3
(ℓ) with the
chemical properties of a 600.-kilogram sample of Al
2
O
3
(ℓ). [1]
75 Write the chemical name for the liquid compound dissolved in the Na
3
AlF
6
(ℓ). [1]
76 What is the melting point of the substance that collects at the bottom of the tank? [
1]
77 Compare the density of the Al(ℓ) with the density of the mixture of Al
2
O
3
(ℓ) and
Na
3
AlF
6
(ℓ). [1]
Base your answers to questions 78 through 80 on the information below and on your knowledge of chemistry.
One process used to manufacture sulfuric acid is called the contact process. One step
in this process, the reaction between sulfur dioxide and oxygen, is represented by the
forward reaction in the system at equilibrium shown below.
2SO
2
(g) ⫹ O
2
(g) ⇌ 2SO
3
(g) ⫹ 394 kJ
A mixture of platinum and vanadium(V) oxide may be used as a catalyst for this
reaction. The sulfur trioxide produced is then used to make sulfuric acid.
78 Determine the amount of energy released when 1.00 mole of sulfur trioxide is
produced. [
1]
79 Write the chemical formula for vanadium(V) oxide. [
1]
80 On the labeled axes in your answer booklet, complete the potential energy diagram for
the forward reaction represented by this equation. [1]
Hall-Heroult Process
Graphite
rod
Graphite-lined tank
Power
source
P.S./Chem.–June ’13 [14]

P.S./Chem.–June ’13 [15]
Base your answers to questions 81 and 82 on the information below and on your knowledge of chemistry.
Two very stable compounds, Freon-12 and Freon-14, are used as liquid refrigerants. A
Freon-12 molecule consists of one carbon atom, two chlorine atoms, and two fluorine
atoms. A Freon-14 molecule consists of one carbon atom and four fluorine atoms.
81 In the space in your answer booklet, draw a structural formula for Freon-12. [
1]
82 To which class of organic compounds do Freon-12 and Freon-14 belong? [1]
Base your answers to questions 83 through 85 on the information below and on your knowledge of chemistry.
Chemical concepts are applied in candy making. A recipe for making lollipops is shown
below.
Hard-Candy Lollipops Recipe
Ingredients:
414 grams of sugar
177 grams of water
158 milliliters of light corn syrup
Step 1: In a saucepan, mix the sugar and water. Heat this mixture, while stirring, until
all of the sugar dissolves.
Step 2: Add the corn syrup and heat the mixture until it boils.
Step 3: Continue boiling the mixture until the temperature reaches 143°C at standard
pressure.
Step 4: Remove the pan from the heat and allow it to stand until the bubbling stops.
Pour the mixture into lollipop molds that have been coated with cooking oil
spray.
83 Explain, in terms of the polarity of sugar molecules, why the sugar dissolves in water. [
1]
84 Determine the concentration, expressed as percent by mass, of the sugar dissolved in
the mixture produced in step 1. [
1]
85 Explain, in terms of the concentration of sugar molecules, why the boiling point of the
mixture in step 3 increases as water evaporates from the mixture. [1]

P.S./CHEMISTRY
P.S./CHEMISTRY
Printed on Recycled Paper

The University of the State of New York
REGENTS HIGH SCHOOL EXAMINATION
PHYSICAL SETTING
CHEMISTRY
Tuesday, June 18, 2013 — 9:15 a.m. to 12:15 p.m., only
ANSWER BOOKLET
Student . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Sex: I Female
Teacher . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
School . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Grade . . . . . . . . .
Record your answers for Part B–2 and Part C in this booklet.
I Male
51 _________ Mg(s) ⫹ _________ N
2
(g) → _________ Mg
3
N
2
(s)
52
53
Part B–2

54
55
56
57
58 kJ
59
P.S./Chem. Answer Booklet–June ’13 [2]

60
61 Temperature: ________________________________________________________________________
Pressure: _____________________________________________________________________________
62
63
64
65
P.S./Chem. Answer Booklet–June ’13 [3] [OVER]

66
67
68
69
70 From __________________ to __________________
71
72
73
P.S./Chem. Answer Booklet–June ’13 [4]
Part C

74
75
76 K
77
78 kJ
79
80
Reaction Coordinate
Potential Energy
P.S./Chem. Answer Booklet–June ’13 [5] [OVER]

81
82
83
84 %
85
P.S./Chem. Answer Booklet–June ’13 [6]


P.S./CHEMISTRY
P.S./CHEMISTRY
Printed on Recycled Paper

FOR TEACHERS ONLY
The University of the State of New York
REGENTS HIGH SCHOOL EXAMINATION
PHYSICAL SETTING/CHEMISTRY
Tuesday, June 18, 2013 — 9:15 a.m. to 12:15 p.m., only
SCORING KEY AND RATING GUIDE
P.S.–CH
Directions to the Teacher:
Refer to the directions on page 2 before rating student papers.
Updated information regarding the rating of this examination may be posted on the New York
State Education Department’s web site during the rating period. Check this web site at:
http://www.p12.nysed.gov/assessment/
and select the link “Scoring Information” for any recently
posted information regarding this examination. This site should be checked before the rating process
for this examination begins and several times throughout the Regents Examination period.
Part A and Part B–1
Allow 1 credit for each correct response.
1 . . . . . 3 . . . . .
2 . . . . . 4 . . . . .
3 . . . . . 3 . . . . .
4 . . . . . 3 . . . . .
5 . . . . . 1 . . . . .
6 . . . . . 2 . . . . .
7 . . . . . 3 . . . . .
8 . . . . . 3 . . . . .
9 . . . . . 4 . . . . .
10 . . . . . 2 . . . . .
11 . . . . . 4 . . . . .
12 . . . . . 4 . . . . .
13 . . . . . 2 . . . . .
14 . . . . . 2 . . . . .
15 . . . . . 2 . . . . .
16 . . . . . 3 . . . . ..
17 . . . . . 3 . . . . .
18 . . . . . 4 . . . . .
19 . . . . . 4 . . . . .
20 . . . . . 2 . . . . .
21 . . . . . 4 . . . . .
22 . . . . . 1 . . . . .
23 . . . . . 1 . . . . .
24 . . . . . 1 . . . . .
25 . . . . . 2 . . . . .
26 . . . . . 2 . . . . .
27 . . . . . 1 . . . . .
28 . . . . . 4 . . . . .
29 . . . . . 4 . . . . .
30 . . . . . 3 . . . . .
31 . . . . . 3 . . . . .
32 . . . . . 2 . . . . .
33 . . . . . 2 . . . . .
34 . . . . . 2 . . . . .
35
. . . . .
3 . . . . .
36 . . . . . 3 . . . . .
37 . . . . . 1 . . . . .
38 . . . . . 3 . . . . .
39 . . . . . 4 . . . . .
40 . . . . . 3 . . . . .
41 . . . . . 1 . . . . .
42 . . . . . 4 . . . . .
43 . . . . . 2 . . . . .
44 . . . . . 2 . . . . .
45 . . . . . 4 . . . . .
46 . . . . . 2 . . . . .
47 . . . . . 1 . . . . .
48 . . . . . 2 . . . . .
49 . . . . . 3 . . . . .
50 . . . . . 1 . . . . .
Part A
Part B–1

P.S./Chem. Rating Guide–June ’13 [2]
Directions to the Teacher
Follow the procedures below for scoring student answer papers for the Regents Examination in
Physical Setting/Chemistry. Additional information about scoring is provided in the publication Information
Booklet for Scoring Regents Examinations in the Sciences.
Do not attempt to correct the student’s work by making insertions or changes of any kind.
If the student’s responses for the multiple-choice questions are being hand scored prior to being
scanned, the scorer must be careful not to make any marks on the answer sheet except to record
the scores in the designated score boxes. Marks elsewhere on the answer sheet will interfere
with the accuracy of the scanning.
Allow 1 credit for each correct response.
At least two science teachers must participate in the scoring of the Part B–2 and Part C open-ended
questions on a student’s paper. Each of these teachers should be responsible for scoring a selected number
of the open-ended questions on each answer paper. No one teacher is to score more than approximately
one-half of the open-ended questions on a student’s answer paper. Teachers may not score their own
students’ answer papers.
Students’ responses must be scored strictly according to the Scoring Key and Rating Guide. For open-
ended questions, credit may be allowed for responses other than those given in the rating guide if the
response is a scientifically accurate answer to the question and demonstrates adequate knowledge, as
indicated by the examples in the rating guide. On the student’s separate answer sheet, for each question,
record the number of credits earned and the teacher’s assigned rater/scorer letter.
Fractional credit is not allowed. Only whole-number credit may be given for a response. If the student
gives more than one answer to a question, only the first answer should be rated. Units need not be given
when the wording of the questions allows such omissions.
For hand scoring, raters should enter the scores earned in the appropriate boxes printed on the
separate answer sheet. Next, the rater should add these scores and enter the total in the box labeled
“Total Raw Score.” Then the student’s raw score should be converted to a scale score by using the
conversion chart that will be posted on the Department’s web site at: http://www.p12.nysed.gov/assessment/
on Tuesday, June 18, 2013. The student’s scale score should be entered in the box labeled “Scale Score” on
the student’s answer sheet. The scale score is the student’s final examination score.
Schools are not permitted to rescore any of the open-ended questions on this exam after each
question has been rated once, regardless of the final exam score. Schools are required to ensure
that the raw scores have been added correctly and that the resulting scale score has been
determined accurately.
Because scale scores corresponding to raw scores in the conversion chart may change from one
administration to another, it is crucial that, for each administration, the conversion chart provided for that
administration be used to determine the student’s final score.

P.S./Chem. Rating Guide–June ’13 [3]
Part B–2
Allow a total of 15 credits for this part. The student must answer all questions in this part.
51 [1] Allow 1 credit for 3___ Mg(s) ⫹ ___ N
2
(g) → ___ Mg
3
N
2
(s).
Allow credit even if the coefficient “1” is written in front of N
2
(g) and/or Mg
3
N
2
(s).
52 [1] Allow 1 credit for Ne or neon.
53 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
An atom of magnesium loses its outer shell electrons to form the Mg
2+
ion.
The electron configuration of a magnesium atom is 2-8-2, and the electron configuration of the
magnesium ion is 2-8.
An atom of the metal loses electrons to form the ion.
54 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
covalent
double covalent
nonpolar
double
55 [1] Allow 1 credit.
Examples of 1-credit responses:
O
XX
X
X
X
X
O
O

P.S./Chem. Rating Guide–June ’13 [4]
56 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Energy is needed to break the bonds in O
2
.
57 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The potential energy remains the same, but the average kinetic energy of the H
2
O molecules
increases.
There is no change in potential energy. There is an increase in the average kinetic energy.
58 [1] Allow 1 credit for 8 kJ ⫾ 1 kJ.
59 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The heat of vaporization of water is 2260 J/g and the heat of fusion for water is only
334 J/g.
The heat of fusion of water is much less than its heat of vaporization.
60 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The number of gas molecules in cylinder A is the same as the number of gas molecules
in cylinder B.
61 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Temperature: above 293 K
Pressure: below 1.2 atm
Temperature: higher
Pressure: lower
62 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
(2773
293
)(1.2)(1.25)
(.
(.
12
293
10
273
atm)(1.25 L)
K
atm)( )
K
⫽
V
2

63 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
alkanes
C
n
H
2n⫹2
64 [1] Allow 1 credit for C
3
H
7
. The order of the elements can vary.
65 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Isomer 2 boils at a lower temperature because it has weaker intermolecular forces than isomer 1.
The intermolecular forces in isomer 1 are stronger.
P.S./Chem. Rating Guide–June ’13 [5]

Part C
Allow a total of 20 credits for this part. The student must answer all questions in this part.
66 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
increasing atomic mass
atomic mass
oxide formulas
67 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
three electrons
three
3
68 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Ag
silver
69 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Since the Group 18 elements tend not to react with other elements, there were no
oxide compounds for Mendeleev to study.
Group 18 elements are generally unreactive.
70 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
From 0 to ⫹1
From zero to one
71 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Pb
2⫹
⫹ 2e
⫺
→ Pb
72 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The glass tube is not a closed system.
Gases are entering and leaving the system.
The reaction is not reversible under these conditions.
P.S./Chem. Rating Guide–June ’13 [6]

73 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Increase the temperature.
Increase the concentration of the hydrogen gas in the tube.
Grind the metal oxide to increase its surface area.
74 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
Both samples have the same chemical properties.
75 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
aluminum oxide
76 [1] Allow 1 credit for 933 K.
77 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The density of the aluminum is greater than the density of the Al
2
O
3
and Na
3
AlF
6
mixture.
The density of Al(ℓ) is greater.
78 [1] Allow 1 credit for 197 kJ.
79 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
V
2
O
5
O
5
V
2
80 [1] Allow 1 credit.
Example of a 1-credit response:
Reaction Coordinate
Potential Energy
P.S./Chem. Rating Guide–June ’13 [7]

81 [1] Allow 1 credit.
Examples of 1-credit responses:
82 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
halide
halocarbon
83 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The polarity of sugar molecules is similar to the polarity of water molecules.
Both substances consist of polar molecules.
84 [1] Allow 1 credit for 70.1%. Significant figures do not need to be shown.
85 [1] Allow 1 credit. Acceptable responses include, but are not limited to:
The boiling point of the mixture increases as water evaporates because the concentration of
dissolved molecules increases.
An increase in the concentration of sugar particles increases the boiling point.
F
Cl
C
CI
F
F
Cl
C
F
Cl
P.S./Chem. Rating Guide–June ’13 [8]

P.S./Chem. Rating Guide–June ’13 [9]
The Chart for Determining the Final Examination Score for the June 2013
Regents Examination in Physical Setting/Chemistry will be posted on the
Department’s web site at: http://www.p12.nysed.gov/assessment/
on Tuesday,
June 18, 2013. Conversion charts provided for previous administrations of the
Regents Examination in Physical Setting/Chemistry must NOT be used to
determine students’ final scores for this administration.
Regents Examination in Physical Setting/Chemistry
June 2013
Chart for Converting Total Test Raw Scores to
Final Examination Scores (Scale Scores)
Online Submission of Teacher Evaluations of the Test to the Department
Suggestions and feedback from teachers provide an important contribution to the test
development process. The Department provides an online evaluation form for State
assessments. It contains spaces for teachers to respond to several specific questions and to
make suggestions. Instructions for completing the evaluation form are as follows:
1. Go to http://www.forms2.nysed.gov/emsc/osa/exameval/reexameval.cfm
.
2. Select the test title.
3. Complete the required demographic fields.
4. Complete each evaluation question and provide comments in the space provided.
5. Click the SUBMIT button at the bottom of the page to submit the completed form.

P.S./Chem. Rating Guide–June ’13 [10]
Map to Core Curriculum
June 2013 Physical Setting/Chemistry
Question Numbers
Key Ideas/Performance Indicators Part A Part B Part C
Standard 1
Math Key Idea 1 39, 62
Math Key Idea 2 51, 58, 60
Math Key Idea 3 32, 36, 40, 46, 49,
53, 62, 64
67, 70, 78, 79, 84
Science Inquiry Key Idea 1 33, 53, 54, 56, 59,
61, 63, 65
66, 68, 69, 72, 73,
74, 77, 83, 85
Science Inquiry Key Idea 2
Science Inquiry Key Idea 3 37, 38, 47, 50, 51,
53, 56, 60, 65
67, 69, 70, 75, 79,
82, 85
Engineering Design Key Idea 1
Standard 2
Key Idea 1 47 76
Key Idea 2 81
Standard 6
Key Idea 1
Key Idea 2 34
Key Idea 3 44
Key Idea 4
Key Idea 5
Standard 7
Key Idea 1
Key Idea 2
Standard 4 Process Skills
Key Idea 3 31, 33, 34, 35, 39,
41, 42, 43, 45, 51,
55, 62, 63, 64
66, 67, 68, 71, 81,
83, 84
Key Idea 4 40, 48, 49, 57, 58,
59
78, 80, 82
Key Idea 5 52, 54, 65
Standard 4
Key Idea 3 1, 2, 3, 4, 5, 7, 8,
9, 11, 15, 16, 18,
19, 20, 21, 22, 23,
24, 25, 26, 27, 28
31, 32, 33, 34, 35,
36, 41, 42, 43, 44,
45, 46, 47, 51, 60,
61, 62, 63, 64
66, 67, 68, 70, 71,
72, 73, 74, 75, 76,
77, 79, 81, 82, 83,
84, 85
Key Idea 4 12, 13, 14, 29, 30 39, 40, 48, 49, 50,
57, 58, 59
78, 80
Key Idea 5 6, 10, 17 37, 38, 52, 53, 54,
55, 56, 65
69
Reference Tables
2011 Edition 2, 7, 8, 10, 17, 22,
28, 30
33, 34, 36, 38, 39,
40, 45, 46, 47, 48,
52, 53, 55, 59, 62,
63
68, 69, 70, 71, 75,
76, 79, 81, 82, 84

Raw Scale Raw Scale Raw Scale Raw Scale
Score Score Score Score Score Score Score Score
85
100
62
73
39
57
16
34
84
98
61
72
38
56
15
32
83
96
60
72
37
56
14
31
82
95
59
71
36
55
13
29
81
93
58
70
35
54
12
28
80
92
57
69
34
53
11
26
79
90
56
69
33
53
10
24
78
89
55
68
32
52
9
22
77
87
54
67
31
51
8
20
76
86
53
67
30
50
7
18
75
85
52
66
29
49
6
16
74
84
51
66
28
48
5
14
73
83
50
65
27
47
4
11
72
82
49
64
26
46
3
9
71
81
48
63
25
45
2
6
70
80
47
63
24
44
1
3
69
79
46
62
23
43
0
0
68
78
45
61
22
42
67
77
44
61
21
41
66
76
43
60
20
39
65
75
42
59
19
38
64
75
41
59
18
37
63
74
40
58
17
35
Schools
are
not permitted to rescore any of the open-ended questions on this exam after each question has
been rated once, regardless of the final exam score. Schools are required to ensure that the raw scores
have been added correctly and that the resulting scale score has been determined accurately.
Because scale scores corresponding to raw scores in the conversion chart change from one administration to
another, it is crucial that for each administration the conversion chart provided for that administration be used to
determine the student’s final score. The chart above is usable only for this administration of the Regents
Examination in Physical Setting/Chemistry.
The State Education Department / The University of the State of New York
Regents Examination in Physical Setting/Chemistry – June 2013
Chart for Converting Total Test Raw Scores to Final Examination Scores (Scale Scores)
To determine the student’s final examination score, find the student’s total test raw score in the column labeled “Raw
Score” and then locate the scale score that corresponds to that raw score. The scale score is the student’s final
examination score. Enter this score in the space labeled “Scale Score” on the student’s answer sheet.
P.S./Chemistry Conversion Chart - June '13 1 of 1
