
Distance sampling with camera traps
Eric J. Howe*
,1
, Stephen T. Buckland
1
, Marie-Lyne Despr
es-Einspenner
2
and
Hjalmar S. K
€
uhl
2,3
1
Centre for Research into Ecological and Environmental Modelling, University of St Andrews, The Observatory, Buchanan
Gardens, St An drews, Fif e KY1 6 9LZ, UK;
2
Max Planck Institute for Evolutionary Anthropology, Deutscher Platz 6, 04103
Leipzig, Germany; and
3
German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Deutscher Platz 5e,
04103 Leipzig, Germany
Summary
1. Reliable estimates of animal density and abundance are essential for effective wildlife c onservation and
management. Camera trapping has proven efficient for sampling multiple species, but statistical estimators
of density from camera trapping data for species that cannot be individually identified are still in develop-
ment.
2. We extend point-transect methods for estimating animal density to accommodate data from camera traps,
allowing researchers to exploit existing distance sampling theory and software for designing studies and ana-
lysing data. We tested it by simulation, and used it to estimate densities of Maxwell’s duikers (Philantomba
maxwellii)inTa
€
ı National Park, C
^
ote d’Ivoire.
3. Densities estimated from simulated data were unbiased when we assumed animals were not available for
detection during long periods of rest. Estimated duiker densities were higher than recent estimates from line tran-
sect surveys, which are believed to underestimate densities of forest ungulates.
4. We expect these methods to provide an effective means to estimate animal density from camera trapping data
and to be applicable in a variety of settings.
Key-words: animal abundance, camera trapping, density, distance sampling, Maxwell’s duiker
Introduction
Remote motion-sensitive photography, or camera trapping,
is increasingly used in wildlife research, and allows multiple
research objectives to be addressed (Sollmann, Mohamed &
Kelly 2013a; Burton et al. 2015; Rovero & Zimmermann
2016). Estimation of population density (D) is a key objec-
tive of many ecological studies and assessments of conser-
vation status employing camera traps (Burton et al. 2015;
Rovero & Zimmermann 2016). If individuals are recognis-
able, density can be estimated using spatially explicit cap-
ture–recaptur e (SECR) models (Efford, Borchers & Byrom
2009), but methods for estimating D from camera trapping
data in the absence of individual identification are still in
development (Sollmann, Mohamed & Kelly 2013a; Burton
et al. 2015; D
enes, Silveira & Beissinger 2015; Rovero &
Zimmermann 2016). Detection rates at camera traps have
been used to index abundance, however, due to spatiotem-
poral variation in detection rates indices can rarely be con-
verted to estimates of absolute density, nor do they provide
reliable evidence of differences or trends in abundance
(Sollmann et al. 2013b; Burton et al. 2015). The random
encounter model (REM) estimates absolute density as a
function of the de tection rate, the dimensions of a sector
within which detection is certain, and the speed of animal
movement; methods for quantifying the latter two parame-
ters from camera trapping data have been described (Row-
cliffe et al. 2008, 2011, 2016). The REM has been
recognised as a potentially us eful model, but its accuracy
and reliability remains to be demonstrated (Rovero & Mar-
shall 2009; Sollmann, Mohamed & Kelly 2013a; Zero et al.
2013; Cusack et al. 2015a; Balestrieri et al. 2016; Caravaggi
et al. 2016). SECR estimators for unmarked populations
estimate the number and location of animals’ activity cen-
tres from the spatial correlation of counts at different sam-
pling locations; sampling must be sufficiently intensive to
detect the same animals at multiple locations, and estimates
lack precision (Chandler & Royle 2013).
Here, we describe how densities of unmarked animal popu-
lations can be estimated by distance sampling (DS) with cam-
era traps, allowing researchers to take advantage of a well-
described theoretical framework complete with software and
advice for designing studies and analysing data (Buckland
et al. 2001, 2004, 2015; Thomas et al. 2010; Miller 2015; dis-
tancesampling.org). Below, we formulate a point transect dis-
tance sampling model specific to camera traps and describe its
assumptions and the estimation of variances. We test for bias
in estimated density (
b
D) and its variance by simulation, and
apply the method to estimate the density of Maxwell’s duikers
(Philantomba maxwellii)inTa
€
ı National Park, C
^
ote d’Ivoire.
*Correspondence author. E-mail: ejh20@st-andrews.ac.uk
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society
Methods in Ecology and Evolution 2017 doi: 10.1111/2041-210X.12790

Materials and methods
FORMULATION OF THE MODEL
Acameratrap(CT)isdeployedatapointk that is independent of ani-
mal density for a period of time T
k
and set to record images for as long
as an animal is present to trigger it. We predetermine a finite set of
snapshot moments within T
k
, t units of time apart, at which an image
of an animal could be obtained. Temporal effort at the point is then
T
k
/t. When images of animals are obtained, we estimate the horizontal
radial distance r
i
between the midpoint of each animal and the camera,
at each snapshot moment, for as long as it remains in view. If the cam-
era covers an angle h radians, then
h
2p
describes the fraction of a circle
covered by the camera, so we define overall sampling effort at point k
as
hT
k
2pt
. We regard the data as a series of snapshots, and density estima-
tion follows by standard point transect methods (Buckland et al.
2001). We estimate D as
b
D ¼
P
K
k¼1
n
k
pw
2
P
K
k¼1
e
k
b
P
k
; eqn 1
where e
k
¼
hT
k
2pt
is the effort expended at point k, K is the set of points, h
is the horizontal angle of view (AOV) of the camera, w is the truncation
distance beyond which any recorded distances are discarded, n
k
is the
number of observations of animals in the population of interest at point
k,and
b
P
k
is the estimated probability of obtaining an image of an ani-
mal that is within h and w in front of the camera at a snapshot moment.
Substituting e
k
in eqn 1, we have
b
D ¼
2t
P
K
k¼1
n
k
hw
2
P
K
k¼1
T
k
b
P
k
: eqn 2
We use the distances r
i
to model the detection function and hence to
estimate P
k
.
ASSUMPTIONS AND PRACTICAL CONSIDERATIONS
The usual DS assumptions apply (see chapter 2 of Buckland et al.
2001). We record distances at instantaneous snapshot moments to
ensure that animal movement does not bias the distribution of detec-
tion distances. Below, we describe an approach for accurately assigning
animals to distance intervals; Rowcliffe et al. (2011) and Caravaggi
et al. (2016) describe methods for measuring continuous distances
betweenCTsanddetectedanimals.
Random designs or systematic designs with random origin are con-
sistent with the assumption that points are placed independently of ani-
mal locations. Selecting camera orientations as part of the design is also
advisable. Orientations could be selected randomly, or the same orien-
tation could be used for all cameras. Deviating slightly from the loca-
tion and orientation selected by design (e.g. to attach the camera to a
nearby tree or to avoid an obscured field of view) would not bias esti-
mates provided field staff do not intentionally target habitat features
known to be either preferred or avoided by the animals.
Empirical, design-based estimators of the encounter rate variance
are robust to violation of the assumption that detections are indepen-
dent events (Fewster et al. 2009; Buckland et al. 2015). However, in
CT surveys we expect violations to be severe because we include multi-
ple detections of the same animal during a single pass through the
detection zone. We can avoid this assumption by estimating variances
using a nonparametric bootstrap, resampling points with replacement
(Buckland 1984; Buckland et al. 2001). Another consequence of lack
of independence is that the usual goodness-of-fit tests and model
selection criteria are invalid (Buckland et al. 2001). Methods for select-
ing among DS models when observations are not independent are in
development.
The assumption that detection is certain at zero distance could be
violated by (i) animals passing beneath the field of view (FOV) of the
camera, (ii) failure to identify the species because only part of the ani-
mal is visible, and possibly (iii) the delay between the time the sensor is
activated and the time the first image is recorded (the ‘trigger speed’), if
animals directly in front of the camera at a snapshot moment do not
yield images. Such violations may be detectable during exploratory
analysis in the form of fewer than expected detections near the point,
and bias can be avoided via left-truncation (Buckland et al. 2001; Mar-
ques et al. 2007). To minimise violations and ensure that detection
probability is certain or high at some distance near the point, cameras
should be set at a height appropriate to the species of main interest
(Rovero & Zimmermann 2016). Lower heights would reduce the
chance of small animals passing beneath the camera at short distances,
but would also reduce the range of distances over which animals could
be detected and therefore sample size and flexibility when modelling
the detection function. Pairs of cameras mounted facing each other
could reveal violations caused by any of the three sources mentioned
above. Paired cameras mounted some distance apart targeting the same
location (but not necessarily facing each other) would also provide the
data needed to apply mark–recapture distance sampling methods,
which avoid this assumption (Buckland et al. 2004; Laake et al. 2011).
In traditional point transect surveys, human observers measure dis-
tances to each detected animal only once during each visit to a point,
and effort at each point is the number of times it was visited. CTs
remain at the point, but the snapshot approach discretises the number
of times we could potentially detect each animal (as T
k
/t as described
above). However, CTs detect only moving animals within the range of
the sensor and the FOV of the camera, and can be programmed to
record multiple still images, or video footage, each time the sensor is
triggered (Rovero & Zimmermann 2016). These characteristics of CTs
as observers must be taken into consideration. Observed distances
upon first detection are expected to be positively biased because ani-
mals entering the detection zone through the arc of the sector would
contribute a disproportionate number of observations at far distances.
Bias would be slight if the time between snapshot moments (t)was
small enough to ensure that the animals did not move far relative to the
range of the sensor between snapshots, as then the observations would
be representative of animals’ continuous paths past the CTs. However,
we prefer to avoid the potential for bias by assuming that the snapshot
moments are selected independently of animal locations, and predeter-
mining them as specific times of day to ensure that the assumption is
met. Practical considerations constrain t.Ift is large, animals that trig-
ger the sensor might leave the detection zone before a snapshot moment
arises, which would not cause bias but wastes data. As t is reduced,
there would be fewer missed detections and larger samples as we record
distance to each animal multiple times during a single pass in front of
the CT. Eventually, improvements in the precision of
b
D with larger
samples would become negligible because variation in the encounter
rate among points would contribute most of the variation in estimated
density. Reducing t further would then needlessly increase the time
required to process and analyse the data. We suggest that values from
025 to 3 s are likely to be useful, with values at the lower end of the
range being more appropriate for faster-moving or rarer animals, and
CTs with faster trigger speeds.
Programming cameras to record time-stamped video would make it
straightforward to record distances at the predetermined snapshot
moments. If still images are preferred, cameras should be programmed
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society, Methods in Ecology and Evolution
2 E. J. Howe et al.
to record an image at the next several snapshot moments when trig-
gered, or, if this is not feasible, to record a rapid series or ‘burst’ of still
images to ensure that images are recorded at times that align with snap-
shot moments. There should always be the potential for the camera to
be triggered again immediately or after a minimal delay. Note that
depending how cameras are programmed, the sample of distances
observed in CT data may or may not comprise a realisation from the
detection function described by the probability that an animal at dis-
tance r triggers the sensor. If cameras record a single image at the subse-
quent snapshot moment, or a rapid series of images for <t second s,
when the sensor is triggered, then each detection of an animal that trig-
gers the sensor several times during a pass in front of a CT is a function
of the sensitivity of the sensor. If cameras are set to record video, or a
series of still images for >2t seconds, then all but the first detection is
certain for as long as the animal remains in the FOV and the camera
continues to record images. Furthermore, regardless of how the camera
is programmed, any other animals in the FOV while the camera is
recording images would contribute observations that do not depend on
the sensitivity of the sensor. These differences do not invalidate the
method provided we define the detection function as representing the
proportion of locations at different distances which are recorded,
regardless of whether an animal triggered the sensor at that distance.
Obviously, we can only estimate the density of populations that are
available for detection by CTs. Similarly, because the sampling dura-
tion at each location (T
k
) is part of the model definition, we expect den-
sities of animals that spend part of their time outside the vertical range
of camera traps to be underestimated, and for the bias to be propor-
tional to time animals are not available for detection. For example,
with T
k
set to the study duration, we expect
b
D of a species that spends
all its time in the canopy to be zero, and of a species that spends half its
time underground and the rest at ground level to be half of the true den-
sity. Negative bias would also result if animals went undetected only
because movement was insufficient to trigger the sensor. To avoid this
bias, either T
k
should be defined as the amount of time that the entire
population was available for detection while cameras were operating,
or, equivalently, the proportion of time when animals were available
for detection should be included as a parameter in the model. Animals
are unavailable for detection when outside the vertical range of CTs,
and may not be available when within this range depending on their
level of activity. We explore this issue further in subsequent sections.
SIMULATIONS
We tested the method using simulations employing simple and complex
models of animal movement and different sampling scenarios (see
Appendix S1, Supporting Information). With the simple model, ani-
mals moved continuously at a constant speed and tended to maintain
their heading. The complex model included variable speeds and tortu-
osities, and all animals rested for the same 12 h of each day. We
recorded the distance between cameras and animals within detection
zones every 2-s, 24 h per day. Where the complex model was used, we
also collected data only when animals were moving, and reduced T
k
by
half accordingly when estimating density.
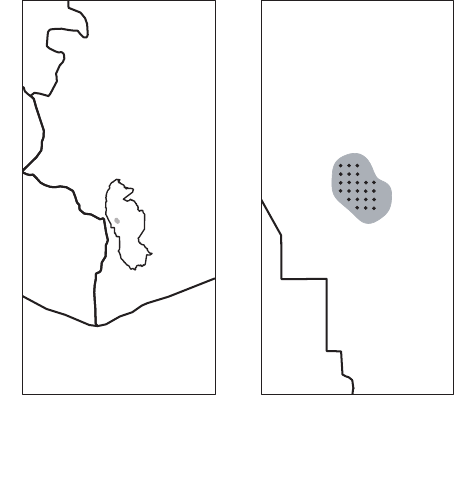
EXAMPLE: MAXWELL’S DUIKERS IN TA
€
INATIONAL PARK
We used point transect DS methods to estimate the density of
Maxwell’s duikers within the territory of the ‘east group’ habituated
chimpanzee community in Ta
€
ı National Park, C
^
ote d’Ivoire
(Despr
es-Einspenner et al. 2017; Fig. 1a). Maxwell’s duikers were
sampled from 28 June through 21 September 2014 at 23 camera traps
(Bushnell Trophy Cam
TM
; Bushnell, Overland Park, MO, USA,
Model 119576C) mounted at a height of 07–10 m and set to high
sensitivity. Cameras were deployed with a fixed orientation of 0˚ at
the intersections of a grid with 1 km spacing and a random origin
superimposed over the study area (Fig. 1b). Realised sampling loca-
tions and orientations deviated from the design by as much as 30 m,
and 40°, respectively, in order to mount cameras on trees and to
ensure there was some chance of detecting animals. During installa-
tion of each camera, we measured horizontal radial distances from
the camera, and recorded videos of researchers holding distance
markers, at 1 m intervals out to 15 m, in the centre and along both
sides of the FOV. We estimated distances to filmed duikers by com-
paring their locations to those of researchers in the reference videos.
We set t = 2 s, and recorded the distance interval within which the
midpoint of each animal fell at 0, 2, 4, ..., 58 s after the minute. Lar-
ger distances were more difficult to measure precisely, so we assigned
animals to 1-m intervals out to 8 m, but binned observations between
8 and 10 m, 10 and 12 m, 12 and 15 m, and beyond 15 m.
We excluded data from one camera because the FOV was lar-
gely obscured by vegetation, and another which was placed on a
slope and failed to detect any animals, but we included data from
a third camera that functioned normally but did not detect any
duikers. Maxwell’s duikers sleep or rest for most of each night and
for shorter periods during the day (Newing 1994, 2001). We
assumed they would not be available for detection overnight and
excluded the hours of darkness (19.00–6.00 h) from T
k
apriori.
We accounted for limited availability during the daytime three dif-
ferent ways. First we naively assumed that all duikers were active
by 6.30.00 h and remained so through 17.59.59 h, included dis-
tances observe d du ring this interval in a ‘daytime’ dataset, and
defined temporal effort at each locatio n (T
k
/t) as the number of 2-s
time steps during that time interval (20 699), multiplied by the num-
ber of sampling days. Second, we assumed that all animals were
available only during apparent times of peak activity (6.30.00–
8.59.59 h and 16.00.00–17.59.59 h) and recalculated temporal effort
and censored distance observations accordingly (T
k
/t per
day = 8098). Third, we defined T
k
and included observations as
8·5°W8°W 7·5°W
7°W
6·5°W6°W
4°N
5°N6°N
7
°N
8°N
Liberia
Cote d'Ivoire
^
TNP
(a)
7·35°W7·3°W7·25°W
7·2°W
5·7
°
N
5·8°
N
5·9°
N6
°
N
(b)
Fig. 1. Location of the study area (grey polygon) in Ta
€
ı National Park
(TNP), C
^
ote d’Ivoire, 2014 (a), and (b) locations of 23 camera traps
deployed in a grid with 1 km spacing within the study area.
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society, Methods in Ecology and Evolution
Distance sampling with camera traps 3
above for the daytime dataset, and included an independent esti-
mate of the proportion of time captive Maxwell’s duikers were
active during the same time interval (064; Newing 2001) in the
denominator of eqn 2. We included only data from complete days
when cameras were operating and not visited by researchers.
We fit point transect models in program Distance (version 7.0;
Thomas et al. 2010), defining survey effort at each location as
hT
k
2pt
.
ThecamerashadanAOVof42°, and a wider effective angle of
the sensor (Trailcampro.com 2015), so we set h = 42° or 0733
radians. We considered models of the detection function with the
half-normal key function with 0, 1 or 2 Hermite polynomial
adjustment terms, the hazard rate key function with 0, 1, or 2
cosine adjustments, and the uniform key function with 1 or 2
cosine adjustments. Adjustment terms were constrained, where nec-
essary, to ensure the detection function was monotonically decreas-
ing. We selected among candidate models of the detection function
by comparing AIC values, acknowledging the potential for overfit-
ting because many observations were not independent. We present
measures of uncertainty derived from design-based variances (‘P2’
of Fewster et al. 2009, Web Appendix B), and from 999 bootstrap
resamples, with replacement, across camera locations.
Results
SIMULATIONS
Where we used the simple model of animal movemen t, and
where we used the complex model of animal movement and
collected data only when animals were active,
b
D was unbi-
ased (Table S1). Results were biased and erratic when we
recorded distances to resting animals (see Appendix S1 for
details). Design-based variances were smaller than the sam-
pling variance of
b
D across iterations, and associated confi-
dence interval coverage was <90% (Table S1). Where we
estimated variance by bootstrapping, the coefficient of vari-
ation was 0119, similar to the sampling variance of
b
D,and
CI coverage was 936% across 1000 iterations. Doubling
spatial sampling effort improved precision, slightly more so
where we doubled the number of locations as opposed to h
(Table S1).
EXAMPLE: MAXWELL’S DUIKERS IN TA
€
INATIONAL PARK
We obtained 11 324 observations of the distance between
Maxwell’s duikers and cameras in 806 different videos. Duik-
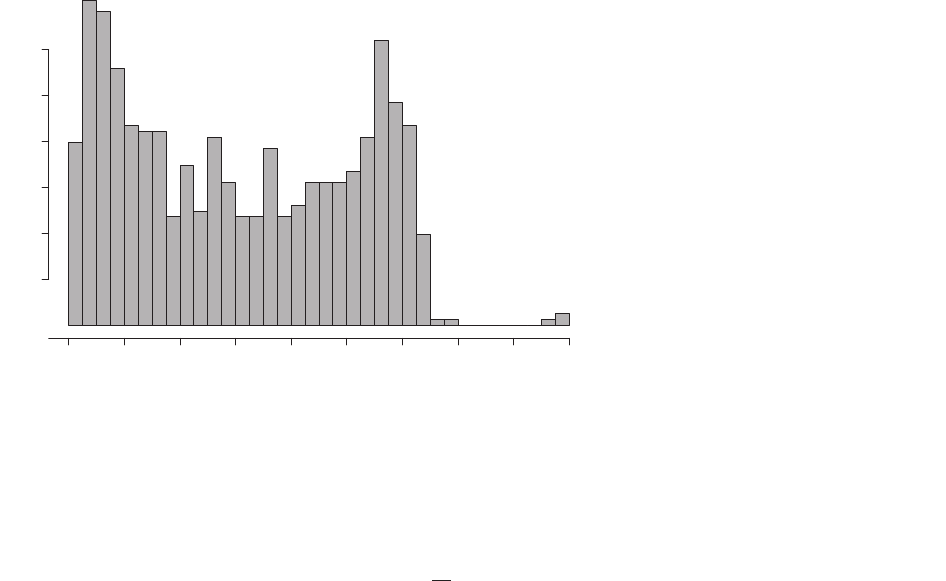
ers were rarely filmed during hours of darkness, and none were
filmed between midnight and 6.00 h. The frequency of detec-
tion increased steadily after 6.00 h to a maximum between
6.30 h and 7.00 h and remained relatively high until 9.30 h,
after which it decreased slightly and remained relatively low
until16.30h,thenincreasedagainandremainedhighuntil
18.00 h, then declined gradually until 19.00 h (Fig. 2). Duikers
were always active when detected; CTs did not record any
duikers that were asleep or stationary for an entire minute. We
recorded 11 180 distances from 6:30:00 h through 17:59:59 h,
and 6274 during times of peak activity.
Exploratory analyses revealed no evidence of data collection
errors, and a paucity of observations between 1 and 2 m but
not between 2 and 3 m, so we left-truncated at 2 m. Fitted
detection functions and probability density functions were
heavy-tailed when distances >15 m were included, so we right-
truncated at 15 m. Truncating removed 8% of observations
from the daytime dataset, leaving n = 10 284, and 65% of
observations from the peak activity dataset, leaving n = 5865.
Mean encounter rates (mean numbers of duikers observed per
2-s time interval) across all points were 327 9 10
4
during the
daytime and 476 9 10
4
during times of peak activity.
Encounter rates were highly variable among locations but did
not exhibit an obvious spatial pattern across the study area,
and there was no evidence of spatial autocorrelation
(Moran’s I P = 047; Fig. 3).
When we fit the hazard rate model with two adjustment
terms to the daytime dataset, the detection function was not
monotonically decreasing, so this model was not considered
for estimation. All models were fitted successfully to the peak
activity dataset. The hazard rate model with no adjustments
minimised AIC and was used to estimate density in both cases.
Probability density functions of observed distances and rela-
tionships between detection probability and distance were sim-
ilar (Fig. 4). Detection probability was ~10within5mand
0·02 0·04 0·06 0·08 0·10 0·12
6:00 8:00 10:00 12:00 14:00 16:00 18:00 20:00 22:00 24:00
Time
Denstiy of video start times
Fig. 2. Histogram of start times of videos of
Maxwell’s duikers in Ta
€
ı National Park, C
^
ote
d’Ivoire, 2014.
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society, Methods in Ecology and Evolution
4 E. J. Howe et al.

005 at 15 m; effective detection radii were 91and94mfrom
the daytime and peak activity datasets, respectively.
We expected to underestimate density where we assumed
duikers were active all da y;
b
D was 37% higher when we
included only data from times of peak activity (Table 1).
Including an independe nt estimate of the proportion of time
active during the daytime as a parameter in the model fit to the
daytime dataset yielded a still higher estimate (‘Active daytime’
in Table 1). Measures of uncertainty in the proportion of time
active were not available (Newing 2001) so did not contribute
to the variance of
b
D. Bootstrap variances were larger than
design-based analytic variances (Table 1). The vast majority
(998%) of the design-based variance of
b
D was attributable to
the variation in encounter rate between locations, and only
02% to detection probability.
Discussion
Simulations demonstrated the potential for the method to yield
unbiased density estimates, but also that animals’ activity pat-
terns must be accounted for. Where simulated animals rested
for half of each day and we set T
k
equal to the survey duration,
the most common scenario was that animals did not rest in
frontofCTsandnegativebiasin
b
D was proportional to the
time spent resting. When we recorded distance at each snap-
shot moment while animals rested in front of CTs, the encoun-
ter rate and therefore
b
D washigheronaverage,buttheshape
of the detection function was strongly affected, leading to erra-
tic estimates and cases where models could not be fitted to the
data. In practice, it is unlikely that we would detect animals
while they sleep or rest because movement will be insufficient
to trigger the sensor. Therefore, estimates of the proportion of
time animals are active within the vertical range of CTs will be
required to avoid negatively biased
b
D. Ideally, this proportion
would be estimated from data collected concurrently with the
distance data to ensure it is representative. Fortunately, the
temporal distribution of camera trap detections is informative
Fig. 3. Variation in encounter rates of Maxwell’s duikers among 21
camera trap locations in Ta
€
ı National Park, C
^
ote d’Ivoire, 2014 (range
000–145 9 10
3
). The areas of the grey circles are proportional to the
encounter rates.
0 5 10 15
0·00 0·05 0·10 0·15
Daytime
Probability density
0 5 10 15
0·00 0·05 0·10 0·15
Peak activity
0 5 10 15
0·0
0·40·8
1·2
Detection probability
Distance
0 5 10 15
0·00·40·81·2
Distance
Fig. 4. Probability density functions of
observed distances (top) and detection proba-
bility as a function of distance (bottom) from
hazard-rate point transect models fit to data
from Maxwell’s duikers in Ta
€
ı National Park,
2014 collected during the daytime (left) and
during times of peak activity (right).
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society, Methods in Ecology and Evolution
Distance sampling with camera traps 5
regarding animal activity patterns (Lynam et al. 2013; Cruz
et al. 2014; Rowcliffe et al. 2014). If it is reasonable to assume
that the entire population is available for detection for any part
of each day, additional data would not be required to estimate
b
D accurately, because we could either (i) analyse only the data
collected at that time, censoring effort and distance data from
other times, or (ii) estimate the overall proportion of time
active directly from the CT data (e.g. Rowcliffe et al. 2014).
Newing’s (1994) data from Ta
€
ı indicated that there was no time
at which all wild duikers could be assumed to be active. If this
was true during our survey, we may have underestimated den-
sity where we did not correct for limited availability within the
time included in T
k
, because even at times of peak activity some
animals may have been resting and unavailable for detection.
Activity data from wild duikers were presented only as figures
and could not be converted into estimates of the overall pro-
portion of time active (Newing 1994). We therefore relied on
the assumption that activity data from captive duikers (New-
ing 1994, 2001) were representative of activity patterns during
our survey. If this assumption held, then the density estimate
calculated using their estimate of the proportion of time active
during the day should not be biased as a result of limited avail-
ability. We suggest that the need to account for availability
should not pose a serious obstacle to reliable estimation of the
density of many species, but for others, notably ectotherms,
and semi-arboreal and fossorial species, it will require careful
consideration, and possibly additional data. We further sug-
gest that combining Rowcliffe et al.’s (2014) or similar meth-
ods for estimating the proportion of time active from detection
times at CTs with the point transect method described here
could yield accurate density estimates for many species from
CT data alone.
Avoidance of, or attraction to, CTs would bias encounter
rates and therefore density estimates. Some species exhibit
complex responses to CTs or are particularly wary of humans
(S
equin et al. 2003). If behavioural responses are expected or
apparent in images of detected animals, CTs could be deployed
prior to the start of the actual survey to allow animals to
become accustomed to them and for signs of human presence
to dissipate. Similarly, effort and distance data from times
when animals may have been displaced from the trap sites by
humans visiting them to download data, replace batteries, etc.,
should be censored.
The probability of detection at PIR CTs is lower at greater
angles from the centre of the FOV, due to a combination of the
trigger speed, the effective horizontal angle of the sensor rela-
tive to the AOV of the camera (which varies among CT mod-
els) and possibly reduced sensitivity of the sensor at the
periphery of its horizontal range (Rowcliffe et al. 2011; Rovero
et al. 2013; Rovero & Zimmermann 2016). This introduces
heterogeneity in the detection function. Fortunately, provided
that detection is certain at the zero distance, the pooling robust-
ness property ensures that estimation is unbiased in the pres-
ence of heterogeneity in detectability among individuals
(Buckland et al. 2004),andthisalsoappliestoheterogeneity
caused by differences in angle at different snapshot moments.
However, if detection probability at high h is much lower than
in the centre, fitted models of the detection function might
show a rapid drop in detection probability near the point,
whereas detection functions with a gradual decrease near the
point are preferred for stable density estimation (Buckland
et al. 2001). The expected distribution of angles within a sector
within which the sensor is fully effective is uniform. We recom-
mend that researchers measure angles as well as distances to
detected animals (e.g. Caravaggi et al. 2016), and test for
departures from the uniformity assumption at increasing
angles as part of their exploratory analysis. If departures are
apparent, the data could be truncated to exclude observations
beyond an angle within which the distribution is approximately
uniform, in which case h should be set to two times the trunca-
tion angle rather than the AOV of the camera in the definition
of effort. An alternative approach that would allow us to retain
all of the data would be to develop a two dimensional detection
function where detection probability depends on both radial
distance and angle from centre, using methods similar to those
developed by Marques et al. (2010). We expect heterogeneity
withangletobemoreseverewithCTmodelswithnarrowhori-
zontal ranges of the sensor relative to the AOV of the camera,
or slow trigger speeds, and where faster-moving animals are
sampled. CTs with fast trigger speeds, short recovery times,
and curved array Fresnel lenses (which provide a wide effective
angle of detection such that the camera begins recording
images as or even before the animal enters the FOV; Rovero &
Zimmermann 2016) could reduce or eliminate differences in
detection probability at different angles in future studies.
The encounter rate variance accounted for the vast majority
of the design-based variance in duiker density, and variances
around
b
D were larger than for simulated data despite similar
sample sizes. Real populations exhibit clumped or patchy dis-
tributions and non-random movement, leading to variable
encounter rates among sampling locations and hence greater
uncertainty in
b
D (Buckland et al. 2001; Fewster et al. 2009);
the small area sampled at each location exacerbates this prob-
lem. Increasing the area sampled will therefore enhance preci-
sion, more so than would increasing temporal effort at a point.
Theory predicts that increasing the number of points will yield
the largest improvements to precision (Buckland 1984; Fewster
et al. 2009). That the improvement in precision in simulations
was only slightly greater where we doubled the number of sam-
pling locations than where we doubled h is not representative
Table 1. Densities of Maxwell’s duikers in Ta
€
ı National Park, 2014,
estimated using different methods to account for limited availability for
detection
Design-based Bootstrap
Availability
^
D CV 95% CI CV 95% CI
Daytime 106027 61– 183040 50– 218
Peak activity 14 5030 78– 269036 61– 269
Active daytime 165027 95– 286040 77– 341
Bootstrap confidence intervals were calculated using the percentile
method.
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society, Methods in Ecology and Evolution
6 E. J. Howe et al.
of real studies because the expected spatial distribution of ani-
mal locations was uniform, and movement was random. Coef-
ficients of variation around
b
D for duikers were >35% despite
large samples of distance observations, so we recommend that
future studies employ more points to improve precision.
The average density of Maxwell’s duikers throughout Ta
€
ı
National Park was recently estimated as 16perkm
2
from line
transect DS surveys (N’Goran 2006). However, line transect
sampling by human observers is believed to severely underesti-
mate densities of forest-dwelling animals in general, and forest
antelopes in particular, due to effects of evasive movement and
behaviour in response to observers on both the encounter rate
and the distribution of observed distances (Koster & Hart 1988;
Jathanna, Karanth & Johnsingh 2003; Rovero & Marshall
2004, 2009; N’Goran 2006; Marshall, Lovett & White 2008;
Marini et al. 2009). For this reason, line transect surveys of sign
are frequently performed, and sign densities converted to ani-
mal densities. This approach is expected to yield biased esti-
mates in the absence of local and concurrent estimates of sign
production and decay rates, which are time-consuming to esti-
mate (Plumptre 2000; Kuehl et al. 2007; Todd et al. 2008).
Dung surveys may further require genetic analysis to identify
the species (Bowkett et al. 2009). Distance sampling with CTs
apparently avoided the underestimation characteristic of line
transect surveys of live animals, in less time than would be
required to obtain reliable estimates from sign surveys.
The recent proliferation of CT studies is providing new
information about wildlife in diverse habitats (Burton et al.
2015; Rovero & Zimmermann 2016). Where estimating the
density of a rare but individually identifiable species is the pri-
mary research objective, it may be preferable to deploy CTs
non-randomly in order to obtain sufficient detections of indi-
viduals to estimate density by SECR (Wearn et al. 2013;
Cusack et al. 2015b; Despr
es-Einspenner et al. 2017). How-
ever, multiple research objectives can be addressed, and useful
data for multiple species obtained, if CTs are deployed
according to a randomised design (MacKenzie & Royle 2005;
Wearn et al. 2013; Burton et al. 2015; D
enes, Silveira & Beis-
singer 2015). The size of unmarked populations can then be
estimated from CT data, using Poisson and negative binomial
GLMs or hierarchical N-mixture models (D
enes, Silveira &
Beissinger 2015), but population density is of greater interest
because it is more biologically relevant and comparable across
studies. Densities of unmarked animal populations can only
be estimated from CT data using SECR models for unmarked
populations, the REM, or DS methods; the latter two require
randomised designs (Buckland et al. 2001; Rowcliffe et al.
2008). SECR methods for unmarked populations require
intensive designs, and even then estimates will often be too
imprecise to be useful unless a subset of the population can be
reliably identified (Chandler & Royle 2013; Saout et al. 2014).
The REM requires an estimate of the average speed of animal
movement, assumes that detection is certain within an estim-
able area in front of the camera, and makes use of only one
observation from each detected animal (Rowcliffe et al.
2008). Our point transect approach requires an estimate of
the proportion of time animals are available for detection,
assumes that detection is certain only at zero distance, and
multiple observations from each detected animal inform
detection probability estimates. We expect the extension of
point transect DS methods to provide an effective and effi-
cient tool for estimating animal density and to enhance the
information derived from CT surveys.
Authors’ contributions
E.J.H. contributed to the development of the point transect model for camera
traps, performed the analyses, and wrote the manuscript. S.T.B. conceived and
formulated the original version of the point transect model for camera traps, and
contributed to the manuscript text. M.-L.D.-E. helped to design and conducted
the field study, estimated distances to Maxwell’s duikers from video footage,
wrote portions of the methods section, and contributed to the manuscript text.
H.S.K. designed the field study and contributed to the manuscript text.
Acknowledgements
We thank the Robert Bosch Foundation, the Max Planck Society and the Univer-
sity of St Andrews for funding, the Minist
ere de l’Enseignement Sup
erieur et de la
Recherche Scientifique and the Minist
ere de l’Environnement et des Eaux et For-
^
ets in C
^
ote d’Ivoire for permission to conduct field research in Ta
€
ı National Park,
and Dr. Roman Wittig for permitting data collection in the area of the Ta
€
ı
Chimpanzee Project.
Data accessibility
The data files from which densities of Maxwell’s duikers were estimated using
Distance software, and data describing start times of videos of Maxwell’s duikers,
have been archived at the Dryad data repository (https://doi.org/10.5061/dryad.
b4c70) (Howe et al. 2017).
References
Balestrieri, A., Ruiz-Gonz
alez, A., Vergara, M., Capelli, E., Tirozzi, P., Alfino,
S., Minuti, G., Prigioni, C. & Saino, N. (2016) Pine marten density in lowland
riparian woods: a test of the random encounter model based on genetic data.
Mammalian Biology-Zeitschrift f
€
ur S
€
augetierkunde, 81,439–446.
Bowkett, A.E., Plowman, A.B., Stevens, J.R., Davenport, T.R. & van Vuu-
ren, B.J. (2009) Genetic testing of dung identification for antelope sur-
veys in the Udzungwa Mountains, Tanzania. Conservation Genetics, 10,
251–255.
Buckland, S.T. (1984) Monte Carlo confidence intervals. Biometrics, 1, 811–817.
Buckland, S.T., Anderson, D.R., Burnham, K.P., Laake, J.L., Borchers, D.L. &
Thomas, L. (2001) Introduction to Distance Sampling: Estimating Abundance
of Biological Populations. Oxford University Press, Oxford, UK.
Buckland, S.T., Anderson, D.R., Burnham, K.P., Laake, J.L., Borchers, D.L. &
Thomas, L. (2004) Advanced Distance Sampling: Estimating Abundance of
Biological Populations. Oxford University Press, Oxford, UK.
Buckland, S.T., Rexstad, E.A., Marques, T.A. & Oedekoven, C.S. (2015) Dis-
tance Sampling: Methods and Applications. Springer, Heidelberg, Germany.
Burton,A.C.,Neilson,E.,Moreira,D.,Ladle, A., Steenweg, R., Fisher, J.T.,
Bayne, E. & Boutin, S. (2015) REVIEW: wildlife camera trapping: a review
and recommendations for linking surveys to ecological processes. Journal of
Applied Ecology, 52,675–685.
Caravaggi, A., Zaccaroni, M., Riga, F., Schai-Braun, S.C., Dick, J.T., Mont-
gomery, W.I. & Reid, N. (2016) An invasive-native mammalian species
replacement process captured by camera trap survey random encounter mod-
els. Remote Sensing in Ecology and Conservation, 2,45–58.
Chandler, R.B. & Royle, J.A. (2013) Spatially explicit models for inference about
density in unmarked or partially marked populations. The Annals of Applies
Statistics, 7, 936–954.
Cruz, P., Paviolo, A., B
o, R.F., Thompson, J.J. & Di Bitetti, M.S. (2014) Daily
activity patterns and habitat use of the lowland tapir (Tapirus terrestris)inthe
Atlantic Forest. Mammalian Biology-Zeitschrift f
€
ur S
€
augetierkunde, 79,376–
383.
Cusack, J.J., Dickman, A.J., Rowcliffe, J.M., Carbone, C., Macdonald, D.W. &
Coulson, T. (2015b) Random versus game trail-based camera trap placement
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society, Methods in Ecology and Evolution
Distance sampling with camera traps 7
strategy for monitoring terrestrialmammalcommunities.PLoS ONE, 10,
e0126373.
Cusack, J.J., Swanson, A., Coulson, T., Packer, C., Carbone, C., Dickman, A.J.,
Kosmala, M., Lintott, C. & Rowcliffe, J.M. (2015a) Applying a random
encounter model to estimate lion density from camera traps in Serengeti
National Park, Tanzania. The Journal of Wildlife Management, 79, 1014–1021 .
D
enes, F.V., Silveira, L.F. & Beissinger, S.R. (2015) Estimating abundance of
unmarked animal populations: accounting for imperfect detection and other
sources of zero inflation. Methods in Ecology and Evolution, 6, 543–556.
Despr
es-Einspenner, M.-L., Howe, E.J., Drapeau, P. & K
€
uhl, H.S. (2017) An
empirical evaluation of camera trapping and capture-recapture methods for
estimating chimpanzee density. American Journal of Primatology, https://doi.
org/10.1002/ajp.2264 7
Efford, M.G., Borchers, D.L. & Byrom, A.E. (2009) Density estimation by spa-
tially explicit capture – recapture: likelihood-based methods. Modelling Demo-
graphic Processes in Marked Populations (eds D.L. Thompson, E.G. Cooch &
M.J. Conroy), pp. 255–269. Springer, New York, NY, USA.
Fewster, R.M., Buckland, S.T., Burnham, K.P., Borchers, D.L., Jupp, P.E.,
Laake, J.L. & Thomas, L. (2009) Estimating the encounter rate variance in dis-
tance sampling. Biometrics, 65, 225–236.
Howe, E.J., Buckland, S.T., Despr
es-Einspenner, M.-L. & K
€
uhl, H.S. (2017)
Data from: Distance sampling with camera traps. Dryad Digital Repository,
https://doi.org/10.5061/dryad.b4c70
Jathanna, D., Karanth, K.U. & Johnsingh, A.J.T. (2003) Estimation of large her-
bivore densities in the tropical forests of southern India using distance sam-
pling. Journal of Zoology, 261, 285–290.
Koster, S.H. & Hart, J.A. (1988) Methods of estimating ungulate populations in
tropical forests. African Journal of Ecology, 26, 117–126.
Kuehl, H.S., Todd, A., Boesch, C. & Walsh, P.D. (2007) Manipulating decay time
for efficient large-mammal density estimation: gorillas and dung height. Eco-
logical Applications, 17, 2403–2 414.
Laake, J.L., Collier, B.A., Morrison, M.L. & Wilkins, R.N. (2011) Point-based
mark-recapture distance sampling. Journal of Agricultural, Biological, and
Environmental Statistics, 16, 389–
408.
Lynam, A.J., Jenks, K.E., Tantipisanuh, N. et al. (2013) Terrestrial activity pat-
ternsofwild cats from camera-trapping.Raffles Bulletin of Zoology, 61,407–415.
MacKenzie, D.I. & Royle, J.A. (2005) Designing occupancy studies: general
advice and allocating survey effort. Journal of Applied Ecology, 42, 1105–1114.
Marini, F., Franzetti, B., Calabrese, A., Cappellini, S. & Focardi, S. (2009)
Response to human presence during nocturnal line transect surveys in fallow
deer (Dama dama) and wild boar (Sus scrofa). European Journal of Wildlife
Research, 55, 107–115.
Marques, T.A., Buckland, S.T., Borchers, D.L., Tosh, D. & McDonald, R.A.
(2010) Point transectsamplingalong linear features. Biometrics,66, 1247–1255.
Marques, T.A., Thomas, L., Fancy, S.G. & Buckland, S.T. (2007) Improving esti-
mates of bird density using multiple-covariate distance sampling. The Auk,
124, 1229–1243.
Marshall, A.R., Lovett, J.C. & White, P.C.L. (2008) Selection of line-transect
methods for estimating the density of group-living animals: lessons from the
primates. American Journal of Primatology, 70,452–462.
Miller, D.L. 2015. Distance: distance sampling detection function and abundance
estimation. R package version 0.9.4. http://CRAN.R-project.org/package=
Distance (accessed 9 November 2016).
Newing, H.S. 1994. Behavioural ecology of duikers (Cephalophus spp.) in forest
and secondary growth, Ta
€
ı, C
^
ote d’Ivoire. PhD thesis, University of Stirling,
Stirling, Scotland.
Newing, H.S. (2001) Bushmeat hunting and management: implications of duiker
ecology and interspecific competition. Biodiversity and Conservation, 10,
99–118.
N’Goran, P.K. 2006 Quelques r
esultats de la premi
ere phase du biomonitoring
au Parc National de Ta
€
ı(ao
^
ut 2005 – mars 2006). Ministere de l’Environ-
nement et des Eaux et Forets, Ministere de l’Enseignement Superieur et de la
Recherce Scientifique, Abidjan, C
^
ote d’Ivoire.
Plumptre, A.J. (2000) Monitoring mammal populations with line transect tech-
niques in African forests. Journal of Applied Ecology, 37, 356
–368.
Rovero, F. & Marshall, A.R. (2004) Estimating the abundance of forest antelopes
by line transect techniques: a case from the Udzungwa Mountains of Tanza-
nia. Tropical Zoology, 17,267–277.
Rovero, F. & Marshall, A.R. (2009) Camera trapping photographic rate as an
index of density in forest ungulates. Journal of Applied Ecology, 46,1011–1017.
Rovero, F. & Zimmermann, F. (2016) Camera Trapping for Wildlife Research.
Pelagic Publishing, Exeter, UK.
Rovero, F., Zimmermann, F., Berzi, D. & Meek, P. (2013) ‘Which camera trap
type and how many do I need?’ A review of camera features and study designs
for a range of wildlife research applications. Hystrix, the Italian Journal of
Mammalogy, 24, 148–156.
Rowcliffe, M.J., Carbone, C., Jansen, P.A., Kays, R. & Kranstauber, B. (2011)
Quantifying the sensitivity of camera traps: an adapted distance sampling
approach. Methods in Ecology and Evolution, 2,464–476.
Rowcliffe, J.M., Field, J., Turvey, S.T. & Carbone, C. (2008) Estimating animal
density using camera traps without the need for individual recognition. Journal
of Applied Ecology, 45, 1228–123 6.
Rowcliffe, J.M., Jansen, P.A., Kays, R., Kranstauber, B. & Carbone, C. (2016)
Wildlife speed cameras: measuring animal travel speed and day range using
camera traps. Remote Sensing in Ecology and Conservation, 2,84–94.
Rowcliffe, M.J., Kays, R., Kranstauber, B., Carbone, C. & Jansen, P.A. (2014)
Quantifying levels of animal activity using camera trap data. Methods in Ecol-
ogy and Evolution, 5, 1170–1179.
Saout, S.L., Chollet, S., Chamaill
e-Jammes, S. et al. (2014) Understanding the
paradox of deer persisting at high abundance in heavily browsed habitats.
Wildlife Biology, 20, 122–135.
S
equin, E.S., Jaeger, M.M., Brussard, P.F. & Barrett, R.H. (2003) Wariness of
coyotes to camera traps relative to social status and territory boundaries.
Canadian Journal of Zoology, 81,2015–2025.
Sollmann, R., Mohamed, A. & Kelly, M.J. (2013a) Camera trapping for the study
and conservation of tropical carnivores. The Raffles Bulletin of Zoology, 28,
21–42.
Sollmann, R., Mohamed, A., Samejima, H. & Wilting, A. (2013b) Risky business
or simple solution – relative abundance indices from camera trapping. Biologi-
cal Conservation, 159, 405–412.
Thomas, L., Buckland, S.T., Rexstad, E.A., Laake, J.L., Strindberg, S., Hedley,
S.L., Bishop, J.R.B., Marques, T.A. & Burnham, K.P. (2010) Distance soft-
ware: design and analysis of distance sampling surveys for estimating popula-
tion size. Journal of Applied Ecology, 47,5–14.
Todd, A.F., Kuehl, H.S., Cipolletta, C. & Walsh, P.D. (2008) Using dung to esti-
mate gorilla density: modeling dung production rate. International Journal of
Primatology, 29, 549–563.
Trailcampro.com (2015) Available at: http://www.trailcampro.com/ (accessed 5
August 2015).
Wearn, O.R., Rowcliffe, J.M., Carbone, C., Bernard, H. & Ewers, R.M. (2013)
Assessing the status of wild felids in a highly-disturbed commercial forest
reserve in Borneo and the implications for camera trap survey design. PLoS
ONE, 8, e77598.
Zero, V.H., Sundaresan, S.R., O’Brien, T.G. & Kinnaird, M.F. (2013) Monitor-
ing an Endangered savannah ungulate, Grevy’s zebra Equus grevyi:choosinga
method for estimating population densities. Oryx, 47, 410–419.
Received 13 January 2017; accepted 15 March 2017
Handling Editor: Jason Matthiopoulos
Supporting Information
Details of electronic Supporting Information are provided below.
Appendix S1. MEE-17-01-032-S1 describes simulation methods and
results in detail.
© 2017 The Authors. Methods in Ecology and Evolution © 2017 British Ecological Society, Methods in Ecology and Evolution
8 E. J. Howe et al.
