
Duquesne University Duquesne University
Duquesne Scholarship Collection Duquesne Scholarship Collection
Electronic Theses and Dissertations
Summer 5-18-2021
Clinical Pharmacy services and medication utilization in Hospice Clinical Pharmacy services and medication utilization in Hospice
Care Care
Aishwarya Kulkarni
Follow this and additional works at: https://dsc.duq.edu/etd
Part of the Pharmacoeconomics and Pharmaceutical Economics Commons, and the Pharmacy
Administration, Policy and Regulation Commons
Recommended Citation Recommended Citation
Kulkarni, A. (2021). Clinical Pharmacy services and medication utilization in Hospice Care (Master's
thesis, Duquesne University). Retrieved from https://dsc.duq.edu/etd/1980
This Immediate Access is brought to you for free and open access by Duquesne Scholarship Collection. It has been
accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Duquesne
Scholarship Collection.
CLINICIAL PHARMACY SERVICES AND MEDICATION UTILIZATION IN
HOSPICE CARE
A Thesis
Submitted to the School of Pharmacy
Duquesne University
In partial fulfillment of the requirements for
the degree of Masters of Science in Pharmacy Administration
By
Aishwarya Kulkarni
May 2021
Copyright by
Aishwarya Kulkarni
2021
iii
CLINICIAL PHARMACY SERVICES AND MEDICATION UTILIZATION IN
HOSPICE CARE
By
Aishwarya Kulkarni
Approved November 18, 2020
________________________________
Jordan R Covvey, PharmD, PhD, BCPS
Associate Prof of Pharm Admin
(Committee Chair)
________________________________
Khalid M Kamal, MPharm, PhD
Prof of Pharm Admin
(Committee Member)
________________________________
Mary Mihalyo, PharmD
(former) Assistant Prof of Pharm Practice
(Committee Member)
________________________________
Vincent Giannetti, PhD
(former) Prof of Pharm Admin
(Committee Member)
________________________________
Carl Anderson, PhD
Interim Assistant Dean, Graduate School of
Pharmaceutical Sciences
________________________________
James Drennen, PhD
Interim Dean, School of Pharmacy and
Graduate School of Pharmaceutical
Sciences
iv
ABSTRACT
CLINICIAL PHARMACY SERVICES AND MEDICATION UTILIZATION IN
HOSPICE CARE
By
Aishwarya Kulkarni
May 2021
Thesis supervised by Dr Jordan Covvey
Background: As discussed within the guidelines from the American Society of
Health-System Pharmacists (ASHP), pharmacists are an integral part of the hospice
multidisciplinary team involved in optimizing the treatments
. Methadone, a long-active
opioid, is particularly useful in this population but may be clinically underutilized.
Additionally,
the cost share of medication utilization in hospice and palliative care is shifting
towards hospice providers. The assessment of medication utilization and methadone use in this
setting can help develop overall cost/clinical optimization strategies.
Thus, there is a need to
understand the use and expenditure of various medications and pharmacists' role in
providing methadone use recommendations in hospice and palliative care settings that
would facilitate the cost containment.
Objectives: The purpose of the study was to (1) identify the prevalence and acceptance of
clinical pharmacists’ methadone recommendation before and after admission to
hospice/palliative care, and (2) identify the frequency, expenditure, and monthly mean cost
of therapeutic medication classes belonging pain, pulmonary and anticoagulant
medications categories.
v
Methods: The study was conducted in two phases. The phase I was conducted in two parts
of data collection at DeltaCareRx hospice and palliative care site. A systematic literature
review formed the basis of clinical pharmacist's role and significance in the
multidisciplinary team of hospice and palliative care. The instruments for data collection
were developed for the clinical pharmacists and student pharmacist researcher. Descriptive
and inferential statistics of the collected data
identified the prevalence of clinical pharmacist
recommendations for methadone upon admission to hospice/palliative care and the acceptance
of the pharmacists’ recommendations for methadone after admission to hospice/palliative care.
Phase II studied medication utilization at the hospice/palliative sites served by the pharmacy
benefit manager (PBM) DeltaCareRx. Pharmacy claims data for six months of the year 2019
was obtained from DeltaCareRx. The data included information on the utilization of individual
medications and their associated therapeutic classes, patient characteristics, and dispensing
cost charged to the patients. Claims data were analyzed to identify the frequency in use, total
expenditure, and the monthly average cost of each therapeutic class and the pattern in the
utilization of therapeutic class based on the sex of the patients. The consumption of individual
medications was calculated using defined daily doses (DDD), a methodology that analyses
medication consumption and enables comparison across different months in a standardized
manner.
Results: In total, the data collected on both instruments included 158 (99.3%) patients. The
prevalence of pharmacist methadone recommendation was 37 (23.4%). The majority (26;
16.5%) of methadone recommendation were for switching to methadone as the maintenance
treatment. Out of the 37 pharmacist recommendations, 5 (13.5%) were accepted by the
physicians, and the physicians themselves implemented 3 (8.1%) recommendations. In phase
II, the pharmacy claims data were obtained for six months (January, June, July, September,
October, and November) of 2019. The data consisted of 487 unique therapeutic classes and
3,189 unique medications. Sympathomimetics, opioid agonists, and coumarin anticoagulants
were the most frequently used therapeutic classes. The average cost per male/female patients
was the highest ($64.82 and $67.70) for pulmonary medications. Medications such as albuterol,
enoxaparin, and morphine had higher consumption levels.
Conclusion: The study provided valuable insights regarding clinical pharmacists' significant
role in hospice and palliative care. A pharmacist's role in providing recommendations on
vi
medication use to the patients can improve clinical/cost optimization in the setting. The data
collection on pharmacists’ recommendations on methadone demonstrates minimum
medication use in the hospice and palliative care setting. There should be an increase in the use
of this cost-effective medicine for pain management among the patients. The pharmacy claims
data analysis implements that the rise in use of cost-effective medications from the individual
therapeutic classes will help in higher cost savings at DeltaCareRx’s client sites and reduce the
provider’s overall cost burden.
vii
DEDICATION
I would like to dedicate this thesis to my newborn nephew and niece, Yunay and
Mrunmayi . Thank you for bringing the joy in our lives.
viii
ACKNOWLEDGEMENT
I would like to thank Dr. Jordan Covvey for her great inputs and constant support throughout
my thesis work and time at Duquesne University. I highly appreciate the timely advice and
direction that you provided. This work wouldn’t have been possible without you. I would like
to thank Dr. Kamal and Dr. Vince Gianneti for serving on my thesis committee and for being
great professors throughout my journey at Duquesne. Thank you to all three for sharing your
great knowledge and helping me solve problems either in professional or personal life.
I extend my appreciation to Dr. Mary Mihalyo for collaborating on my thesis project and
providing insightful data from DeltaCareRx. I would also like to thank DeltaCareRx staff and
Madison Hawkins for their great contribution to the thesis work.
This thesis work and the journey away from home for completing my master’s wouldn’t have
possible without the encouragement, love and support of my close and extended family, mom,
dad, sister and brother in law. A big thankyou to my friend and my roommate Yashika for
being there always. Your patience and motivational talks helped me throughout. I would like
to thank all my friends in the states and in India. They have helped me excel and motivated me
always to reach greater heights. Snigdha and Rachana have played an important role in my life.
I am thankful to have such great friends in my life. I wouldn’t have come this far without you
guys.
ix
TABLE OF CONTENTS
Page
Abstract ..................................................................................................................................... iv
Dedication .................................................................................................................................. v
Acknowledgement .................................................................................................................... vi
List of Tables ......................................................................................................................... viii
List of Figures ........................................................................................................................... ix
List of Abbreviations ................................................................................................................. x
CHAPTER 1: BACKGROUND..............................................................................................1
I. Hospice and palliative care..........................................................................................1
a. Definition and prevalence .........................................................................................1
b. Clinical importance ...............................................................................................2
c. Costs within the healthcare system ..........................................................................3
II. Medication use in hospice setting ...............................................................................4
a. Policy changes with Medicare billing ......................................................................4
III. General role of pharmacists ......................................................................................5
IV. Pain management within hospice/palliative care ....................................................5
a. Clinical guidelines and outcomes of interest ...........................................................6
b. Medications used in pain management ................................................................6
c. Utilization of methadone in hospice care ................................................................6
d. Role of pharmacists specifically in pain management ........................................7
V. DeltaCareRx .................................................................................................................7
a. Description of the organization ................................................................................8
b. Services provided to hospices...............................................................................8
VI. Problem statement .....................................................................................................8
VII. Study objectives/aims ...............................................................................................9
CHAPTER 2: LITERATURE REVIEW ..............................................................................10
I. Rationale .....................................................................................................................10
II. Objective.....................................................................................................................11
III. Search strategy ........................................................................................................11
x
a. PubMed ....................................................................................................................11
b. Embase .................................................................................................................12
c. Scopus ......................................................................................................................12
IV. Inclusion/exclusion criteria.....................................................................................13
V. Data extraction ...........................................................................................................13
VI. Results ......................................................................................................................15
VII. Extraction .................................................................................................................15
a. Prospective evaluations of pharmacist-led interventions......................................27
b. Retrospective evaluations of pharmacist impact ...............................................31
c. Survey questionnaires regarding pharmacists in hospice and palliative care......33
VIII. Overall summary .................................................................................................34
IX. Limitations ...............................................................................................................35
CHAPTER 3: METHODS .....................................................................................................36
I. Phase I of the study ....................................................................................................36
a. Phase aim .................................................................................................................36
b. Overview ..............................................................................................................36
1. Rationale ..............................................................................................................36
a. Study sample ...........................................................................................................37
b. Protection of human subjects ..............................................................................37
c. Developing instruments for data collection ...........................................................37
1. Instrument #1 - Pharmacist data collection tool ................................................37
2. Instrument #2 - Researcher data collection tool ................................................38
d. Data collection process .......................................................................................39
e. Data management and statistical analysis..............................................................40
1. Research questions ..............................................................................................40
II. Phase II of the study...................................................................................................41
a. Phase aim .................................................................................................................41
b. Overview ..............................................................................................................41
c. Rationale ..................................................................................................................42
d. Data source ..........................................................................................................42
e. Database structure ...................................................................................................42
1. Therapeutic classification of drugs ....................................................................43
2. Drug names ..........................................................................................................43
3. Sex ........................................................................................................................43
xi
4. Generic long name ..............................................................................................43
5. DeltaCareRx medication cost .............................................................................44
f. Utilization of medications.......................................................................................44
g. Selection of medication categories .....................................................................45
1. Impact of missing data ...............................................................................................46
2. Research questions .....................................................................................................46
CHAPTER 4: RESULTS .......................................................................................................48
I. Phase I of the study ....................................................................................................48
a. Study aims ...............................................................................................................48
b. Overview ..............................................................................................................48
c. Demographic and clinical characteristics of the sample .......................................48
1. Sample size ......................................................................................................48
2. Patient demographic variables ........................................................................48
3. Patient clinical characteristic variables ..........................................................49
d. Research question 1 .............................................................................................52
1. Provided methadone recommendations .........................................................52
2. Indications/contraindication of provided methadone recommendations .....52
3. Accepted methadone recommendations .........................................................53
e. Research question 2.................................................................................................53
1. Sample stratification ........................................................................................53
2. Methadone recommendations by demographic/clinical characteristics .......54
f. Characteristics of accepted methadone recommendation patients .......................56
II. Phase II of the study .......................................................................................................57
a. Study aims ...............................................................................................................57
b. Overview ..............................................................................................................57
c. Sample characteristics .............................................................................................57
d. Research question 1 .............................................................................................57
1. Frequency in the use and expenditure of each therapeutic class in different
months .............................................................................................................................57
i. January .....................................................................................................................58
ii. June.......................................................................................................................60
iii. July .......................................................................................................................62
iv. September ............................................................................................................63
v. October .................................................................................................................66
vi. November.............................................................................................................68
xii
e. Overall costs per therapeutic class in the combined dataset of all the months....70
i. Pain medication category ........................................................................................70
ii. Pulmonary medication category .........................................................................70
iii. Anticoagulant medication category ....................................................................71
f. Research question 2.................................................................................................72
1. Trends in utilization as per patients’ sex............................................................72
2. The difference in mean cost per patients across individual months and all the
months .............................................................................................................................74
g. Research question 3 .............................................................................................75
CHAPTER 5: DISCUSSION ................................................................................................79
I. Key findings ...............................................................................................................79
II. Limitations and future considerations ......................................................................84
a. Clinical outcomes of methadone use .....................................................................84
b. Generalizability ...................................................................................................85
c. Limitation of using PBM claims database .............................................................85
d. Impact of missing data ........................................................................................85
III. Study implications and conclusion.........................................................................85
REFERENCES .......................................................................................................................87
APPENDICES ........................................................................................................................93
Appendix 1: Instrument #1 – Pharmacist Data Collection tool .......................................93
Appendix 2: Instrument #2 – Researcher Data Collection tool .......................................94
Appendix 3: Examples of categorization of variables .....................................................95
Appendix 4: List of DDD values .......................................................................................97
xiii
LIST OF TABLES
Table 1. List of indications and contraindications of using methadone..............................38
Table 2: Demographic and clinical characteristics of hospice/palliative care patients ......50
Table 3: Overall distribution of pain variables in patients before and after the
admission ................................................................................................................................51
Table 4: Overall distribution of allergies and comorbidities in study sample ....................52
Table 5: Indications and contraindications for using methadone in the study sample .......53
Table 7: Differences in categorical variables based on methadone recommendation .......55
Table 8: Characteristics of patients with accepted methadone recommendations .............56
Table 9. Frequency and expenditure of pulmonary medications in January ......................58
Table 10. Frequency and expenditure of pain medications in January ...............................59
Table 11. Frequency and expenditure of anticoagulant medications in January ................59
Table 12. Frequency and expenditure of pulmonary medications in June .........................60
Table 13. Frequency and expenditure of pain medications in June ....................................61
Table 14. Frequency and expenditure of anticoagulant medications in June .....................61
Table 15. Frequency and expenditure of pulmonary medications in July ..........................62
Table 16. Frequency and expenditure of pain medications in July .....................................63
Table 17. Frequency and expenditure of anticoagulant medications in July ......................63
Table 18. Frequency and expenditure of pulmonary medications in September ...............64
Table 19. Frequency and expenditure of pain medications in September ..........................65
Table 21. Frequency and expenditure of pulmonary medications in October ....................66
Table 22. Frequency of use and expenditure of pain medications in October ..................67
Table 23. Frequency and expenditure of anticoagulant medications in October ...............67
Table 24. Frequency and expenditure of pulmonary medications in November ...............68
Table 25. Frequency and expenditure of pain medications in November ..........................69
Table 26. Frequency and expenditure of anticoagulant medications in November ...........69
Table 27. Overall cost descriptive statistics for pain medication category.........................70
Table 28. Overall cost descriptive statistics for pulmonary medication category ..............71
Table 29. Overall cost descriptive statistics for anticoagulant medication category .........71
Table 30: Trends in anticoagulant medication utilization as per patients’ sex ...................72
Table 31: Trends in pain medication utilization as per patients’ sex ..................................73
Table 32: Trends in pulmonary medication utilization as per patients’ sex .......................74
Table 33. Differences in per male patient mean cost across three medical categories ......75
Table 34: Differences in per female patient mean cost across three medical categories ...75
Table 35. Total anticoagulant medication DDDs dispensed................................................76
Table 36. Total pain medication DDDs dispensed ...............................................................77
Table 37. Total pulmonary medication DDDs dispensed ....................................................78
1
CHAPTER 1: BACKGROUND
I. Hospice and palliative care
a. Definition and prevalence
Hospice is compassionate care for patients who are in their terminal phase of life, defined as less than six
months by the Medicare program.
1
It includes mainly pain and symptom management, and providing
emotional and spiritual support as per the patient’s needs. Palliative care is defined by World Health
Organization (WHO) as “a service which improves quality of life of patients through by means of early
identification and impeccable assessment and treatment of pain and other problems, physical,
psychosocial and spiritual”.
2
Hospice and palliative care services are paid for through both public and private insurance plans in the
United States (US). For patients in the Medicare program, hospice is covered by Part A (Hospital
Insurance) under the Medicare Hospice Benefit, established in 1982. Beneficiaries are eligible for the
hospice benefit only if the hospice provider and their regular provider certify that the beneficiary is
terminally ill (defined as a life expectancy of less than six months). Hospice care coverage for Medicaid
patients depends on the life expectancy period established by the respective state in which they reside.
3
Palliative care is covered for beneficiaries under Medicare Part B (Medical Insurance). Medicaid patients
can avail some palliative coverage as well, depending on the treatment they receive. Patients who are not
eligible for hospice services due to a life expectancy of more than six months qualify for palliative care
service.
Medicare defines four levels/types of hospice care, which varying needs of the patients. The first level of
care is Routine Hospice Care (RHC) which is provided at the patient’s residence, also known as routine
nursing home care. The second level of care is Continuous Hospice Care (CHC), which is predominantly
nursing care that focuses on maintaining pain control or addressing symptom crisis situations at the
patient’s home. The third level of care is Inpatient Respite Care (IRC), which provides a temporary support
to the patient’s primary caregiver. It can be offered in various settings, such as the hospital, hospice
facility, or a long-term care facility that has enough 24-hour nursing personnel present. The fourth level
of care is General Inpatient Care (GIP) which is offered for pain control or other acute symptom
management that cannot feasibly be supplied in any other setting. GIP is offered when an additional care
is required for managing symptoms of the patients. Among all levels of care, RHC service is the most

2
utilized type. In 2017, among all the hospice care in US, 98.2% of days of care were provided at RHC
level, compared to CHC (0.2%), IRC (0.3%) and GIC (1.3%).
4,5
In 2017, National Hospice and Palliative Care Organization (NHPCO) reported that 1.49 million Medicare
beneficiaries received hospice care services, an increase of 4.5% from 2016.
4,5
The proportion of enrollees
under Medicare Advantage plans who utilized hospice benefits have drastically increased from 26.8% in
2012 to 34.7% in 2017. This has resulted in a $18.99 billion payment by Medicare to hospice care
providers in 2017, a 6.3% increase compared to $17.86 billion paid in 2016. The maximum spending
based on level of care was on RHC service at 89.31%, and lowest on CHC at 1.77% of total spending.
5
In case of palliative care services, fast growth has been observed as well. One of the reasons for this is the
ability of palliative care services to improve quality of life (QoL) for both patients and their families. In
2019, 72% of hospitals with 50 or more beds were identified to have a palliative team, compared to 67%
in 2015.
6,7
A report published by the Center to Advance Palliative Care (CAPC) assigned a letter grade
(A to F) to almost all the states in the US as per the number of beds in the hospital and availability of a
palliative care team in the same hospital. The letter grading rubric applied includes: “A grade is assigned
to a state in which over 80% of hospitals had palliative care programs, B grade to states with 61%–80%
of hospitals with palliative care programs, C grade to states with 41%–60% of hospitals having palliative
care programs, D grades to states with 21%–40% of hospitals having palliative care programs, and F
grades to states with 20% or fewer hospitals having palliative care programs”.
7
As per the grading, three
quarters of states in the US have either A or B grade, with more than 60% of hospitals with a palliative
care team. The percentage of annual hospital admissions for palliative care increased slightly from 5.0%
in 2016 to 5.3% in 2017.
7
The availability of palliative care depends on geography of the hospital and
hospital size in terms of number of beds and tax status. Tax status is the predictor of access to palliative
care. In 2019, as per the proportion of hospitals with palliative care based on tax status. Nonprofit hospitals
regardless of hospital size (i.e. hospital beds facility 50-150, 151-300 and 301-350) were found to have
higher proportion of palliative care services available. Access to palliative care was lowest for the for
profit hospitals.
8
b. Clinical importance
Hospice and palliative care focus on relieving pain and other symptoms in patients. The services offered
in hospice setting focus on improving patient’s remaining time left by providing comfort. Care
additionally aids patients’ families and caregivers during the time of illness by providing a support system.
3
One of the main aspects the care revolves around is providing quality of life to all the patients. All of the
patient’s needs are addressed during the time of illness, including physical, social, physiological and
spiritual.
2
Hospice improves end-of-life outcomes. The link between hospice care services and outcomes was
identified through a research study, “Quality of life matters: end of life care news and clinical findings for
physicians,” with data acquired from the Dartmouth Atlas of Healthcare Report (2012) and the American
Hospital Association Survey (2012). It found that hospice care service was associated with high values of
end-of-life care outcomes such as less hospital deaths (p=0.01), hospital stays (p=0.01), better pain control
(p=0.01) and good patient ratings (P=0.01).
9
Unlike hospice care, palliative care does not depend on prognosis of terminal illness. It mainly focuses on
symptom management and psychological support. It achieves the desired clinical outcomes by
comprehensive assessment and treating patient’s physical, psychological, and spiritual symptoms.
Palliative care plays a role in decreasing symptom burden, increasing communication between
multidisciplinary teams and improving patient’s treatment regimen. Initiating early palliative care in
cancer patients has shown improvement in quality of life,
10,11
symptoms
11,12
and survival rates.
12,13
c. Costs within the healthcare system
In 2017, 1.5 billion Medicare beneficiaries received hospice services, with a dramatic increase in resources
utilized from $2.9 billion in 2000 to $17.9 billion in 2017.
14
Hospice and palliative care costs are shared
by multiple entities, including Medicare, Medicaid, managed care or private insurance and other (such as
charity and self-pay). The cost breakdown for each of these entities is 85.4%, 5%, 6.9% and 2.7% of the
total expenditure, respectively.
15
Medicare patients receive hospice coverage through their Medicare advantage plan. Payments are made
from Medicare to hospice providers in a form of daily rates. As soon as a patient is enrolled in the hospice
setting, the providers receive a fixed amount payment from Medicare, based on the four levels of care.
For 2019, RHC for days 1-60 has a base rate of $196 per day and days 61+ has a base rate of $145, while
CHC is $42 per hour, IRC is $176 per day and GIC is $758 per day.
14
The payment rates are changed
annually by the inpatient hospice market basket index. The rate for the most common level of hospice
care, RHC, was reformed in 2016 by CMS.
16,17
Originally, RHC was paid at a single rate, but now
Medicare pays two per diem rates for RHC that includes a higher rate for the first 60 days of a hospice
episode and a lower rate for days 61+, at $196 and $154 per day, respectively, in 2019.
16
The change in
4
the payment rates were made because hospices provide maximum care during the beginning and end of
the episode and less during the middle phase.
Medicare pays the hospice/palliative care provider as per diem rate for the assigned level of care provided.
The payment is fixed is regardless of the service provided to the patients.
18
Additionally, the payments
are designed to limit the costs. An overall cap on the aggregate payment is applied to the Medicare hospice
reimbursements along with caps on inpatient cap to limit the number of inpatient days.
19
Hospice care services has proven to be cost effective compared to the care acquired in a hospital during
the patient’s last 180 days of survival, with the per diem expenditure in the inpatient setting far exceeding
that for palliative/hospice care.
20
A cost-effectiveness study was conducted to evaluate the cost savings
due to palliative inpatient admissions. The results of this study showed it to be more cost effective than
the standard/usual inpatient care service in the hospital.
21
A retrospective data analysis of Medicare claims
data demonstrated that beneficiaries enrolled in hospice 53–105 days before death saved $2,561 compared
to a matched, nonhospice control population ($22,083 vs $26,466 p<0.01).
22
II. Medication use in hospice setting
Beneficiaries covered under Medicare Hospice Benefit receive treatment to address symptoms, maximize
comfort and improve quality of life. Under Medicare Part A, beneficiaries have access to only those drugs
that are used for pain relief and other terminal illness conditions (inclusive of biologics which have
palliative roles). Hospices utilize formularies, wherein all commonly used drugs for palliation and
terminal illness management are included.
a. Policy changes with Medicare billing
There are instances when beneficiaries require medications not on the hospice formulary and/or covered
by Part A. In this case, previous Medicare guidelines allowed inappropriate payments for these
medications required to treat hospice related symptoms under the Medicare Part D benefit. However, in
2013, policy was changed to prevent these inappropriate payments. The letter stated that drugs and
biologics when used primarily for the relief of pain and symptom control related to the terminal condition
will be covered under the Medicare Part A per-diem payment. The medication will be covered under Part
D only when it is not related to the patient’s terminal illness.
23
5
III. General role of pharmacists
Pharmacists are highly educated with regards to medications. They conduct comprehensive medical
history reviews and perform medication reconciliation. Special attention is given to pharmacotherapy
history, symptom assessment, and identification of drug-related problems. Pharmacists also provide
patient counseling where they can identify inadequate treatment response or treatment-related adverse
events.
24
In 2010, CMS published a certification process manual for hospice providers, recommending
that each hospice care facility have a clinical pharmacist. The functions carried out by this pharmacist
may include educating and training patients regarding drug management and assisting patients in
treatment selection. They can also conduct outcome assessment for ensuring the quality of the service
provided. They play a significant role in managing adverse effects and proving recommendation wherever
necessary.
25
The 2000 American Society Health System Pharmacist guidelines detailed pharmacist responsibilities and
their scope of practice in contributing to the hospice care.
26
The listed roles of pharmacist included: (1)
symptom management, (2) counseling and education of staff and family members, (3) ensuring adherence
to the drug, (4) addressing financial concerns of the patients and (5) disposal of medication after patient’s
death. Further, ASHP published an updated report exploring extended involvement of the pharmacist in
hospice and palliative care (PHC)
27
. It describes PHC services in two parts, including essential and
desirable services. Roles and responsibilities for essential services include: (1) direct patient care, (2)
medical review and reconciliation, (3) education and medication counseling and (4) administrative roles.
Desirable services include: (1) direct patient care, (2) education, (3) scholarship and (4) administrative
roles.
IV. Pain management within hospice/palliative care
Pain is a multidimensional experience of emotional and physical dimensions. The concept of total pain
for terminally ill patients is made up of four components, including: (1) physical noxious stimuli, (2)
emotional discomfort, (3) interpersonal conflicts, and (4) nonacceptance of one’s own dying.
28
The
ultimate key of pain management in end-of-life care is pain assessment. They focus on not only treating
physical pain but also the emotional and interpersonal pain of the patient. The objective of end of life care
is assisting the patient with pain reduction interventions and improving their functioning abilities as much
as possible. There are organizations such as National Hospice and Palliative Care Organization (NHPCO),
American Academy of Hospice and Palliative Medicine (AAHMA) , Center to Advance palliative Care

6
(CAPC) and others which publish guidelines/reports which can be useful for delivering hospice and
palliative care.
29,30
a. Clinical guidelines and outcomes of interest
Pain is classified in an unstructured manner despite being a common ailment among patients. A task force
on taxonomy initiated by International Association for Study of Pain (IASP) provides a detailed
classification of chronic pain.
31
It classifies chronic pain in two types; chronic primary pain and secondary
pain. Chronic primary pain is characterized by disability or emotional stress, a more “nonspecific” pain.
International Classification of Diseases 11
th
revision, “defines the universe of diseases, disorders, injuries
and other related health conditions, listed in a comprehensive, hierarchical fashion”.
32
Chronic secondary
pain is more specific pain represented by ICD-11. Additionally, WHO guidelines on “Palliative care:
symptom management and end of life care” explains pain management in detail.
33
It recommends
conducting pain assessment, assigning treatment based on the assessment and later on managing the
symptoms due the treatments assigned. In 2017, the National Coalition for Hospice and Palliative Care
published the ‘Clinical Practice Guidelines for Quality Palliative Care, 4th edition,’ aimed to improve
palliative care access to patients with serious illness by guiding the healthcare organizations across to
integrate principles in their routine assessment and delivery of quality care.
29
b. Medications used in pain management
Pain management in an end-of-life care setting begins with determining the patient’s pain type.
Assessment can be in terms of location of pain (visceral, somatic, neuropathic or nociceptive) or intensity
of pain.
34
Determination of the type of pain assists providers in assigning appropriate pharmacotherapy.
WHO’s Pain Ladder provides guidelines for achieving pain management in cancer patients as well as
patient with chronic and acute nonmalignant pain. The first line agents are non-opioids drugs such as
acetaminophen and/or NSAIDS, used to treat mild type of pain. For treating moderate pain, the guidelines
include administering an opioid such as hydrocodone or oxycodone along with or without
acetaminophen/NSAID.
35
Patients with severe pain are advised to use stronger opioid therapies, due to
their analgesic effect.
36
Some of the common opioid used in hospice care include morphine,
buprenorphine, fentanyl, etc. An essential medication list for palliative care includes ibuprofen and
morphine as a treatment for pharmacological pain management.
37
c. Utilization of methadone in hospice care
Traditionally, methadone, a synthetic opioid, has been used for treating opioid dependence, but it also has
significant utility in the treatment of chronic pain, where it has clinical and cost benefits over other opioids
7
which are used for pain management.
34,38
Methadone is considered to have a high bioavailability, long
duration of action and is available in multiple dosage forms (oral, rectal, parenteral).
39
It also provides an
option for treatment in patients with morphine allergy. Furthermore, it acts as a N-methyl-D-aspartate
(NMDA) receptor antagonist, which provides utility in the treatment of neuropathic pain and a reduced
propensity to develop opioid tolerance.
40,41,42
Methadone is a high-risk medication, which makes it important for its administration and use to be
monitored closely by a pharmacist. The medication can cause several important adverse effects, including
QTc interval prolongation, respiratory depression, and drug accumulation. The long half-life of the drug
is the consequence of accumulation in the body relative to amount of drug eliminated from the body.
43
The American Pain Society (APS) and the College on Problems of Drug Dependence (CPDD), in
collaboration with the Heart Rhythm Society (HRS), consulted an interdisciplinary panel to develop a
clinical practice guideline on safer prescribing of methadone for treatment of opioid addiction and chronic
pain. The guidelines recommend careful assessment and selection of patient prior to administering
methadone, inclusive of patient education/counseling and a baseline ECG assessment.
44,45
d. Role of pharmacists specifically in pain management
Pharmacists are a great source of timely advice to patients. They are available without appointments and
at convenient locations to discuss any patient’s onset of pain episodes.
46
A pharmacist-managed pain clinic
resulted in a decrease in waiting for appointments and elimination of unscheduled visits for narcotic
prescription. Their involvement resulted in close monitoring of pharmacotherapy, adverse events, and
medication dosages. Pharmacists helped in facilitating communication between pain clinic staff,
pharmacy department and physicians.
47
A systematic review and meta-analysis analyzed pharmacist role
in chronic pain management at community and hospital settings. The identified roles included conducting
medication reviews, specialized prescription delivery service, face to face consultation and providing
recommendations to the physicians. These resulted in reduced pain intensity, improvement of physical
functioning and patient satisfaction.
48
V. DeltaCareRx
DeltaCareRx is a locally based pharmacy services organization that works with hospice and palliative care
providers across the US. Duquesne University has a collaboration with the organization through Dr Mary
Mihalyo, a clinical pharmacist faculty member in the School of Pharmacy.
8
a. Description of the organization
DeltaCareRx is a pharmacist-founded, pharmacist-owned, and pharmacist-operated pharmacy benefit
management (PBM) company that works exclusively with and for hospice and palliative care providers,
primarily community-based, not-for-profit hospice and palliative care organizations. Pharmaceutical care
is provided through their mail order pharmacy and a nationwide network of highly regarded local retail
pharmacies. DeltaCareRx has a mission to transform and improve the hospice pharmacy industry through
business transparency, innovation, consistent customer service and community pharmacy relationships.
The transparency is maintained through the creation of pricing models and innovative technologies
designed to support clinicians. Furthermore, the organization is known for delivering quality,
compassionate, cost-effective pharmaceutical care for patients with a life-limiting diagnosis.
49
b. Services provided to hospices
DeltaCareRx provides local PBMs services to hospices. It facilitates medication availability from local
pharmacies and optimizes cost-effectiveness. Additionally, they provide a facility known as inpatient
innovation™ which is a Delta Care pharmacy installed in an inpatient unit. Mail order delivery is provided
to the patient’s house which is useful in case of medical crisis. They provide patient monitoring through
their branch of service known as ADAPT (remote patient monitoring and, pharmaceutical care at home).
It is created for remote patient monitoring and proving pharmaceutical care at home. ADAPT delivers
therapeutic expertise, prescription dispensing options and technological innovation to improve clinical
outcomes and effective cost control mechanisms. Additionally, report generation is made easy through
their innovative web-based technology Deltalytics. These reports assist in understanding trends in
prescription, matching benchmarking values and quality initiatives. They provide their clients with newest
technology, education support and cost containment strategies.
VI. Problem statement
Pharmacists play an important role in hospice and palliative care. Their involvement in pain management
has demonstrated desired clinical and cost outcomes. Despite many advantages of pharmacist’s
involvement and recommendations by ASHP, many hospice and palliative care organizations remain
reluctant to include pharmacist in their multidisciplinary team. The practical demonstration of importance
of a pharmacist recommendation and their role will make the idea of adding a pharmacist to the team
stronger. Therefore, it is necessary to assess their involvement in the daily routine of hospice and palliative
care. Their involvement can be assessed through the recommendation they provide to the patients
9
suffering with pain. Methadone is an effective medication to treat chronic pain and generates cost savings.
Still it is found to be underutilized in hospice and palliative care settings. Therefore, it is necessary to
understand whether pharmacist recommend this drug for pain management. If the provided
recommendations have not been accepted by the physicians without a reason, it would demonstrate a
gap in knowledge transfer regarding the appropriate use of the medication. One of the opportunities to
understand the trend in use of methadone at hospice and palliative care sites is through Deltalytics. It is a
web- based report generator innovated by DeltaCareRx. The reports generated contains the utilization
value of each medication for each one of the DeltaCareRx’s clients. These values are then compared to
the industry benchmarking. This activity decreases unnecessary utilization of medication and encourages
cost saving. Analyzing the reports would assist in understanding the pattern in use of methadone and
other medications post the Medicare policy changes in 2013. Additionally, it will encourage the use of
cost-effective drugs at the client sites.
VII. Study objectives/aims
Therefore, to further understand the importance and impact of pharmacist services in hospice/palliative
care, the specific aims of the studies are as follows:
Identify the impact of clinical pharmacist recommendations for methadone upon admission to
hospice/palliative care services
Evaluate differences in medication utilization across different hospice settings served by
DeltaCareRx
10
CHAPTER 2: LITERATURE REVIEW
I. Rationale
ASHP guidelines describe the expansion of the clinical pharmacist roles in hospice and palliative care
service.
27
Clinical pharmacists play different roles in patient counseling, optimizing medication use, and
recommending or terminating medications in the provision of care.
26,50
The inclusion of a pharmacist in
the hospice/palliative care has potential to improve clinical outcomes and demonstrate cost savings.
51
Pharmacists are involved in direct contact of care in pain management given their accessibility compared
to other healthcare professionals. Clinical pharmacists can conduct clinical pain assessments before and
after administration of the treatment. They also can play an important role while selecting and monitoring
appropriate therapeutic regimens for patients. Valgus et al. evaluated the impact of a pharmacist-led,
interdisciplinary team intervention on cancer patients in an ambulatory cancer clinic setting. The
intervention was found to improve symptom scores and use of medications in these patients.
52
A
pharmacist is also an integral part of educating/training other staff members and family members. A
systematic review and meta-analysis conducted by Benneth et al. reported the positive impact of
pharmacist-led educational interventions on chronic pain patients.
53
As per the ASHP guidelines, roles
11
and responsibilities of a pharmacist in a hospice and palliative care multidisciplinary team can assist in
improving patient’s quality of life.
27
There are many studies that have provided information on roles of a pharmacist in hospice and palliative
care settings. However, the specific impact of clinical pharmacists in pain management in these settings
has not been fully evaluated. Accordingly, it is necessary to identify the impact of pharmacist-led
interventions or pharmacist involvement on clinical/cost outcomes in the treatment of pain in hospice and
palliative care.
II. Objective
The objective of the systematic review was to: (1) identify available information on the extent of clinical
pharmacist involvement in the pain management of hospice and palliative care patients, and (2) explore
relevant roles of a pharmacist in achieving clinical/cost outcomes while participating in pain management
of end-of-life care patients.
III. Search strategy
The systemic review was conducted using the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines.
54
The articles were retrieved/assessed on three databases: PubMed,
Embase and Scopus. The first search was done on PubMed using keywords including ‘pharmacist,’
‘hospice care,’ ‘palliative care,’ ‘pain’ and ‘pain management.’ The search strategy from PubMed was
then modified to suit Embase and Scopus search strategies. The keywords utilized in all the three databases
are included below.
a. PubMed
((("Pharmacists"[Mesh] OR Pharmacist*[tiab] OR Pharmacist*[ot] OR "Pharmacy Service,
Hospital"[Mesh] OR "Pharmacy Service”[tiab] OR "Pharmacy Service”[ot] OR "Pharmacist service"[ot]
OR "Pharmacist service"[tiab])
AND
("Pain"[Mesh] OR "Pain"[ot] OR "Pain"[tiab] OR "Pain Management"[Mesh] OR "Pain
Management"[tiab] OR "Pain Management"[ot] OR “Hospice and palliative Care Nursing”[MeSH] OR
“Analgesics”[Mesh] OR Analgesic*[tiab] OR Analgesic*[ot])
AND
12
("Hospice Care"[Mesh] OR "Respite Care"[Mesh] OR "Respite Care"[ot] OR "Respite Care"[tiab] OR
"Home Care Services"[Mesh] OR "Home Care Services"[ot] OR "Home Care Services"[tiab] OR
"Home Care Service"[ot] OR "Palliative Care"[Mesh] OR "Palliative"[tiab] OR "Palliative"[ot] OR
"supportive care"[tiab] OR "supportive care"[ot] OR "home hospice"[ot] OR "home hospice"[tiab] OR
“Hospices”[Mesh] OR Hospice*[ot] OR Hospice*[tiab] OR “Bereavement care”[ot] OR “Bereavement
care”[tiab])))
b. Embase
('pharmacist'/exp OR 'pharmacist*’ OR 'hospital pharmacy'/exp OR 'hospital pharmac*’ OR 'pharmacist
intervention'/exp)
AND
('analgesia'/exp OR 'analgesi*' OR 'pain management index'/exp OR 'pain'/exp OR 'pain*' OR 'palliative
nursing'/exp OR 'palliative nursing')
AND
('hospice care'/exp OR 'hospice care' OR 'hospice'/exp OR 'hospice*' OR 'palliative therapy'/exp OR
'palliative therapy' OR 'terminal care'/exp OR 'terminal care' OR 'respite care'/exp OR 'respite care' OR
'home care'/exp OR 'home care' OR 'supportive care'/exp OR 'supportive care' OR 'bereavement
care'/exp OR 'bereavement care')
c. Scopus
(INDEXTERMS(Pharmacist*) OR TITLE-ABS-KEY(Pharmacist*) OR INDEXTERMS(“Pharmacy
Service, Hospital”) OR INDEXTERMS(“hospital pharmacy”) OR INDEXTERMS (“pharmacist
interventions”) OR TITLE-ABS-KEY(“Pharmacy Service”) OR TITLE-ABS-KEY(“Pharmacist
service”))
AND
(INDEXTERMS(“Pain”) OR TITLE-ABS-KEY(“Pain”) OR INDEXTERMS(“Pain Management”) OR
TITLE-ABS-KEY(“Pain Management”) OR INDEXTERMS(“Hospice and palliative Care Nursing”)
OR INDEXTERMS(“Analgesics”) OR TITLE-ABS-KEY(Analgesic*) OR INDEXTERMS(analgesia)
OR INDEXTERMS(“pain management index”) OR INDEXTERMS(“palliative nursing”))
AND
(INDEXTERMS (“Home Care Services”) OR INDEXTERMS(“bereavement care”) OR
INDEXTERMS(“Hospice Care”) OR INDEXTERMS(“Hospices”) OR INDEXTERMS(“Palliative
Care”) OR INDEXTERMS(“palliative therapy”) OR INDEXTERMS(“Respite Care”) OR
13
INDEXTERMS(“supportive care”) OR INDEXTERMS(“terminal care”) OR TITLE-ABS-KEY
(“Bereavement care”) OR TITLE-ABS-KEY (“home hospice”) OR TITLE-ABS-KEY (“Hospices”) OR
TITLE-ABS-KEY(“Home Care Services”) OR TITLE-ABS-KEY(“Palliative Care”) OR TITLE-ABS-
KEY(“Respite Care”) OR TITLE-ABS-KEY(“supportive care”))
IV. Inclusion/exclusion criteria
All studies published in English between January 2010 to January 2019 were evaluated in the systematic
review. Included articles described studies that evaluated the treatment for pain (chronic, cancer,
neuralgia, visceral etc.) in the hospice and/or palliative care setting (e.g. routine home, continuous home,
general inpatient, respite care). Furthermore, the studies were required to detail the pharmacist role in
hospice/palliative care pain management, either alone or as a part of a multidisciplinary team. Finally, the
study was required to report some form of measurable outcome, either from a clinical or cost perspective,
such as recommendations, modifications to medication dosage, reduction in adverse events, value of
pharmacist, etc. Articles focused on pediatric hospice/palliative care were excluded from the study, and
medical conditions other than pain for which outcomes were evaluated were not discussed.
V. Data extraction
The compiled included citations were imported in a reference manager, EndNote X8 (Clarivate Analytics;
Philadelphia, PA). There were total 702 articles which were scanned for duplicates. A total of 515 articles
remained after removal of the duplicates, carried forward for title/abstract screening and full text review
in a systematic review manager, Covidence (Melbourne, Australia). The full text for the articles was
searched and downloaded online; in case of non-availability, they were requested through the Duquesne
University interlibrary loan service. One reviewer was involved in assessing the eligibility of the articles
throughout the systematic review. In case of any ambiguity, it was resolved through discussion between
thesis advisor and the reviewer.
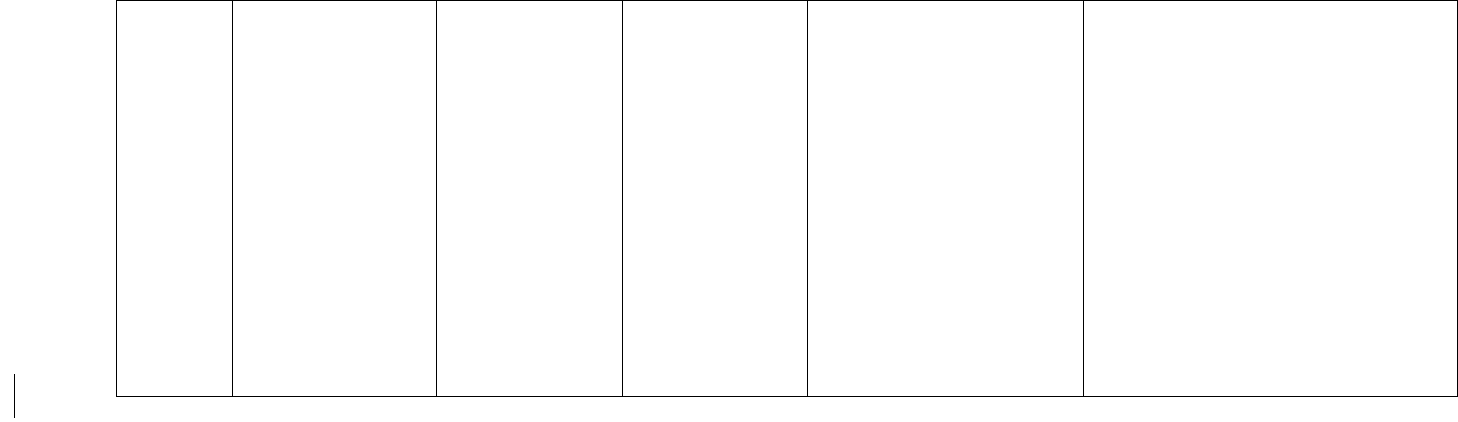
The PRISMA diagram showing the search strategy is shown in Figure 1.

14
Figure 1. PRISMA chart
Further definitions of reasons for exclusion: (1) pharmacist role not specified: study did not include pharmacist
involvement in pain management, (2) outcomes differ from the review: study did not detail measurable
clinical/economic outcomes, (3) non-research studies: descriptive analysis only without research intervention, (4) not
found: full texts were not available via online/inter-library loan, (5) duplicates: articles previously failed to be excluded
in duplicate removal procedure, (6) reports: official announcements
Records identified through searching PubMed,
Embase and Scopus
(n = 529)
Screening
Included
Eligibility
Identification
Records after duplicates removed
(n = 515)
Records excluded
(n = 346)
Full-text articles assessed
for eligibility
(n = 169)
Full-text articles excluded (n = 156)
41 pharmacist role not specified
36 outcomes differ from review aim
25 abstracts from symposium/conferences
13 non-research studies
11 non-hospice setting
10 not found
6 non-pain-oriented intervention
4 non-English
4 duplicates
3 results not mentioned in the article
2 reports
1 pediatric sample
Studies included in
qualitative synthesis
(n = 13)
Records screened
(n = 515)
15
VI. Results
A total of 169 full texts were scanned for inclusion, with Figure 1 describing the reasons for exclusion.
Finally, a total of 13 articles were finalized for qualitative synthesis. Of all these articles, six were
conducted in the US,
51,52,55-58
two in Japan
59,60
and other countries (one each in China,
61
Poland,
62
UK,
63
Qatar,
64
and Korea
65
).
VII. Extraction
Table 1 gives an overview of included studies, regarding the setting, pharmacist role, outcomes
assessment, study sample, key findings, and limitations. Out of the 13 studies, seven (54%) prospectively
evaluated the impact of a clinical pharmacist on patient pain control. Four studies (31%) retrospectively
evaluated a pharmacist-led intervention in hospice and palliative care and two studies (15%) conducted
survey research among pharmacist/hospital staff to understand pharmacist’s contribution to hospice and
palliative care.

17
Table 1. Extraction results of the studies
Author
Year
Country
Study Aim Study Design Patient/study sample Pharmacist role Res ults
Atayee
56
2018
(US)
Describe an inpatient
palliative care
pharmacist’s
interventions and
outcomes; Evaluate the
impact on length of stay
(LOS), length from
admission to palliative
care consult (LTC), and
time from consult to
discharge and death
(CTD).
Retrospective study of
patients under part-
time palliative care
clinical pharmacist
care as part of a
consultation team
Hospitalized patients
seen by pharmacist
September 1, 2015,
and March 30, 2017.
(1) guiding the transdisciplinary
team on medication selection,
dosing adjustments and titrations,
(2) educating on medications,
importance of adherence,
symptoms, (3) recommending
changes to medication orders, labs,
and diagnostic testing, (4) serving
as liaison between the palliative
care team and the department of
pharmacy, (5) providing home
medication supply at discharge,
and (6) follow up communication
with the outpatient palliative care
team.
Pharmacist invo lvement resulted in a significant
difference in pain consultation and days from
consult to discharge VS to the patients seen by
the palliative are team. In total, patients received
an average of 3.5 interventions and 4.1
documented outcomes. Most common
interventions and outcomes: optimized symptom
drug regimen (92.75%), education of
patient/provider (90%) and change in med
therapy implemented (90%), healthcare
professionals educated (84.5%). There was a
significant difference between patients seen by
pharmacist VS palliative team for: consultation
of pain (80.9% vs 39.4%, p<0.005).
Comparison based on pharmacist visit time
within 3 days of hospitalization VS 3 or more
days after hospitalization: LOS (10 VS 25,
p<0.005), LTC (3.79 VS 9.48, p<0.05), and
CTD (6.1 VS 14.59, p<0.005).

18
Chen
61
2014
(China)
Compare the
effectiveness of opioid
treatment between
cancer patients receiving
interventions from
Clinical Pharmacist Led
Guidance team (CPGTs)
and a comparable control
group.
Prospective,
multicenter, double
armed controlled
cohort study
18 years or older,
diagnosed with cancer
pain by an oncologist,
and able to receive
opioid treatment for
more than two weeks.
Patients previously
treated with opioids
were also included
(1) physician and patient
education, drug-use monitoring,
evaluation of drug responses, (2)
consultation in cases of pain or in
case of complications (without
prescribing ), (3) monitoring drug
efficacy and toxicity (follow-up)
Outcomes for standardization of opioid
administration broadly improved through the use
of the CPGT intervention, including more
frequent pain assessments before therapy
(OR:3.39 [2.78-4.14]), dose titrations before SR
formulations (OR: 8.12 [6.34-10.78]) and
dosage increases (OR: 9.67 [8.11-11.02]). Fewer
inappropriate prescriptions and conversions
were utilized, while SR formulation use
increased. CPGT resulted in better pain control
(scale 1-10) by site (bone [3.1 vs. 4.2, P=0.038],
body [1.2 vs. 3.6, P=0.041], visceral [1.9 vs. 3.1,
P=0.024), nerve [2.7 vs. 4.8, P=0.045]) and
improved QOL (48.3 vs. 37.6, P=0.032, scale 0-
60). Adverse events were significantly reduced
in the CPGT group for constipation, nausea, and
vomiting.
Edwards
63
2019
(UK)
Determine whether
medicines consultations
for patients with
advanced cancer pain are
feasible and acceptable.
Prospective,
multicenter study.
Patients with cancer
received consultation
regarding medication
use from the
pharmacist along with
baseline and post
consultation
questionnaires.
Patients with
advanced cancer pain
between November
2015 and March 2017
aged 16 years or
older, aware of their
diagnosis, on a
prescribed opioid, not
on any anticipatory
medicines for end-of-
life care and with
capacity to provide
informed consent and
complete
questionnaires.
Provide medicine consultation and
recommendations to the patients,
identify drug-related problems,
and provide intervention
A mean of 2.5 drug-related problems per patient
were identified, most commonly including
effects of drug not optimal (n=25) and unclear
problem/complaints (n=7). Lack of information
(n=15) and non-adherence (n=16) were the main
causes reported. Intervention provided for most
of DRPs and their causes was patient
counselling (n=35). The intervention has a
positive impact on the mean pain score pre vs
post consultation (4.1 vs 4.0).

19
Geum
65
2019
(Korea)
Evaluate the impact on
pain management by
multidisciplinary
palliative care team
(mPCT) and the team
pharmacist.
Retrospective analysis
of the medical chart
review. Patient
reported pain intensity
was recorded three
times: (1) seven days
before palliative care
unit (PCU) admission
(day -7), (2) on the
day of admission (day
0), and (3) seven days
after admission (day
7)
18 years or older,
hospitalized for 7+
days between April
2014 and December
2015, after being
transferred from the
wards, emergency
center, or outpatient
clinics due to
worsening of
oncologic pain.
(1) recommending medications
and evaluating analgesics, (2)
validation and intervention of
analgesic prescriptions based on
the type and severity of pain, dose,
routes, and schedule, (3)
assessment of contraindications,
drug interactions, and adverse
effects, (4) patient counseling for
nonadherent patients, (5)
educating staff on evidence-based
treatment with new analgesics
Mean pain intensity and appropriate use of
analgesic improved gradually for patients
admitted in the PCU with the mPCT.
Appropriate analgesic use was higher when
compared to patients who were taken care by
mPCT (35.04% on day -7, 34.19% on day 0 and
75.21% on day 7) (P<0.001). Appropriate opioid
use was 76.9% on day 7, 35.9% on day 0 and
35.9 on day -7 (P<0.001) and mean pain
intensity score was 2.66 on day 7 of PCU and
4.05, 3.16 on day 0 and day -7, respectively.
Decrease in inappropriate use of opioid was
observed on PCU admissions. As per the Korean
Cancer pain management guidelines,
appropriateness of analgesic doses (for chronic
pain: 87.2%, 80.3%, and 95.7% on day 7, day 0,
and day 7, respectively; P= 003; for
breakthrough pain: 88.9%, 88.9%, and 96.6% on
day 7, day 0, and day 7, respectively; P=0.049)
and the rate of reassessment of each patient’s
pain to adjust the medication for breakthrough
pain (63.2%, 68.4%, 91.5% on day 7, day 0, day
7, P<0.001) both significantly improved over
time.

20
Ise
60
2014
(Japan)
Examine the clinical,
educational and research
activities of pharmacist
in a palliative care team,
their perceived
contribution to the team
or why they do not
contribute.
Multicenter,
prospective study
using questionnaires
mailed to pharmacists
in cancer hospitals
across the country
Pharmacists working
in the palliative care
for cancer patients
from November 2012
to January 2013.
(1) ward rounds, counselling
patients, managing adverse drug
effects, drug interactions,
strategies for titration and rotation
of drugs, provided
information/suggestions about the
efficacy, adverse effects, and
interactions of drugs used to
alleviate symptoms, informed the
primary pharmacists about patient
pharmacotherapy requests, (2)
education and research activity of
palliative care: organizing
conferences, presenting research
work.
Clinical activity provided by pharmacist were
direct counselling of the patients regarding
opioids (29%) and adverse effects due to opioids
(19%). As a part of the palliative care team they
provided suggestions to the team regarding
managing adverse effects of opioids (35%),
rotation of opioids (34%), pharmacology of
opioids (34%), drug interaction of opioids (33%)
and managing adverse effects of opioids (21%),
pharmaceutical production of opioids (21%).
Pharmacist are most commonly involved in
providing suggestion to team’s primary
pharmacist sometimes (35%) and often/always
(24%), 70% pharmacist agreed on some level of
contribution to the palliative care team, 16%
reported they could not contribute and main
perceived reasons for no contribution were
insufficient time (90%) and/or staff (68%).
Ma
58
2016
(US)
Evaluate pharmacist
interventions and patient
outcomes of a
pharmacist-led
outpatient palliative care
practice.
Single-center,
retrospective analysis
of medical records
conducted at cancer
center with a
transdisciplinary
clinic with two
pharmacists.
18 years or older, with
a diagnosis of cancer
between March 2011-
2012.
(1) assess, initiate, stop, and or
adjust therapy for the management
of pain, nausea/vomiting, under
physician direction, (2) optimize
medication therapy, (3) compose
clinical encounter and documented
recommendations for therapy in
the electronic medical record, (4)
schedule follow-up visits to
monitor symptoms and medication
use.
Patients with severe pain (48%) showed gradual
decrease in pain over the four visits. More
patients (64%) were found in the stable pain
state by the end of the four visits. Pain
medication problems identified by the
pharmacist included lack of efficacy, nausea,
vomiting decreased with increase number of
patient visits to the setting. Majority of patients
(61%) were assigned a change in the opioid
dosage as an intervention.

21
Mancini
55
2012
(US)
Describe the operational
aspects of
multidisciplinary
supportive oncology
clinic.
Prospective,
multicenter study of
pharmacist
assessments as part of
the clinic regarding
drug interaction,
duplication in therapy,
lack of efficacy and
untreated condition.
Oncology patients
referred to the clinic
for early palliative
care based on
National
Comprehensive
Cancer Network
(NCCN) guidelines.
Prior to visit: evaluation of
medication list; check for drug
interaction; assess for duplication
therapy; form a patient-friendly
medication list
On the day of visit: reviewing
patient's medication containers;
assess for drug interaction, adverse
effects, adverse effects and
untreated conditions; provide
recommendations and consultation
service
After visit: provides
recommendations to team
regarding medication changes,
provides updated medication list ,
fill out the assessment, providing
consultation
The results of the assessment were reported as
follows: (1) Access to medication: higher cost
(53.5%), transportation issues (20%), lack
healthcare access (32%), (2) adherence to
medication: missing at least one dose (62.7%),
(3) medication therapy review: most common
problems were duplication of therapy (46.7%)
for breakthrough of sleep (25.6%) and pain
(20.5%), drug interaction (44%) with the
majority due to warfarin (24.3%) and
metoclopramide (21.6%), side effects (74.7%)
with most common being constipation (27.9%),
lack of efficiency of drugs (94.7%) mostly the
drug used for pain (31.9%), and untreated
conditions (73.3%) such as fatigue (25.5%) and
constipation (12.7%). Positive feedback was
acquired from the patient for involvement of a
pharmacist in their pain management.
22
Naidu
57
2018
(US)
Evaluate pharmacist-
initiated interventions
and validate the
pharmacist’s role on a
transdisciplinary
palliative care team at a
community hospital.
Single center,
retrospective analysis
of medical records
related to patient
interactions with a
palliative care
pharmacist.
Patients who had a
palliative care consult
order and a
pharmacist-generated
clinical note in the
medical record
between November 1,
2013 and October 31,
2014.
(1) participate in palliative care
team rounds three times each
week; (2) contribute to
management plan for all patients
with symptom issues; (3)
providing medication education to
patients, families, and staff; (4)
coordinating interventions for
pharmacy-related issues with
discharge planners and physicians;
(5) participating in family
meetings with physicians and
other palliative care team
members; (6) initiate and adjust
opioid doses, including transitions
from parenteral to oral agents, and
participate in pain and dyspnea
management for end of life care
patients.
Pharmacist intervention resulted in reduction
(4.6 to 2.0) of pain score in acute and chronic
pain suffers (5.7 to 2.5 points). Patients with
interventions for moderate to severe signs of
symptoms showed improvement in their
condition; nausea 42/44 (95.4%), dyspnea 82/92
(89%) and anxiety 39/45 (86%), Pharmacist
participated in family meeting (n=142),
completed a total of 58 advance care directories
and forms. A considerable cost saving was
observed through direct cost reduction of
$100,000 due to treatment discontinuation
initiated by the palliative care pharmacist.

23
Pawłowska
62
2015
(Poland)
To provide an overview
of the current state of
pharmacy practice at
Polish residential
hospices.
Cross-sectional survey
with three types of
questionnaires
addressed to
pharmacists, hospice
directors and hospice
physicians.
Pharmacists, hospice
directors and hospice
physicians at 93
residential hospices
identified through a
web database in 2012.
(1) the most common service
provided by the pharmacists was
providing advices on drugs and
medical devices (75%) followed
by various other responsibilities
such as dispensing of drugs and
medical devices, co-participation
in therapy management,
participating in rationalizing of
drug therapy and monitoring
adverse reactions, (2) as per the
hospice directors expected role of
pharmacist was participation in
clinical trials and training hospice
care staff, preparing sterile drug
formulations, preparing enteral
feeding solutions and
compounding drugs, (3) other
roles such as advise members of
the therapeutic team, providing
opinions to physicians, advise on
pharmacotherapy choices.
Ten (63%) pharmacists estimated their
involvement in this service at a level of 100%.
The hospice directors and physicians indicated
the necessity for including the pharmacist within
the therapeutic team more frequently than
respondents employed at hospices where there
was no pharmacist contribution (p=0.02480 and
0.003, respectively). There was no statistically
significant difference between the opinions of in
the three groups of respondents regarding the
benefits associated with providing
pharmaceutical services at a hospice except for
better selection of drugs for individual patients
was indicated more often by hospice
pharmacists than hospice directors (p=0.03) and
physicians (p=0.02). Majority of the opinions
regarding benefits employing pharmacist in
hospice care were improved access, decrease
cost of the pharmacotherapy, and proper drug
storage.

24
Richter
51
2018
(US)
Investigate clinical and
financial impacts of
adding a clinical
pharmacist to the
hospice care team
Prospective, single-
center study. A
clinical pharmacist
was added to the
interdisciplinary
group (IDG)
Hospice care patients,
comparing 2016 (pre)
and 2017 (post) data
(1) attend IDG meetings, (2)
formulary management and
adherence for hospice patients, (3)
chair for the P&T committee, (4)
prevention of controlled substance
diversion, (5) education of clinical
staff, (6) general drug reference
for physicians and nurses, (7)
consultation, (8) present emerging
trends in drug therapy for hospice
patients.
Financial impact: As per the time spent in the
IDG meeting and its preparation, value of
pharmacist was about $138 per hour. Average
PPD drug cost decreased from $5.44 to $4.07,
resulting in direct drug cost saving of $329,797
from baseline and cost benefit per intervention
per month of $72.52. Total cost saving was
$427,705 including indirect cost saving
($15,750) and outside consultant pharmacist
($60,000).
Clinical impact: Major impacts in reducing
unnecessary medications on the patient's
medication list, improving medication use
during drug shortages and eliminating medicine
increasing fall risk in patients.
Valgus
52
2010
(US)
Describe a pharmacist-
led, interdisciplinary
method of care delivery
begun at the University
of North Carolina;
describe the
characteristics of the
population seen and the
role of the individual
members of the
interdisciplinary team,
and provide an early
analysis of the program’s
impact on symptom
improvement
Prospective analysis
and retrospective
medical chart review
for studying the
impact of a
pharmacist led multi-
disciplinary team for
supportive care in
cancer patients
Adult cancer clinics:
radiation, surgical,
gynecologic,
hematology, and
medical
Clinical pharmacist practitioner
approved to provide drug therapy
management under physician
direction. Two delivery models:
(1) consult service, with care
provided at clinic where patient
already seen, and (2) structured
visits at separate clinic with initial
assessment (cognitive/medication)
by pharmacist. Encounters studied
included consultation by
nurse/pharmacist (28.6%) or
nurse/pharmacist/physician
(22.7%); 10.3% included
pharmacist consultation alone
Across first 18 months of service, patient
volume and encounters increased from 4.5 to 6.6
per month, and 13 to 20 per month, respectively.
Among a subset of 54 patients assessed on pain
medication, encounters with the service resulted
in 40% of patients receiving an increased dose,
23% receiving a new medication, 15% switched
to another opioid, and 15% with no change;
methadone was the most common
addition/switch. Among a subset of 49 patients
assessed, reductions in symptom scores for pain,
nausea and constipation decreased and
maintained across three visits.

25
Wilby
64
2014
(Qatar)
Create a baseline
inventory of clinical
pharmacy interventions
after establishment of an
academic cross-
appointment in palliative
care and to assess the
perceived importance of
interventions made.
Prospective, single-
center characterization
study. Data collected
included: (1) number
of patients admitted to
palliative care while
study pharmacists
were on service, (2)
actual or potential
drug therapy problem,
(3) clin ical pharmacist
intervention for
resolution of
identified drug
therapy problem, and
(4) acceptance by the
prescriber, if
applicable. Responses
of an online survey
from the pharmacist in
Qatar and Canada
were compared for
assessing importance
of each type of
recommendation.
Palliative care service
between September 1,
2013 and December 1,
2013.
Additional data was
also collected via
pharmacist survey.
(1) identifying actual or potential
drug therapy problem (2)
assignment of an
intervention/recommendation for
the identified problem (3) ranking
of the perceived importance of
each of the recommendation given
by study pharmacists
32 patients were seen (the average intervention
rate of 3.0 per intervention per patient ). On
removal of education-related interventions, 81%
of pharmacist's recommendations were accepted
by physicians. Discontinuation of drug therapy
(29%) and initiation of drug therapy (25%)
recommendations were most common while
referral to other professionals (2%) was least
common. A significant difference existed
between overall rankings for each question
between pharmacists in Canada and Qatar
(p<0.05). Initiation of drug therapy (10)
(p=0.955), discounting of drug therapy (10)
(p=0.758) and, physician/nurse education (10)
(p=0.918) were among highly rated
interventions/recommendations among the
pharmacists from Qatar and Canada.

26
Yamada
59
2018
(Japan)
Evaluate the effect of
continuous interventions
for pain management
and opioid-induced side
effects in outpatients
with cancer.
Single-center,
prospective study.
Four pharmacist
interviewed patients
from the first visit for
opioid introduction to
interventions via
telephone to assess
pain patterns, doses,
side effects, and
recommendation
acceptance rates.
Outpatients
administered opioid
treatments for cancer
pain relief and who
received pharmacist
interventions from
October 2014 to
March 2016.
(1) introduction to opioids at the
first visit, (2) interventions through
telephonic interviews between 3-7
days of first visit, (3) daily patient
counseling, (4) training patient to
assess pain intensity and pain
response to analgesics, how to
treat breakthrough pain using
rescue doses, and how to prevent
or treat side effects caused by
analgesics, (5) increasing opioid
doses or administering alternative
opioids was recommended to the
physicians in case of need for
titration of analgesic preparation
for pain control, (6) recommended
adequate antiemetic or laxative
drugs for symptom management
Pain intensity decreased gradually along with
increase of visits (occasion) with the
pharmacists. A significant change in the worst,
average, and least pain scores at visits 2 and 3
compared with those at occasion 1 (p<0.001).
Side effects scores showed a significant
difference only between visit 1 and 3 (n=18,
p=0.030). Pharmacist provided 48 new
recommendations with an acceptance rate of
85.4%; maximum accepted (21/25)
recommendations were change of dose (n=25)
out of which (n=20) there were dose changes for
opioids.
CPGT- clinical pharmacist-led guidance team, CTD- time from consult to discharge and death, DRPs- drug related problems, IDG- interdisciplinary group, LOS- length of stay, LTC-
length from admission to palliative care consult, mPCT- multidisciplinary palliative care team, NCCN- National Comprehensive Cancer Network guideline, OR- odds ratio, PCU- palliative
care unit, PPD- per patient day, QOL- quality of life, SR- sustained release.
27
a. Prospective evaluations of pharmacist-led interventions
These seven identified studies contain an intervention led by a pharmacist or detail a pharmacist
involved in the hospice and/or palliative care of patients. Outcomes of administered
interventions were assessed by evaluating pain intensity. Six out of the seven studies focused
on the management of pain among patients with cancer in a hospital/clinic setting
51,52,55,61,63,64
and one study was conducted in an outpatient setting.
59
A study by Chen et al
61
compared the effectiveness of opioid treatment between patients with
cancer receiving care from a clinical pharmacist-led guidance team (CPGT) and a control
group. One of the important pharmacist roles within the CPGT was evaluation of pain and
follow-up with patients. Pharmacists were involved in selecting drug therapy for patients along
with physicians. The procedures included in selection of drug therapy were initial pain
assessment, dose conversion, selection and titration, all of which were referred to as process
parameters. Similarly, outcome parameters contained results of pain evaluation before and
after medication administration, occurrence of adverse events and quality of life measurements.
Pharmacists collaborated with nurses in following up regarding management of adverse
effects. The effect of a clinical pharmacist in the CPGT group was assessed for both the process
and outcome parameters. Results from the study show that there was a higher rate of accurate
assessment of pain severity in the CPGT group (97.4% vs 71.8%, p<0.001). Process parameters
such as standardized dose titration, changes in specific opioids and errors in dose conversion
improved significantly in the CPGT group vs control group (p<0.001). The pain scores
assessed using a numerical/visual scale for the CPGT group demonstrated better control for
bone pain (3.1 vs. 4.2, p=0.038), body pain (1.2 vs. 3.6, p=0.041), visceral pain (1.9 vs. 3.1,
p=0.024), and nerve pain (2.7 vs. 4.8, p=0.045). The rates of adverse events among the patients
in the CPGT intervention group were lower than the control group, with a significant difference
in rates of constipation, nausea, and vomiting (p=0.041, 0.028, 0.035). Further, quality of life
(QOL; on a scale from 0-60) scores in the CPGT group were found to be better compared to
the control group (48.3 vs 37.6, p=0.032).
Mancini
55
evaluated the value of adding a part-time pharmacist for the palliative care of
patients with cancer. The service provided by the pharmacist was distributed among pre-visit,
visit and follow-up services. Before the visit, the pharmacist evaluated the medication list of
the patients from the electronic medical records and checked for drug interactions and
28
duplications of therapy. During the visit, in coordination with the nurse, the pharmacist went
through the medication list and discussed any difficulties with adherence, making
recommendations when necessary. After the visit, the pharmacist guided the team regarding
changes in drug therapy for the patient, if applicable, and filled out the assessment. The
pharmacists under nurse practitioner’s guidance made necessary medication recommendations.
The assessment evaluated five areas of medication management, including: (1) medication
adherence, (2) access to medication, (3) continuity of care, (4) medication reconciliation, and
(5) education. Patients reported various concerns regarding access including cost issues (n=40,
53.5%), transportation costs (n=15, 20%), and access to healthcare (n=24, 34%). The
medication therapy review of the patients allowed the pharmacists to go through drug
interactions, adverse effects, lack of drug efficacy and untreated conditions. The results showed
most common duplication therapies included sleeping meds (n=9, 25.6%) and breakthrough
pain meds (n=4, 26.5%), side effects included constipation (n=16, 27.9%), and lack of efficacy
in controlling pain (n=23, 31.9%). The most common untreated condition found during the
assessment was fatigue (n=14, 25.5%).
Valgus et al
52
evaluated the integration of a pharmacist in ambulatory care for an oncology
supportive service on a team with a nurse and a physician. Typically, a structured visit was
arranged for patients on acquiring approval from the primary oncologist. An initial cognitive
assessment and detailed medication history review was conducted by the pharmacist, and then
physicians and nurses went through a detailed symptoms management assessment. Finally, a
team meeting was held for discussing treatment recommendations, medication changes,
symptoms interventions or any referral services to be provided to the patient. The collected
data included demographics, symptoms scores (scored on a scale from 1=no pain to 5=most
severe) and medication for symptoms (pain, nausea, vomiting and constipation). Based on
referral encounter data from a total of 292 patients, 30 (10.3%) patients were consulted alone
by the pharmacist, and in 99 (34%) encounters, the pharmacist worked as a team with
physician/nurse. Out of total 89 patients, 88 (75%) visited the outpatient service for pain
management. Out of total 54 patients with pain, 52 (96.29%) were taking either methadone or
another long-acting opioid. After the initial visit, it was found that 40% of the patients had
increases in their medication doses, 23% had a new medication added, and 15% switched to
other opioids or had methadone started as a new therapy. The initial analysis of records of first
29
49 patients showed an improvement in the mean symptoms scores of pain management across
the three visits evaluated, although this was not statistically evaluated.
Richter
51
studied the clinical and financial impacts of adding a clinical pharmacist in the
hospice care interdisciplinary group (IDG). The clinical pharmacist carried out functions such
as preventing controlled substance diversion, attending IDG meetings, educating other clinical
staff, consulting pharmacist of the care centers, promoting formulary management and
adherence. The financial benefits showed a decrease in per patient drug (PPD) cost from 2016
to 2017 from $5.44 to $4.07 and a direct drug cost savings from the interventions made in the
IDG meeting of $329,729. The month-to-month intervention cost saving was estimated at
$75.52. Interventions not made during the IDG meeting demonstrated savings of
approximately $22,189. Overall, there was decrease in number of emergency visits and patient
falls. Overall, including a clinical pharmacist in the hospice care team benefited by saving
$427,705 annually. In terms of clinical functions, the clinical pharmacist was involved in
patient consultation and accompanying physicians or nurses during patient visits. This assisted
in patient education regarding medication use, exploring alternative medication options in case
of lack of access to drugs and reducing use of unnecessary medications. The major clinical and
financial impact of the clinical pharmacist was through their involvement in optimizing therapy
regimens for patients and formulary management. The pharmacists consulted patients on topics
such as drug dosing and selection strategies in a variety of settings from inpatient care centers
to the patient’s home. Overall, there was a positive impact of adding the pharmacist to the
clinical team as formulary adherence and accuracy was observed in the medical lists of the
patients.
Yamada et al
59
explored a pharmacist-led intervention for pain management among patients
with cancer within an outpatient clinic setting. The intervention was provided before the
physician visit and during every follow-up visit. The service involved patient counseling
through face-to-face interviews and telephone. During the session, patients were taught how to
assess pain intensity and response to analgesics, how to treat breakthrough pain using rescue
doses, and how to prevent or treat side effects caused by analgesics. A gradual decrease of the
proportion of patients reporting severe pain after pharmacist intervention occurred across
visits: 15/26 (51.7%), 10/27 (37.0%), 7/24 (29.2%), 4/14 (28.6%), 1/5 (20.0%), and 1/5
(20.0%), on visits one to six, respectively. Apart from this, the pharmacist also made
30
recommendations regarding change of dose, introduction of new medications and termination
of existing medication if necessary. Out of 48 total recommendations made, 41 (85.4%) were
accepted by the physicians.
Edward et al
63
evaluated the feasibility and acceptability of pharmacist-delivered medicine
consultation for patients with cancer. Community pharmacists were directly accessible by the
patients in case of emergency or untreatable conditions, and the study quantified drug-related
problems and the recommendations provided by the pharmacists. The pharmacists carried out
telephonic or face-to-face medication utilization reviews (MUR), followed by patient and
pharmacist feedback regarding change in intensity of pain before and after the consultation (on
a scale of 0=no pain to 10=pain as bad as they could imagine). In total, 47 drug-related
problems were identified in 17 patients with a mean of 2.5 per patient. The problems were
classified based on Pharmaceutical Care Network Europe Foundation (PCNE) classification.
The most common drug-related problem encountered during the consultation was pain due to
reasons such as no effect of drug treatment (P1.1) (n=1, 7%), effect of drug treatment not
optimal (P1.2) (n=12, 80%) or result of untreated symptoms (P1.3) (n=3, 13%). The most
common cause was lack of information regarding side effects of the drug (C7.1) (n=6/15, 40%)
and advice (C5.2) (n=8/16, 50%). The most common intervention provided for the common
drug related problems was patient counseling for pain (I2.1) (n=12/35, 34%). The post-
consultation pain score was found to be improved over that of the pre-consultation pain score
(mean: 3.45 vs 3.95, p-value not specified). The telephonic consultation found to be highly
acceptable amongst the patients and healthcare professionals.
Wilby et al
64
described the modernization that took place in the palliative care setting in Qatar
as a part of National Care Strategy. Clinical pharmacists were added as a core component of
the palliative care multidisciplinary team. The pharmacist underwent academic cross-
appointment training which was accredited by Canadian Council of Accreditation of Pharmacy
Programs (CAPP). Along with enrollment in a clinical program, they worked in a palliative
care setting. An inventory list of recommendations was made based on consultation provided
by these cross-appointed pharmacists. The perceived importance of these recommendations
was ranked by both the pharmacists in Canada and Qatar (on a scale of 1=lowest importance
to 10=highest importance). The recommendations list most frequently identified
discontinuation of drug therapy (29%) and initiation of drug therapy (25%). There was no
31
significant difference found between overall rankings for each question between pharmacists
in Canada and Qatar (p>0.05). The perceived importance of the interventions between the
pharmacist at Qatar and Canada was as follows: initiation of drug therapy (10, p=0.955),
discounting of drug therapy (10, p=0.758) and, physician/nurse education (10, p=0.918). The
study provides a strong rationale of adding a clinical pharmacist in a palliative care setting as
an evidence of the services they can offer.
b. Retrospective evaluations of pharmacist impact
Atayee et al
56
described the outcomes associated with adding a clinical pharmacist to a
palliative care service in an inpatient setting. The study retrospectively assessed hospitalized
patients evaluated by a specialist palliative pharmacist at University of California, San Diego.
The analysis focused on identifying the inpatient pharmacist interventions provided to the
patients, as well as evaluating the outcomes related to the interventions provided as a part of
the primary assessment. The study also evaluated length of hospital stay (LOS), reason for
consult to palliative care team, length from admission to palliative care consult (LTC), and
time from consult to discharge or death (CTD) of the patients. In the inpatient setting,
pharmacists were involved in educating/training other team members and patient family
members, dose changing, and medication selection. Pharmacists served as liaisons between the
palliative care team and the department of pharmacy at the medical center. The most common
documented intervention and outcome found were optimizing palliative care medications
(n=371, 92.75%) and change in the medical therapy implemented (n=300, 90%). Early
exposure of clinical pharmacists to the patients (i.e. within >3 days of hospitalization) was
found to improve LOS, LTC and CTD (10, 3.79 and 6.09) compared to exposure after >3 days
of hospitalization wherein LOS, LTD and CTD was 24 (p=0.00004) , 9.48 (p=0.013) and 14.59
(p=0.000009).
Naidu
57
assessed the role of a palliative care pharmacist in a community hospital. Pharmacist
responsibilities include participating in team rounds, forming symptom management plans,
educating staff and family members, coordinating pharmacy related interventions, and
completing Physician Orders for Life Sustaining Treatment (POLST) forms. Alongside
physicians, pharmacists were able to initiate, adjust or transition medications as per patient
pain relief requirements. A retrospective cohort study was conducted and evaluated medical
records of patients who had palliative consult orders and a clinical note from a pharmacist in
32
their clinical record. The data included patient’s pain scores (on scale of 1 to 10) before and
after 24 hours, of intervention administered. A reduction in pain score from 4.6 to 2.0 points
was seen among acute pain patients (n=125, 47%) and 5.7 to 2.5 in chronic pain (n=140, 57%)
patients after administration of pharmacist intervention. Out of the total patients who stated a
numerical pain value (n=191), 174 (91%) met their pain goal within 24 hours. In case of
symptom management, nausea and anxiety scores were improved post-intervention. The most
common interventions provided by the pharmacist were education, counseling patients and
making proper medications available for them. The pharmacist service had a positive financial
impact due to discontinuation of unnecessary medications, tests, or procedures. Palliative
pharmacists achieved a direct cost reduction of $1000 due to treatment discontinuation. In line
with published literature, a reduction in cost per day of $279 for patients discharged alive and
$374 for patients who died as inpatients was achieved through the consultation program.
Ma et al
58
described the role of pharmacist on a palliative care team in an outpatient setting,
providing consultation during visits for pain management and involved in documentation and
interventions on medication problems. Advanced care planning was provided to assess, initiate,
stop, and/or adjust therapy for the management of pain, nausea/vomiting, and other symptoms
due to lack of efficacy, adverse effects, nonadherence or missed doses, drug interactions,
evaluating duplications in therapy and providing recommendations regarding medications. All
patients assessed by the palliative care pharmacists were included in the study, with their pain
(on a scale of 1-10) scored as mild (1-3), moderate (4-5) or severe (6-10). During the first visit
(n=80), 38 (48%) were classified with severe pain, and at the second visit (n=59), 21 (36%)
reported improvement in pain. At third (n=43) and fourth visits (n=33), the number of patients
with stable pain were consistent (21 [49%] and 14 [42%]). All the pharmacists identified
constipation as an adverse effect in the subsequent visit and the most common intervention
provided was starting a new medication.
Geum et al
65
explored the impact of a multidisciplinary palliative care team (mPCT) and
pharmacist on pain management. Data from medical records of the patients admitted in the
palliative care unit (PCU) was retrospectively collected. The mPCT team, along with the
pharmacist, conducted medical rounds collecting information regarding pain severity and other
symptoms. The pain intensity (scored on a scale of 0 to 10) was documented seven days before
PCU admission (day -7), on the PCU admission day (day 0), and seven days after the PCU
33
admission (day 7). Pharmacists were involved in providing medical therapy recommendations
and evaluating use of analgesics. The analgesic use followed the Korean Cancer Pain
Management Guidelines and the National Comprehensive Cancer Network (NCCN)
guidelines. A medication was deemed eligible for use if it satisfied all six categories of
recommendations within the guideline, including: (1) drug selection based on the type and
severity of the pain, (2) dosage for chronic pain, (3) for breakthrough pain, (4) reassessing each
patient’s pain to adjust the pain medication to meet the patient-specific goals for comfort,
function, and safety, (5) analgesic use that reflects renal or hepatic function, and (6) monitoring
adverse effects. The results of the study showed that pain scores were worst on day 0 (4.05),
compared to day -7 (3.16) and day 7 (2.66). The appropriateness of analgesic used improved
along the days of the admission, from day -7 (35.0%), day zero (34.2%) to day 7 (5.2%)
(p<0.001). The analgesic use as per the six categories recommendations improved over time
(day -7, day 0, day 7) for chronic pain (87.2%, 80.3%, and 95.7%) (p<0.003), break through
pain (88.9%, 88.9%, and 96.6%) (p<0.049) and monitoring of the side effects (65.0%, 65.8%,
and 86.3%).
c. Survey questionnaires regarding pharmacists in hospice and palliative care
Ise et al
60
examined responses from palliative pharmacist surveyed regarding their
understanding of their activities on the palliative care team. The pharmacists were asked
questions regarding their clinical, education, and research contributions in a palliative care
setting and their perception of their contribution to the service. Clinical activities were rated
on a five-point Likert scale (one=rarely to five=everyday). The highlighted clinical activities
identified from the responses were: (1) direct counseling of patients about opioid information
(18%) and adverse events of opioids (19%), (2) provision of information to the palliative care
staff about managing adverse events of opioids (21%) and pharmacology of opioids (20%), (3)
attending wards (79%) and conferences (94%). Their contribution to education and research
activities was measured through a yes/no question. Approximately 80% of pharmacists
organized a conference in their own designated cancer hospital. The perception of pharmacist
contributions to the palliative care team was assessed using a yes/no question and associated
reasons were rated on five-point Likert scale (strongly agree to strongly disagree). Out of 304
pharmacists, (n=212) 70% of pharmacists rated their contribution to palliative care services as
34
100%. Those who did not perceive their contribution to the fullest identified a shortage of time
(90%) and staff (68%) for their lacking contribution.
Pawlowska et al
62
administered a survey regarding current and future roles of clinical
pharmacists and their collaborations with physicians in a residential hospice among three sets
of responders: pharmacists, physicians and hospice directors. Each responder had a different
set of questions to answer; the hospice directors and physicians were asked about their attitudes
towards the contribution of a pharmacist in the residential hospice. Pharmacists were asked
questions regarding the services they provided, their role in solving drug-related problems and
making the therapy more cost-effective. The majority of the respondents supported the idea of
including a pharmacist in the palliative hospice care team. Specific reasons for this as per the
pharmacists were delivering cost-effective therapy, while hospice directors identified better
drug management and decision-making regarding therapies as the reason. A need for advice
from the pharmacist was expressed by 53% of physicians (n=16/30) on the following topics:
new drugs, rationalization and cost of pharmacotherapy, reimbursement, generic drugs,
availability of drugs on the pharmaceutical market, drug interactions and compounding. All
the respondents thought that adding a pharmacist to the hospice team would be beneficial for
proper storage of drugs (61%), decreasing cost of the therapy (57%) and improving access to
the drugs (53%).
VIII. Overall summary
The breadth of studies focused on evaluating the impact of adding the clinical pharmacist on
the hospice and palliative care team for pain management of patients. The outcomes of
pharmacist-led interventions or pharmacist involvement in palliative care were found to be
associated with better pain control among the patients.
51,52,55,59,61,63,64
The studies in the review
assessed the effect of study interventions on patient’s pain via evaluating pain intensity. Most
commonly pain intensity was recorded utilizing a numerical pain scale throughout a patient’s
visit to the healthcare setting. Interventions played an important role in optimizing a patient’s
therapeutic regimen, identifying, and solving adverse effects related problems. Furthermore,
Richter
51
mentioned financial benefits gained due to the roles carried out by a pharmacist in a
palliative multidisciplinary team.
35
The current review also provides insights regarding clinical pharmacist’s role in conducting
counseling sessions, medical rounds and completing patient’s health assessment forms.
56-58
It
also endorses pharmacist involvement in medication changes, recommendations or
intervention suggestions as a response to the symptoms experienced by the patients.
65
Ise et
al
60
and Pawlowska et al
62
highlighted how pharmacists perceive their importance in the
hospice and palliative care through administration of surveys.
The results of the systematic literature review provide rationale to the aim of the study to
understand the extent of involvement the clinical pharmacists have in hospice and palliative
care settings.
IX. Limitations
Most of the studies had data from the initial phases of service implementation, where
adding the pharmacist to the multidisciplinary team was just initiated. Therefore,
outcomes resulting from a well-established palliative care team are not as well detailed in
the review. Moreover, the current review largely did not take into consideration effects of
pharmacist involvement or pharmacist-led interventions on humanistic outcomes, such as
quality of life and improved functioning. Regarding clinical setting limitations, articles
based on a pharmacist’s role in hospice care multidisciplinary team were comparatively
fewer than palliative care setting. Furthermore, non-English studies and
seminar/conference data without full text were not included; therefore, studies written in
foreign languages and containing relevant data may have been left out of the review.
36
CHAPTER 3: METHODS
I. Phase I of the study
a. Phase aim
To identify the prevalence of clinical pharmacist recommendations for methadone upon
admission to hospice/palliative care. Further, to assess the acceptance of the pharmacists’
recommendations for methadone after admission to hospice/palliative care.
b. Overview
Phase I of the study was conducted in two parts of data collection. ‘Instrument #1 - Pharmacist
data collection tool’ identified whether a recommendation for methadone was made by the
pharmacist based on individual indications/contraindications of patients. ‘Instrument #2 -
Researcher data collection tool’ was utilized to follow up the patients for evaluating whether
the physicians accepted provided recommendations. Descriptive and inferential statistical
analysis was performed on the data collected by the tools.
1. Rationale
The involvement of a pharmacist in the hospice and palliative care has shown improvement in
pain management and optimizing other patient clinical outcomes. A study performed by Lee
et al. documented all recommendations made by the pharmacists and their effects on the
patient’s clinical outcomes in a palliative care setting. Out of the 87 recommendations, 73
(84%) were accepted by physicians. The patient’s clinical outcomes were positively influenced
by the pharmacists’ pharmacotherapeutic recommendations.
66
Another study by Wilson et al.
demonstrates that the desired clinical outcomes were achieved when the pharmacist’s
recommendations were accepted by the physicians.
67
Methadone’s utilization in hospice and palliative care settings has not been optimal.
41
This has
been the case despite the medication gaining popularity for its pain management attribute
among the patients. The medication has been studied for use in various pain states, especially
pertaining to patients with cancer pain. The indications of using methadone found from those
studies were: (1) management of uncontrolled pain, (2) alternative in case of opioid
allergy/opioid adverse effects, (3) management of neuropathic pain, and (4) pain refractory to
other opioids.
68
Similarly, use of this treatment for pain management has various
37
contraindications. A clinical practice guideline on safe use of methadone suggests
contraindications for using methadone including: (1) prolongation of QTc interval, (2)
potential risk factors of QTc prolongation like electrolyte abnormalities, impaired liver
function, etc., (3) drug-related arrythmia, (4) multiple drug-drug interactions, and (5)
respiratory depression.
44
Pharmacist involvement in hospice interdisciplinary teams is highly
endorsed, with medication reconciliation one of their roles and responsibilities in hospice and
palliative care setting. This understanding assists in using their clinical judgment to provide
methadone recommendations, keeping its indications and contraindications in mind. Therefore,
the strategy of obtaining pharmacists recommendation on methadone utilization will be useful
in demonstrating the medication’s use in this setting. Moreover, evaluating the acceptance of
these recommendations will provide information on the impact of pharmacists’
recommendations in hospice and palliative care setting.
a. Study sample
The sample of patients for the first part of this study included adults (18+ years old) admitted
to hospices served by DeltaCareRx. These patients were newly admitted between October 2019
to December 2019.
b. Protection of human subjects
The study did not involve any direct interactions with patients (and therefore posed minimal
risk); therefore, it was granted an exemption by the IRB review. Patient information was
protected by usage of anonymized study ID to identify patients on research documents,
corresponding to a unique patient ID used onsite at DeltaCareRx. A master sheet matching the
study ID and DeltaCareRx patient ID was accessible only by DeltaCareRx staff and remained
onsite at the facility. An additional master sheet was maintained onsite to keep a record of the
forms filled out by pharmacists, including the names of pharmacists and numbers of forms they
were assigned (e.g. the patients they collected).
c. Developing instruments for data collection
1. Instrument #1 - Pharmacist data collection tool
Preliminary literature search aided in identification of important variables in pain
management.
44,69,70
The following area were chosen to characterize and evaluate methadone
recommendations for pain management: (1) demographics of the patient, (2) type of pain

38
(classified as nociceptive, neuropathic or both), (3) pain intensity at the admission (measured
on a numerical scale 1=no pain to 10=worst pain), (4) current pain medication regimen,
prescribed at the time of admission to hospice/palliative care at DeltaCareRx, (5) previous
recommendations of methadone, (5) breakthrough medications used in past, (6) indications
for prescribing methadone, and (7) contraindications of prescribing methadone. The list of
indications and contraindications are included in Table 1. If the pharmacist provided a
recommendation for methadone, they were requested to specify the type of recommendation,
including: (1) switch to methadone as maintenance treatment, (2) addition of methadone as
adjunctive/adjuvant treatment, (3) discontinue methadone previously prescribed, or (4) other.
These criteria were used to develop instrument #1, available in Appendix 1.
Table 1. List of indications and contraindications of using methadone
Indications Contraindications
High opioid tolerance Clinically unstable
Refractory to other opioids Limited prognosis (< 5 days)
Morphine allergy Drug interactions
Severe renal impairment QTc prolongation/structural heart disease
Neuropathic pain Severe liver impairment
Substance use disorder
Use of other long-acting CNS depressants
2. Instrument #2 - Researcher data collection tool
This data collection tool was utilized to evaluate whether the patients who had
recommendations for methadone were accepted or not, and to collect further data on these
patients. All new admission patients from the first phase of data collection who acquired
recommendations from the pharmacist were followed up by a student pharmacist. The
instrument also collected additional patient information including: (1) allergies, (2)
comorbidities, (3) hepatic/renal dysfunction recorded as presence or absence of these
conditions or any clinical value if provided, (4) nutritional status was recorded as it, (5) pain
medication history of the patient prior to admission in hospice/palliative care at DeltaCareRx,
and (6) pain intensity prior to and during the admission to DeltaCareRx setting, classified on a
39
categorical scale moderate to severe, and (7) number of days of interval from the day of
admission to the date of filling the instrument #2. This gave an idea of the number of days
patients were admitted to the service. Further, if the recommendation was accepted by the
physicians, questions exploring the therapeutic regimen were explored in the tool. Additional
data was collected regarding which day of the week the recommendation was provided in order
to understand site and staff’s functionality over a week’s time. Further, data on interval of days
passed from the day of recommendation to implementation was collected. The alignment of
the accepted dose/frequency with the pharmacist recommended dose/frequency was assessed
through this tool if mentioned. A copy of instrument #2 is available in Appendix 2.
The instruments underwent various revisions before use for data collection. Discussions with
DeltaCareRx staff helped in getting insights on availability of the information for completion
of instrument #2. The revisions included additional questions related to patient’s clinical
characteristics. The palliative prognosis scores (PPS) of the patients at the time of admission
and during completing instrument #2, it was measured on a numerical scale 0=death to
100=normal. It is used to predict patient’s prognosis and survival.
71
A PPS is assigned based
on patient’s total bed bound time, extent of diseases, ability to carry out self-care, food intake
and conscious level
72
. Morphine milligram equivalents (MME)
73
patient was on during and
prior to the admission was evaluated through addition of respective questions. The MME
conversion factor was used to calculate the total opioid dosage prescribed to the
patients.(conversion scale included in instrument #2. Appendix 2). Additionally, a question
exploring the day of the week when recommendation was provided was also added in the tool.
This assisted in assessing facility functionality based on the days of the week.
d. Data collection process
The data collection took place from October to December 2019 at DeltaCareRx. A total of four
pharmacists and a student pharmacist collected data using the tools in paper format. A
researcher designed the instruments for data collection and analyzed the collected data. All
newly admitted patients to DeltaCareRx care underwent their usual clinical review by
pharmacists at DeltaCareRx. After this, the four pharmacists filled out instrument #1 for each
patient they processed. Later in the data collection, information from instrument #1 was used
for follow-up if a recommendation for methadone was rendered. The patients who had
instrument #1 filled, a student pharmacist filled out instrument #2 for those patients using data
40
available in the DeltaCareRx system. The student pharmacist calculated MME of the
medications that were administered by the patients on their individual forms. Additionally,
patients provided with a methadone recommendation had the acceptance section filled on
instrument #2. All paper instruments contained a top section listing the DeltaCareRx patient
ID and study ID (both necessary to achieve follow-up); this section was trimmed off prior to
leaving the DeltaCareRx site to ensure anonymization, and then were sent to Duquesne
University for analysis. The information from both the tools was transferred to a Microsoft
Excel spreadsheet.
e. Data management and statistical analysis
The data from this phase was analyzed using SPSS Statistics 25 (IBM Corp; Armonk, NY).
The individualized data collected for most variables was categorized to ease analysis.
Examples of these categorization is provided in Appendix 3. Selected variables were
categorized as follows. Age was classified in two classes, < 60 and ≥ 60, for the purpose of
statistical analysis. The classification was made because majority of the patients admitted in
hospice care are between the age 60 and higher
5
. Classes for terminal diagnosis/indication
were: (1) cancer, (2) dementia, (3) cardiovascular, (4) respiratory, (5) liver, (6) kidney, (7)
neurodegenerative, and (8) other. Classes for pain medications (prior to and at the time of
admission) were: (1) opioid, (2) NSAID, (3) opioid/APAP, (4) gabapentin, and (5) other.
Allergies were categorized as: (1) opioid, (2) antibiotic, (3) topical, and (4) other.
Comorbidities included: (1) cancer, (2) dementia, (3) cardiovascular, (4) respiratory, (5) liver,
(6) kidney, (7) endocrine, (8) psychiatric, (9) gastrointestinal, (10) neurological, and (11) other.
The response to nutritional status was categorized as: (1) very poor, (2) poor, (3) good, (4) fair,
and (5) good. Similarly, renal, and hepatic dysfunction was categorized as: (1) yes, (2) no, or
(3) not known. Recoding of string variable into numerical characters was done using the
transform function on SPSS.
Character string variables like sex (F=1, M=2), methadone
recommendation (Y=1, N=2), acceptance of recommendation (Y=1, N=2) and type of pain
(1=nociceptive, 2=neuropathic or 3=both) were recoded to numerical string.
1. Research questions
Research question 1: To identify the prevalence of clinical pharmacist recommendations and
acceptance for methadone use among hospice/palliative care patients
41
Descriptive statistics was utilized to calculate the prevalence of the methadone
recommendation provided to the patients. Additionally, demographic, and clinical
characteristics was evaluated for the patient sample such as patient’s mean age, height, weight,
BMI, and sex. Standard deviation, median, maximum, and minimum values were calculated
for these variables. Individual frequencies were measured for clinical characteristics such as
hospice type, pain intensity, medications, palliative prognosis scores. Range was calculated for
days from the date of admission variable. Prevalence was calculated using frequency
evaluation of questions regarding (1) pharmacist recommendations, and (2) acceptance of the
provided recommendations.
Research questions 2: To evaluate the differences in demographic and clinical characteristic
of patients provided with a methadone recommendation and patients who were not.
Inferential statistics was utilized to evaluate the difference between the study sample
recommended for methadone and patients who were not. The groups were compared using
independent t-test analysis for continuous variables and Chi square test analysis for categorical
variables.
II. Phase II of the study
a. Phase aim
To identify the frequency in the use and monthly expenditure of three categories of
medications: pain, pulmonary and anticoagulants at various DeltaCare Rx client sites. The
use of the medications was stratified as per the therapeutic class of their medication
category and sex of the patient across a pharmacy claims database.
b. Overview
Medication utilization data for six months (January, June, July, September, October and
November) of the year 2019 was obtained from DeltaCareRx. The data consist of month
wise prescription drug information, including date of claim, drug names, quantity, cost,
days of supply, and patient sex. Frequency in use, total expenditure and monthly average
cost was calculated for each therapeutic class belonging to the three medication categories.
42
The data was stratified based on the different therapeutic class and patient’s sex. The total
cost for each stratified subgroup was calculated. Additionally, consumption of each
medication from the medication categories of interest were retrospectively evaluated from
the database.
c. Rationale
Hospice and palliative care providers are assisted by DeltaCareRx in cost containment.
DeltaCareRx uses a unique Rx purchasing model to achieve this for their clients. They
obtain the medications from the pharmacy at highly discounted rates and provide them to
their client with transparency in their pricing. The prescription drug data is generated
through Deltalytics, which reports monthly utilization of medications by the hospice and
palliative care providers.
DeltaCareRx staff articulated categories of interest for the analysis, including the selected
three broad categories of pain, pulmonary and anticoagulants. The selection was made
because of the high medication utilization and expenditure belonging to these three-
medicine categories at all the hospice care sites served by DeltaCareRx .Therefore, the
analysis aimed to identify the highly utilized and costly drugs from those medication
categories. The evidence generated will be useful in cost/utilization optimization and
developing strategies of utilizing cost effective drugs by the hospice providers served by
DeltaCareRx.
d. Data source
Prescription claims data was obtained from a pharmacy benefit manager, DeltaCareRx.
The organization provides services to hospice and palliative care clients. The data includes
unique prescription claim of the medications dispensed to a patient at the setting.
e. Database structure
Full prescription drug data for January, June, July, September, October, November months
of the year 2019 was obtained from DeltaCareRx and made available to the researcher in
Microsoft Excel via multiple sheet downloads. The six months for data analysis were
43
selected because of availability of complete data for these months and to maintain
uniformity in utilization data. These multiple sheets were merged together and named. The
primary identifiers in the dataset were the prescription number and unique identification
number of each patient. The data set also includes other variables such as drug name,
therapeutic class (generic and standard), quantity, average wholesale price (AWP),
DeltaCareRx cost, days of supply, patient sex, and denotation of new/refill medication. The
variables for the study were defined as below.
The month-wise data sheets differed in content, with not all datasets inclusive of all
variables.
1. Therapeutic classification of drugs
There were two (standard and generic) therapeutic class variables present in the data. For
the present analysis, generic therapeutic classes of drugs were taken into consideration.
There were 445 different generic therapeutic classes of medications utilized at different
client sites of DeltaCareRx for the dataset provided.
2. Drug names
Drug names are the prescribed medications dispensed to hospice patients at DeltaCareRx
client sites. The data includes information 3189 medications.
3. Sex
The data includes the sex of patients who were administered each medication. As per the
information in data two nomenclatures were used to describe sex of the patients. Numerical
‘1’ was coded for males and ‘2’ was coded for females in some of the data sheets and others
had letters ‘M’ and ‘F’ to denote sex of the patients.
4. Generic long name
Each drug had a generic long name provided in the data. This information was useful in
segregating the drugs based on their generic names, and not the drug names, which had a
44
high degree of variability. For example, the generic long name, ‘methadone’ had various
drug names in the database such as “methadone 5mg”, “methadone solution 5mg/ml”,
“methadone con 10mg/ml”.
5. DeltaCareRx medication cost
Each prescription in the database was associated with the cost charged to the patients. This
was the cost charged by DeltaCareRx while dispensing the medications to the patients.
f. Utilization of medications
To assess the trends in utilization of medication of interest at DeltaCareRx client sites, a
unit measurement called ‘defined daily dose (DDD)’ was used. The World Health
Organization’s definition for DDD is “the assumed average maintenance dose per day for
a drug used for its main indication in adults.”
74
Drug utilization data presented in DDDs
gives a rough estimate of consumption of medications. Each medication has a DDD
assigned as per its route of administration provided it has a designated Anatomical
Therapeutic Chemical (ATC) Classification code. ATC codes classify the active
ingredients of medications according to the organ or system they act on and their
therapeutically, pharmacological, and chemical properties.
75
These codes are maintained
by WHO Center for Drug Statistical Methodology. There are no DDDs assigned for topical
products, vaccines, antineoplastic agents, allergen extracts, general and local anesthetics,
and contrast media.
74
The recommendations of average maintenance doses are made
depending on: (1) the recommended dose referring to a body weighing 70 kg, (2) the
maintenance dose not differing from an initial dose, (3) an assignment based on the content
(strength) of a product, with different salts of a product not having different DDDs, and (4)
prodrugs and various dosage forms of a same drug not having been assigned a separate
DDD value.
The trends in consumption of medications from three broad classes, (1) pain, (2) pulmonary
and (3) anticoagulants were evaluated. A list of medications was prepared with their
assigned DDD values referenced from the ATC/DDD Index 2020 website (Appendix 4).
76
DDD dispensed was calculated for each medication if it had the following information, (1)
45
quantity of medication, (2) strength of medication, and (3) DDD value. The formula for
DDD dispensed used was:
= ∗ ℎ// ℎ
g. Selection of medication categories
Researchers categorized the relevant therapeutic class of medications from the database
into three medication categories. A student pharmacist reviewed the selected classes to
avoid any errors in the selection process.
h. Data analysis
The frequency of each therapeutic class of medications and their cost was found from the
database. Cost of each therapeutic classes was identified and compared across individual
therapeutic classes. Next, all the therapeutic class in the three medication categories were
stratified as per patients’ sex. Cost per patients across their sex was identified for each
month. SUMIF and COUNIF Excel functions were used for obtaining the stratified values
as per sex of the patient and cost values. SPSS (Version 25.0) was used to carry out the
descriptive statistics. The Explore function was used to calculate the mean and +/- standard
deviation values of the costs of various therapeutic classes. For the purpose of this
descriptive analysis, the medical categories were assigned numbers such as anticoagulant
=1, pulmonary = 2 and pain = 3 for the purpose of this analysis. The dependent variables
for the analysis were cost per male/female patients and independent variable was the
numerical medication categories. It demonstrated the different monthly mean cost and the
associated +/- standard deviation value of all the therapeutic class.
Utilization of each medications with available strength and quantity values was calculated
expressed as total DDDs dispensed. SUMIF and COUNTIF functions were used across the
Excel workbook to calculate the strength and quantity values. Individual DDD values of
drug names were grouped under their generic long names. These values represented the
sum of total DDDs dispensed.
46
The missing cost and quantity data in the database were filled by imputing the missing
values. The missing values were substituted with the average of known cost/quantity values
for the prescription. For example, in the case of missing cost value for the drug name
MAPAP (acetaminophen) tablet 500 mg. The average of all the available cost values for
the same tablet was substituted in the place of the missing value. In the case of missing
quantity values in the database. The average of the highest and the lowest quantity value
of a particular drug name was imputed for the missing value.
1. Impact of missing data
The missing values in the datasets impacted analyses in the phase II of the study. All the
listed generic therapeutic classes in the table were not present in all the data of the months.
Hence, those may not be uniform. The missing values of drug names and quantities did not
allow for DDD calculation for those drugs. Additionally, combined products were excluded
from utilization analysis because of DDD values were unavailable. The data regarding
patient’s sex was missing in many datasets. Therefore, the stratification analysis was not
performed for that data. The quantity of missing data and its implication on the overall
results is specified throughout the results of phase II.
2. Research questions
Research question 1: To identify the most frequently utilized generic therapeutic class
and their expenditure from the medication categories of pain, pulmonary and
anticoagulants.
Descriptive statistics was utilized to identify the values to answer the research question.
The mean average cost of each therapeutic class and the standard deviation values were
calculated.
Research question 2: To identify the pattern in medication utilization on the basis of sex
of the patient and therapeutic class.
47
The difference in expenditure of various therapeutic class based on the sex of the patient
was calculated for each month.
Research question 3: To identify the month wise medication consumption at DeltaCare
sites.
The WHO DDD values were identified for the identified medications from the
therapeutic class. Consumption values for individual medications was calculated using
the dispensed DDD formula.
48
CHAPTER 4: RESULTS
I. Phase I of the study
a. Study aims
To assess the data collected to identify prevalence of pharmacist methadone recommendations
and acceptance. Further, to evaluate the difference in the characteristics of patients provided
with the recommendation vs those who were not.
b. Overview
Descriptive analysis resulted in generation of individual frequency tables of patient
demographics and clinical characteristics variables. Prevalence of the provided pharmacist
recommendation and accepted recommendations were calculated. The difference between the
patient characteristics of patients provided with recommendations vs those who were not,
analyzed using inferential statistical analysis.
c. Demographic and clinical characteristics of the sample
1. Sample size
In total, 159 instruments #1 and #2 were filled out, with 158 (99.3%) usable forms analyzed
based on inclusion criteria.
2. Patient demographic variables
A total of 156 (98.7%) out of 158 newly admitted patients to the facility had their age
documented on instrument #2 (Table 2). A total of 45 (28.5%) patients were of age between
80-89 years old, 41 (26.0%) were between 90-99 years old and 3 (1.9%) were between 100-
110 years old. Patients aged 18-59 accounted for only 12 (7.6%) of the study sample. The mean
age for the overall sample was 79.5 years (SD: 13.8 years). The sample had a slightly higher
proportion of females (89; 56.3%) compared to males. BMI was calculated for 125 (79.1%)
patients based on the available height and weight variables. From the total sample, 16 (12.8%)
were classified as obese (≥30 kg/m
2
), 27 (17.0%) patients were overweight (25.0-29.9 kg/m
2
),
64 (51.2%) had a normal BMI (18.5-24.9 kg/m
2
) and 18 (14.4%) were underweight (<18.5
kg/m
2
). The mean BMI for the sample was 23.7 kg/m
2
.
49
3. Patient clinical characteristic variables
Hospice type data was collected through instrument #1. Hospice type information was included
in 157 (99.3%) of the patient sample. Home hospice, for 121 (76.6%) patients, was the most
utilized type reported (Table 2). Similarly, type of pain data was collected for 101 (63.9%) of
patient sample. The majority of patients (62; 39.2%) reported having nociceptive pain. The
change in palliative score was recorded from day of admission (from instrument #1) to the day
of data collection (from instrument #2). The majority of values (141; 89.2%) had no difference
recorded. The mean of difference of 12 was found between palliative prognosis scores pre- and
post-admission scores. Overall, mean scores during the admission and after the admission were
37.20 and 37.44, respectively. The mean values of morphine milligram equivalent (MME)
during the admission and after the admission were 1131.4 and 1160.3, respectively. The
MME/day during the admission and after the admission was found to be 37.71 and 38.67. The
data collected from the date of admission to the date of filling out the instrument ranged from
0 to 189 days.

50
Table 2: Demographic and clinical characteristics of hospice/palliative care patients
Age: categorization provides distribution of elderly (≥60) patients; BMI: Body Mass Index; PPS: Palliative prognosis
score; MME: Morphine Milligram Equivalent; n: number of study sample reported the information; SD: Standard
deviation
A total of 124 (78.5%) patients included data regarding pain intensity score at the time of
admission. Most of the patients (113; 71.5%) classified their pain between the scale 1 to 6
(Table 3). The medications used during and prior to admission were categorized into respective
therapeutic classes. In counting the number of medications patients were administering, one
patient may belong to more than one medication category. The most common medication
utilized in the hospice care setting was opioids (153; 97.0%) followed by APAP (55; 35.0%).
Similarly, most medications in the pain medication history were opioids (122; 77.2%).
n (%) Mean (SD)
Sex
Female
Male
89 (56.3)
66 (41.8)
-
Height (m)
126 (80.0)
1.6 (0.1)
Weight (kg) 133 (84.2) 65.6 (15.0)
BMI (kg per m
2
) 125 (79.1) 23.7 (5.0)
Age (years)
< 60
≥ 60
12 (7.6)
144 (91.5)
79.5 (13.8)
PPS at admission
150 (95.0)
37.2 (12.1)
PPS after admission 147 (93.0) 37.4 (12.0)
Hospice type
Inpatient
Assisted living
Nursing home
Home
4 (2.5)
10 (6.3)
22 (13.9)
121 (76.6)
-
Pain type
Neuropathic
Nociceptive
Both
5 (3.2)
34 (21.5)
62 (39.2)
-
MME prior to admission 51 (32.3) 1131.4 (2261.0)
MME after admission 67 (42.4) 1160.3 (2332.5)

51
Table 3: Overall distribution of pain variables in patients before and after the admission
APAP: Acetaminophen; NSAID: Nonsteroidal anti-inflammatory drugs; Opioid/APAP: opioid/acetaminophen
combination
In total, 95 (60.1%) of patients had their allergies documented using Instrument #2. As per the
categorization, the most frequent allergy was antibiotics (47; 23.6%) followed by opioids (31;
15.3%). Although 97% of the sample was administering opioids for their treatment, alternative
opioids outside of their specific allergy may have been utilized for pain management. The
allergic conditions were opioid specific and alternate opioids were administrated for pain
management respectively (Table 4). Similarly, in total 147 (93.0%) of patients had their
comorbidities documented. The comorbidities were categorized as per different disease
n (%)
Pain intensity before admission
Mild
Moderate
Severe
68 (43.0)
29 (18.3)
11 (7.0)
Pain intensity scores at admission
1
2
3
4
5
6
7
8
9
21 (13.3)
38 (24.1)
16 (10.1)
8 (5.1)
18 (11.4)
12 (7.6)
5 (3.2)
5 (3.2)
1 (0.6)
-
Pain medication history of patients prior to admission
Opioids
APAP
Opioid/APAP
Gabapentin
NSAIDS
Other
122 (77.2)
14 (9.0)
12 (7.5)
8 (5.1)
2 (1.3)
2 (1.3)
Pain medications at the time of admission
Opioids
APAP
Opioid/APAP
Gabapentin
NSAID
Other
153 (97.0)
55 (35.0)
19 (12.0)
16 (10.1)
7 (4.4)
13 (8.2)

52
conditions. The most common comorbidity encountered among the study sample was
cardiovascular disease (116; 73.4%).
Table 4: Overall distribution of allergies and comorbidities in study sample
GI: gastrointestinal; *one patient may be categorized in more than one class
d. Research question 1
1. Provided methadone recommendations
In total, 37 (23.4%) patients had a methadone recommendation provided by the pharmacists.
The majority (26; 16.5%) of reasons of methadone recommendation was switching to
methadone as the maintenance treatment. Further, other reasons included addition of
methadone as adjunctive treatment (7; 4.4%) and other potential reason listed by the
pharmacists (3; 1.9%).
2. Indications/contraindication of provided methadone recommendations
The recommendations provided were based on the patient’s indication and/or contraindication
for administering methadone. In total, 50 (31.6%) out of 158 patients had indications for
recommending methadone reported. One patient may have one or more indications or
Allergies/comorbidities
n (%)
Allergies *
Antibiotic
Opioid
Topical
Other
None
47 (30.0)
31 (20.0)
7 (4.4)
54 (34.2)
63 (40.0)
Comorbidities *
Cardiovascular
Endocrine
Cancer
Respiratory
Kidney
Dementia
Psychiatric
Neurological
Liver
GI
Other
116 (73.4)
61 (39.0)
53 (33.5)
53 (33.5)
44 (28.0)
30 (19.0)
29 (18.3)
25 (16.0
19 (12.0)
51 (32.3)
73 (46.2)

53
contraindications listed on the tool. The most common indication for methadone was identified
as neuropathic pain (27; 17.0%) (Table 5). Contraindications were documented for 68 (43.0%)
patients. QTc prolongation/structural heart disease was one of the most common
contraindications for methadone (38; 24.0%) (Table 5).
Table 5: Indications and contraindications for using methadone in the study sample
CNS: Central nervous system; QTc: Corrected QT interval
3. Accepted methadone recommendations
Out of the 37 pharmacist recommendations, 6 (16.21%) were accepted by the physicians and
2 (8.10%) were implemented by the physicians themselves. Most recommendations provided
by pharmacists and physician implemented were provided on Thursday (13; 8.2%).
e. Research question 2
1. Sample stratification
Two groups compared for the inferential analysis were patients provided with pharmacist
methadone recommendation (37; 23.4%) and those with no methadone recommendation (121;
76.5%).
Indications/contraindications n (%)
Indications
Neuropathic pain
Severe renal impairment
Morphine allergy
Refractory to other opioids
High opioid tolerance
Other
QTc prolongation/structural heart disease
Limited prognosis
27 (17.0)
11 (7.0)
4 (2.5)
5 (3.1)
5 (3.1)
6 (4.0)
38 (24.0)
12 (6.0)
Contradictions
Clinically unstable
Severe liver impairment
Use of other long-acting CNS depressants
Drug interactions
Limited prognosis (<5 days)
Substance use disorder
Other
10 (6.3)
7 (4.4)
5 (3.2)
5 (3.2)
3 (2.0)
1 (0.6)
5 (3.2)

54
2. Methadone recommendations by demographic/clinical characteristics
As per the independent t-test analysis, there was a significant difference in pharmacist
methadone recommendations based on the patient’s pain intensity score (p<0.05). Patients with
a high pain intensity score received higher numbers of methadone recommendations compared
to patients with lower pain intensity scores (Table 6).
Table 6: Differences in continuous variables based on methadone recommendation
Mean (SD)
No methadone
recommendation
(n=121)
Methadone
recommendation
(n=37)
T
statistic
p-value
Age 81.0 (13.3) 74.6 (14.4) -2.362 0.091
BMI 24.0 (5.0) 23.0 (5.0) -1.020 0.310
Pain intensity score 3.0 (1.9) 5.1 (1.5) 6.527 <0.05*
PPS at admission 36.5 (12.0) 39.4 (13.4) 1.241 0.217
PPS after admission
37.0 (12.0)
39.6 (13.0)
1.249
0.214
Days from admission 20.0 (20.0) 28.2 (35.0) 1.362 0.181
* Significant results (>0.05), PPS: Palliative prognosis score; SD: Standard deviation
As per the Chi-square analysis, there was a significant difference between hospice type,
terminal indication category, pain type, indication of methadone and whether the pharmacist
provides methadone recommendation or not (p<0.05). The majority (34; 91.9%) of patients
who had an acceptance for methadone recommendation received home hospice service. Cancer
patients received higher numbers (25; 67.6%) of methadone recommendations for pain
management as compared to other terminal diagnosis. Patients who had both nociceptive and
neuropathic type of pain had higher number (16; 43.2%) of methadone recommendations. The
most common (15; 40.5%) indication for which methadone recommendation provided was
neuropathic pain in patients (Table 7).

55
Table 7: Differences in categorical variables based on methadone recommendation
* Significant result (>0.05); CNS: Central nervous system; QTc: Corrected QT interval
n (%)
No methadone
recommendation
(n=121)
Methadone
recommendation
(n=37)
Chi
statistic
p-value
Sex
Female
Male
72 (46.0)
47 (30.0)
17 (11.0)
19 (12.0)
2.150
0.341
Hospice type
Home
Assisted living
Inpatient
Nursing home
87 (55.1)
10 (6.3)
0 (0.0)
20 (13.0)
34 (21.5)
0 (0.0)
0 (0.0)
2 (1.3)
11.548
0.021*
Terminal indication
Cancer
Dementia
Cardiovascular
Respiratory
Liver
Kidney
Neurodegenerative
Other
37 (23.4)
21 (13.3)
36 (23.0)
3 (1.9)
3 (1.9)
3 (1.9)
1 (0.6)
1 (0.6)
25 (16.0)
3 (1.9)
5 (3.2)
0 (0.0)
0 (0.0)
0 (0.0)
5 (3.2)
6 (3.8)
16.972
0.018*
Pain type
Nociceptive
Neuropathic
Both
49 (31.0)
1 (0.6)
18 (11.4)
13 (8.2)
4 (2.5)
16 (0.1)
28.278
0.00*
Indication
Neuropathic
High opioid
Morphine allergy
Refractory opioids
Several renal impairment
8 (5.1)
0 (0.0)
1 (0.6)
2 (1.3)
9 (5.7)
15 (9.5)
2 (1.3)
2 (1.3)
0 (0.0)
0 (0.0)
79.704
0.00*
Contraindications
Clinically stable
Drug interactions
Limited diagnosis (<5)
QTc prolongation
Severe liver impairment
Other CNS depressant
1 (0.6)
1 (0.6)
3 (1.9)
29 (18.4)
6 (3.8)
4 (2.5)
0 (0.0)
1 (0.6)
0 (0.0)
4 (2.5)
0 (0.0)
1 (0.6)
15.045
0.18

56
f. Characteristics of accepted methadone recommendation patients
In total, eight (21.6%) of 32 pharmacist recommendations were accepted by the physicians.
Assessment of the characteristics of all the eight patients in terms of their type of hospice,
type and intensity of pain, terminal diagnosis, overall medication history, indication, and
contraindication of using methadone is summarized in Table 8.
Table 8: Characteristics of patients with accepted methadone recommendations
AML: Adult acute myeloid leukemia; APAP: Acetaminophen; COPD: Chronic obstructive pulmonary diseases; HIV:
Human immunodeficiency virus
Patients with study ID 70 and 94 did not receive pharmacist methadone
recommendations, but it was implemented by the physician themselves. All of the
patients with accepted methadone recommendation were utilizing home hospice. In total,
six (75%) out of eight patients had cancer as their terminal diagnosis. Most
recommendations for methadone were under the indication of neuropathic pain
management (4; 50%); the frequency of pain intensity scores ranging between 5-8 (75%)
was high. The allergic conditions of these patients were either not known or were not
classified under the categorization used in this study. The listed comorbidities of these
patients included cardiovascular disease (6; 75%), respiratory (4; 50%) and kidney (4;
Study
ID
Hospice
type
Pain type/
intensity
Terminal
diagnosis
Medication history Indication
52 Home
Both
5
AML Opioid, APAP, Gabapentin
Neuropathic,
other
54 Home
Nociceptive
5
Prostate
cancer
Opioid Other
67 Home
Nociceptive
7
Colon cancer Opioid, APAP Other
70 Home 2 Throat cancer Opioid
94 Home 3 Lymphoma Opioid
100 Home
Both
5
COPD Opioid Neuropathic
152 Home
Neuropathic
8
Leukemia Opioid, Gabapentin, Other Neuropathic
153 Home
Neuropathic
8
HIV Opioid Neuropathic
57
50%) conditions. The majority (6; 75%) of the nutritional statuses of these patients was
found to be poor.
II. Phase II of the study
a. Study aims
To identify the frequency and expenditure of medications at various hospice and palliative
care settings served by DeltaCareRx. The use of the medications will be evaluated as per
therapeutic class and sex of the patient across the pharmacy claims data.
b. Overview
Individual month PBM data from DeltaCareRx sites were employed to analyze the
therapeutic class and medication utilization. The trends in the utilization were stratified on
patients’ sex and the therapeutic class. The cost per patient depending on their sex was
calculated for each therapeutic class belonging to the medication categories of interest.
Individual drug consumption was evaluated by calculating the total DDD dispensed for the
medicines.
c. Sample characteristics
Overall, the dataset consisted of 445 therapeutic class and 3189 medication names. In total
183,450 medications were identified from the categories of interest in the combined dataset
of all the six months.
d. Research question 1
1. Frequency in the use and expenditure of each therapeutic class in different
months
Descriptive analyses were run using individual month pharmacy claims data to identify the
frequency in use of medications, the total cost and monthly mean cost of each therapeutic
class along with their standard deviation values (± SD).

58
i. January
The total number of prescriptions identified in January was 97,260. Tables 9, 10, and 11
show each therapeutic class's frequency, total costs, and monthly mean average cost with
standard deviation associated with each class.
Overall, in the case of pulmonary medications, there was 4.5% of missing cost data. The
majority of missing cost data was for sympathomimetic medications (101; 96.2%). Table
9 shows the frequency, total, and mean monthly cost expenditure for pulmonary
medications in January. The average monthly cost was highest for bronchodilator-
anticholinergics medications ($171.40) and steroid inhalants ($172.89).
Table 9. Frequency and expenditure of pulmonary medications in January
The amount of cost missing data in the case of pain medication was 4.3% and the majority
(804; 82.76%) of the cost missing data was found for opioid agonists. Table 10
demonstrates the frequency in use and cost data for pain medications in January. The
medications belonging to the opioid agonist therapeutic class were found to have the
highest frequency and highest expenditure.
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Steroid inhalants 97 $16,770.00 $172.89 ($90.57) $154.28 ($120.77)
Sympathomimetics 2,169 $79,851.03 $38.61 ($83.43) $16.87 ($21.93)
Xanthines 26 $2625.06 $58.33 ($50.88) $31.44 ($78.66)
Bronchodilator-
anticholinergic
69 $11,826.80 $171.40 ($196.10) $23.0 ($411.25)
Leukotriene modulators 41 $277.31 $6.76 ($4.19) $5.13 ($5.21)

59
Table 10. Frequency and expenditure of pain medications in January
In the case of anticoagulant medication, there was missing cost data for 3.15% of
prescriptions in the database. The majority of cost missing data was found for heparin and
heparinoid like agents. Table 11 demonstrates the frequency in use and cost data for
anticoagulant medications in January. The total cost and monthly average cost were higher
for direct factor Xa inhibitors than other therapeutic classes in the medication category.
Table 11. Frequency and expenditure of anticoagulant medications in January
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Analgesics 3,722 $16,499.11 $4.54 ($9.70) $3.21 ($2.67)
Anesthetics 27 $171.72 $6.90 ($6.40) $6.58 ($6.02)
Anesthetics topical 337 $8,585.59 $26.17 ($32.64) $26.13 ($19.16)
Opioid agonists 16,448 $321,495.80
$20.41 ($31.38) $13.99 ($13.76)
Opioid
combinations
1,698 $37,142.27 $22.12 ($21.06) $16.26 ($15.67)
Opioid partial
agonists
3 $314.28 $104.80 ($46.06) $110.88 (-)
NSAIDS 411 $4,466.11 $11.36 ($55.31) $11.36 ($4.88)
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Platelet aggregation
inhibitors
123 $1,395.41 $11.43 ($32.18) $3.96 ($3.67)
Direct factor Xa
inhibitors
143 $27,424.42 $194.50 ($72.71) $211.03 ($39.47)
Coumarin
anticoagulants
220 $1239.91 $5.74 ($3.55) $4.94 ($4.41)
Heparin 117 $1135.49 $10.91 ($30.00) $10.91 ($3.57)
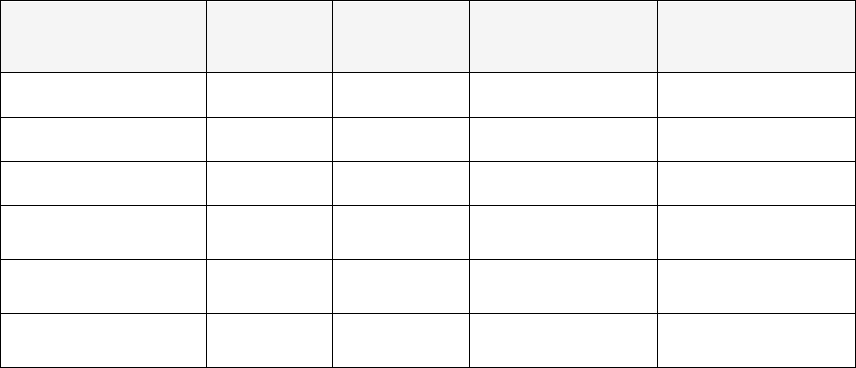
60
ii. June
The total number of prescriptions identified in June was 128,786. Tables 12, 13, and 14
show each therapeutic class's frequency, total costs, and monthly mean average cost with
standard deviation associated with each class.
Table 12 shows the total expenditure of each class belonging to the pulmonary medication
category. The most frequently used therapeutic class was sympathomimetics with higher
total expenditure, and the lowest monthly mean cost.
Table 12. Frequency and expenditure of pulmonary medications in June
In the case of the pain medication category, there was 0.14% of missing cost data. The
opioid agonist therapeutic class had the majority (39; 97.5%) of missing cost data. Table
13 demonstrates the frequency and total expenditure with the average cost per therapeutic
class in the pain medication category.
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Steroid inhalants 143 $20,944.71 $146.5 ($104.91) $135.80 ($206.52)
Sympathomimetics 3360 $124,613.04 $37.09 ($68.59) $18.77 ($19.02)
Xanthines 26 $1,724.29 $45.37 ($42.01) $26.07 ($39.93)
Bronchodilator-
anticholinergic
107 $18,083.71
$169.00
($198.93)
$20.91 ($412.09)
Leukotriene
modulators
41 $701.97 $7.16 ($4.96) $5.20 ($5.20)
Nasal
anticholinergic
6 $227.46 $37.91 ($7.00) $37.21 ($13.36)

61
Table 13. Frequency and expenditure of pain medications in June
Table 14 demonstrates the frequency and total expenditure with the average cost per
therapeutic class in the anticoagulant medication category. The total cost and monthly
average cost were higher for Direct factor Xa inhibitors than other therapeutic classes in
the medication category.
Table 14. Frequency and expenditure of anticoagulant medications in June
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Analgesics 4,598 $23,111.33 $5.02 ($11.90) $3.15 ($2.18)
Anesthetics 33 $396.83 $11.20 ($20.38) $5.13 ($6.49)
Anesthetics topical 579 $15,449.47 $26.68 ($30.84) $17.38 ($25.0)
Opioid agonists 18,609 $412,862.6 $22.186 ($40.21) $14.24 ($13.72)
Opioid
combinations
2,268 $51,164.52 $22.56 ($83.43) $16.24 ($17.31)
Opioid partial
agonists
7 $2,007.06 $286.81 ($119.73) $307.01 ($271.66)
NSAIDS 726 $6,818.57 $9.39 ($16.22) $3.96 ($4.66)
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Platelet
aggregation
inhibitors
249 $2,472.34 $9.93 ($27.21) $5.15 ($3.60)
Direct factor Xa
inhibitors
254 $53,945.07 $212.38 ($65.94) $219.83 ($16.08)
Coumarin
anticoagulants
403 $2,392.38 $5.93 ($3.52) $4.96 ($3.93)
Heparin 143 $1,406.68 $9.84 ($23.05) $4.23 ($4.34)

62
iii. July
In July, there were in total 78,273 prescriptions. Tables 14, 15, and 16 show each
therapeutic class's frequency, total costs, and monthly mean average cost with standard
deviation associated with each class.
The data for pulmonary medication had missing cost data for 0.63% (n=14) prescriptions.
Sympathomimetics and bronchodilator-anticholinergics have the majority of the missing
cost data. Table 15 demonstrates the monthly mean cost for each therapeutic class and the
standard of deviation.
Table 15. Frequency and expenditure of pulmonary medications in July
In the case of pain medications, 0.22% of cost data was missing. Medications belonging
to the opioid agonist had the majority (31; 88.6%) of missing cost data. Table 16 shows
the frequency, total cost, and average monthly expenditure on every therapeutic class of
pain medication category.
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Steroid inhalants 68 $10,611.36 $156.00 ($118.71) $138.0 ($102.4)
Sympathomimetics 1,991 $75,933.73 $38.00 ($77.73) $18.7 ($20.39)
Xanthines 22 $1,221.96 $56.00 ($53) $36.71 ($68.33)
Bronchodilator-
anticholinergic
78 $11,973.79 $169.00 ($216.54) $20.91 ($413.0)
Leukotriene
modulators
64 $465.34 $7.00 ($5) $5.20 ($5.81)
Nasal
anticholinergic
1 $44.59 - -

63
Table 16. Frequency and expenditure of pain medications in July
Table 17 demonstrates the frequency of each therapeutic class of anticoagulant medication in
July. The total cost and month mean cost is higher for direct factor Xa inhibitors. Coumarin
anticoagulants were found to have the lowest mean average expenditure in the month.
Table 17. Frequency and expenditure of anticoagulant medications in July
iv. September
In total, 134,478 the number of prescriptions were identified in September. Tables 18, 19,
and 20 demonstrate the frequency of each therapeutic class, total costs, and monthly mean
average cost with standard deviation associated with each class.
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Analgesics 2633 $9,894.83 $3.76 ($4.31) $3.12 ($1.95)
Anesthetics 14 $228.80 $16.34 ($25.11) $5.13 ($12.48)
Anesthetics
topical
413 $12,887.99 $31.20 ($59.28) $16.80 ($24.10)
Opioid agonists 11,060 $242,094.09 $21.94 ($37.32) $14.20 ($13.86)
Opioid
combinations
1,353 $29,050.80 $21.47 ($19.67) $16.80 ($16.91)
Opioid partial
agonists
2 $448.21 $224.10 ($108.39) $224.10 (-)
NSAIDS 444 $3,938.72 $8.87 ($14.4) $4.36 ($5.18)
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Platelet
aggregation
inhibitors
154 $1,874.12 $12.12 ($32.93) $5.29 ($4.43)
Direct factor Xa
inhibitors
159 $32,307.50 $203.19 ($62.66) $219.70 ($19.70)
Coumarin
anticoagulants
248 $1,428.83 $5.76 ($3.56) $4.75 ($3.73)
Heparin 88 $1,357.40 $15.43 ($51.6) $3.48 ($3.29)

64
The majority of therapeutic class utilized was found to be sympathomimetics (90.38%)
than other therapeutic classes in the pulmonary medication category. The mean average
cost lowest for leukotriene modulators ($9). The sympathomimetics had the highest
frequency in use (90.30%) and expenditure ($135,346) among all the other therapeutic
classes.
Table 18. Frequency and expenditure of pulmonary medications in September
In the case of pain medications, there was missing cost data for 0.11% of medications. The
majority (25; 83.3%) of the missing cost data was found for the opioid agonist therapeutic
class medications. Table 19 shows the frequency and mean average cost of each
therapeutic class belonging to pain medications. Opioid partial agonists constitute the
highest monthly average cost ($164.25) and the lowest frequency (0.2%).
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Steroid inhalants 104 $20,967 $151.60 ($111.72) $155.80 ($229.11)
Sympathomimetics 3,648 $135,846 $37.00 ($71.68) $18.80 ($19.02)
Xanthines 32 $1,425 $45.00 ($48.81) $21.79 ($35.46)
Bronchodilator-
anticholinergic
137 $21,476 $160.00 ($191.44) $21.34 ($412.12)
Leukotriene
modulators
107 $959 $9.00 ($8.35) $5.57 ($6.48)
Nasal
anticholinergic
9 $374 $42.00 ($7.63) $44.60 ($11.0)

65
Table 19. Frequency and expenditure of pain medications in September
Table 20 demonstrates the frequency of therapeutic classes in the anticoagulant medication
category and their average monthly cost. Coumarin anticoagulants have the highest
frequency (37%) and the lowest monthly average cost ($5.85).
Table 20. Frequency and expenditure of anticoagulant medications in September
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Analgesics 4,655 $17,481.75 $3.75 ($4.08) $3.10 ($1.85)
Anesthetics 24 $499.20 $20.80 ($32.16) $5.13 ($18.70)
Anesthetics topical 729 $16,541.09 $22.69 ($26.72) $15.39 ($20.0)
Opioid agonists 18,936 $411,022.38
$21.70 ($36.62) $14.24 ($13.70)
Opioid
combinations
2,369 $51,420.55 $21.70 ($19.53) $16.70 ($16.83)
Opioid partial
agonists
7 $1,149.78 $164.25 ($115.66) $147.46 ($42.00)
NSAIDS 847 $8,660.30 $10.22 ($16.78) $4.43 ($5.95)
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Platelet aggregation
inhibitors
258 $3,370.06 $13.06 ($32.86) $4.49 ($3.34)
Direct factor Xa
inhibitors
306 $61,918.32
$202.34 ($55.51) $22.58 ($20.96)
Coumarin
anticoagulants
411 $2,404.12 $5.85 ($3.62) $4.94 ($3.34)
Heparin 143 $2,337.63 $16.34 ($45.75) $3.82 ($3.60)

66
v. October
In total, there were 158,830 prescriptions identified in the month of October. Tables 21,
22, and 23 demonstrate the frequency of each therapeutic class, total costs, and monthly
mean average cost with standard deviation associated with each class.
In the case of pulmonary medications, there was 0.9% of missing cost data found for the
identified prescriptions. In total, 71.4% of missing cost data was populated by imputing the
data from known values in the database for the pulmonary medications.
Table 21 shows the frequency, cost of the expenditure, and average monthly cost of the
therapeutic lasses in the pulmonary medication category of October. Sympathomimetics
were found to have the highest frequency of use (90.36%) and the highest expenditure
($135,846) with moderately low ($37) monthly mean cost.
Table 21. Frequency and expenditure of pulmonary medications in October
In October's pain medication prescriptions, the cost data were missing for 0.7% of the
prescriptions. In total, 20% of missing data was filled by imputing the values from the
existing prescription data. Table 22 shows the frequency, total cost, and monthly average
Therapeutic class Frequency Total cost
Monthly mean
cost (SD)
Monthly median
cost (IQR)
Steroid inhalants 197 $24,547.01 $131.26 ($87.50) $84.70 ($144.50)
Sympathomimetics 4151 $146,610.45 $35.25 ($70.00) $17.42 ($21.14)
Xanthines 29 $1,658.71 $57.20 ($51.22) $47.36 ($71.25)
Bronchodilator-
anticholinergic
183 $28,578.8 $160.55 ($212.40) $21.24 ($400.13)
Leukotriene
modulators
109 $942.95 $9 ($8.21) $5.41 ($5.81)
Nasal
anticholinergic
14 $487.2 $34.80 ($4.50) $33.10 (-)

67
cost data, which consisted of 0.58% of missing cost data. The highest frequency of use and
expenditure was found for the opioid agonist therapeutic class.
Table 22. Frequency of use and expenditure of pain medications in October
Table 23 demonstrates the frequency and cost data for the anticoagulant medications in the
month of October. Direct factor Xa inhibitors were found to have the highest expenditure and
higher monthly mean cost ($202.34) compared to other therapeutic classes in the medication
category.
Table 23. Frequency and expenditure of anticoagulant medications in October
Therapeutic class Frequency Total cost
Monthly
mean cost (SD)
Monthly
median cost (IQR)
Analgesics 5,427 $24,170.05 $3.84 ($5.35) $3.10 ($1.97)
Anesthetics 61 $430.72 $7.55 ($9.40) $4.68 ($4.49)
Anesthetics topical 864 $19,402.18 $22.45 ($27.88) $15.52 ($16.13)
Opioid agonists 22,600 $475,431.75 $21.20 ($35.72) $13.45 ($14.36)
Opioid
combinations
2596 $58,755.08 $22.63 ($21.51) $17.71 ($17.83)
Opioid partial
agonists
4 $1,481.06 $370.26 ($176.98) $325.04 ($323.81)
NSAIDS 965 $9,481.32 $9.82 ($16.00) $5.03 ($6.20)
Therapeutic class Frequency Total cost
Monthly
mean cost (SD)
Monthly
median cost (IQR)
Platelet aggregation
inhibitors
289 $4,267.05 $14.81 ($38.03) $5.29 ($3.68)
Direct factor Xa
inhibitors
330 $69,484.23
$210.55
($58.49)
$220.51 ($19.83)
Coumarin
anticoagulants
472 $2,668.22 $5.74 ($3.57) $5.04 ($4.27)
Heparin 193 $5,521.28 $28.90 ($75.04) $3.48 ($4.44)

68
vi. November
In total, there were 134,840 prescriptions identified in the month of November. Tables 24,
25, and 26 demonstrate the frequency of each therapeutic class, total costs, and monthly
mean average cost with standard deviation associated with each medication category.
In the pulmonary medication category, 12.3% of cost data was found to be missing for the
prescriptions. In total (425; 25.4%) of missing cost data was filled with the help of imputing
technique. Table 24 consists of frequency, total cost, and mean average cost from the
pulmonary medication data, which has (337; 9.18%) of missing cost data. The
sympathomimetics were found to have the highest utilization and expenditure with
moderate month mean cost. The overall expenditure of pulmonary medications in
November was the highest ($200,430.90) compared to other months' data.
Table 24. Frequency and expenditure of pulmonary medications in November
In the pain medication category, (7311; 34%) of cost data was found to be missing for the
prescriptions. From the total missing data (6045; 23.24%) of cost data was filled by
imputing the values. Table 25 shows the values from the database, which consisted of 28%
of missing cost data. Opioid agonists had the highest utilization and expenditure among
all the therapeutic classes. The analgesics medication had the lowest ($3.90) mean average
cost value.
Therapeutic class Frequency Total cost
Monthly
mean cost (SD)
Monthly
median cost (IQR)
Steroid inhalants 200 $27,697.12 $152.18 ($114.70) $137.50 ($52.85)
Sympathomimetics 4,943 $152,986.75 $32.90 ($55.61) $19.25 ($15.22)
Xanthines 38 $1,502.88 $44.20 ($43.87) $21.79 ($48.0)
Bronchodilator-
anticholinergic
147 $17,395.88 $168.89 ($196.66) $21.29 ($412.39)
Leukotriene
modulators
130 $766.46 $7.36 ($4.93) $6.0 ($5.01)
Nasal
anticholinergic
2 $81.8 $41 ($6.30) -

69
Table 25. Frequency and expenditure of pain medications in November
In the case of the anticoagulant medication category, the prescription had 60% of missing
data for cost. The missing data filled by imputing was about 26%. A high percentage (50%)
of missing quantity data caused higher missing cost data and a low number of imputed
values in the database. Table 26 demonstrates the values from the database, which
consisted of 47% of missing cost data. The majority (38.03%) of missing cost data was
for coumarin anticoagulant’s prescriptions. Direct factor Xa inhibitors were found to have
the highest utilization, expenditure, and mean average cost value.
Table 26. Frequency and expenditure of anticoagulant medications in November
Therapeutic class Frequency Total cost
Monthly
mean cost (SD)
Monthly
median cost (IQR)
Analgesics 6,613 $24,079.25 $3.90 ($4.31) $3.32 ($1.62)
Anesthetics 26 $301.47 $17.73 ($29.64) $5.87 ($9.37)
Anesthetics
topical
1,032 $16,217.97 $23.04 ($24.70) $16.06 ($17.97)
Opioid agonists 26,223 $478,817.92
$22.74 ($35.89) $15.22 ($12.78)
Opioid
combinations
3,286 $50,281.78 $22.16 ($19.98) $16.82 ($16.59)
Opioid partial
agonists
8 $2,231.58 $278.94 ($166.55) $231.0 ($141.11)
NSAIDS 1,178 $7,665.50 $9.12 ($16.74) $4.30 ($5.0)
Therapeutic class Frequency Total cost
Monthly
mean cost (SD)
Monthly
median cost (IQR)
Platelet aggregation
inhibitors
269 $4123.84 $15.38 ($37.03) $5.29 ($4.31)
Direct factor Xa
inhibitors
461 $67,795.13
$214.54 ($69.93) $220.32 ($19.86)
Coumarin
anticoagulants
421 $2394.0 $6.0 ($3.36) $5.05 ($3.50)
Heparin 128 $10,968.53
$86.36 ($169.41) $4.85 ($24.90)

70
e. Overall costs per therapeutic class in the combined dataset of all the months
i. Pain medication category
In total 154,576 pain medication claims were identified from the combined dataset. The
data consisted of missing cost data (8574, 5.54%). Table 27 shows the descriptive
statistics of pain medication category in the combined data set from all the months.
Table 27. Overall cost descriptive statistics for pain medication category
ii. Pulmonary medication category
In total, 22,523 pulmonary prescription data were found in the combined dataset of all the
months. The data consisted of missing cost data (484, 2.15%). Table 28 shows the
descriptive statistics of pain medication category in the combined data set from all the
months.
Therapeutic class Mean (SD) Median (IQR)
Analgesics $4.28 ($8.04) $3.16 ($2.00)
Anesthetics $11.77 ($20.37) $5.13 ($6.60)
NSAIDS $9.71 ($24.54) $4.34 ($4.94)
Opioid combinations $22.16 ($20.75) $16.48 ($16.63)
Opioids agonists $21.75 ($36.33) $14.24 ($13.76)
Partial agonists $246.21 ($230.97) $230.97 ($159.55)
Topical anesthetics $24.62 ($19.50) $16.53 ($19.50)

71
Table 28. Overall cost descriptive statistics for pulmonary medication category
iii. Anticoagulant medication category
In total, 6,351 pulmonary prescription data were found in the combined dataset of all the
months. The data consisted of missing cost data (570, 9%). Table 29 shows the
descriptive statistics of pain medication category in the combined data set from all the
months.
Table 29. Overall cost descriptive statistics for anticoagulant medication category
Therapeutic class Mean (SD) Median (IQR)
Coumarin anticoagulants $6.00 ($3.52) $4.94 ($3.84)
Direct factor Xa $207.75 ($63.80) $219.85 ($19.64)
Heparin $28.55 ($3.74) $3.74 ($4.19)
Platelet aggregation inhibitors $13.07 ($5.29) $5.30 ($8.84)
Therapeutic class Mean (SD) Median (IQR)
Bronchodilator – anticholinergic $165.16 ($201.87) $21.28 ($412.40)
Leukotriene modulators $7.89 ($6.51) $5.36 ($5.81)
Nasal anticholinergic $37.53 ($6.61) $33.31 ($11.79)
Steroid inhalants $140.67 ($106.94) $137.50 ($122.56)
Sympathomimetics $36.04 ($69.44) $18.50 ($19.53)
Xanthines $51.00 ($47.93) $26.07 ($68.00)

72
f. Research question 2
The dataset of each month was stratified as per the patient’s sex. The cost for each subset
was calculated. Total data of dispensed medications of interest were found for 88,601 male
and 124,389 female patients
1. Trends in utilization as per patients’ sex
Table 30 demonstrates the stratification of frequency data as per the patient’s sex for all
the anticoagulant medications. The patient’s sex data was missing for certain prescriptions
in the month of January (1.7%), June (50%), and for the month of November due to 47%
of missing cost data the expenditure was found to be lower compared to the frequency of
the use of certain medications. In the majority of months, the frequency of anticoagulants
used in female patients was found to be higher. The therapeutic class most frequently used
in all the months in male and female patients was coumarin anticoagulants. In terms of
expenditure, direct factor Xa inhibitors constituted the highest expenditure in all the months
for both males and females.
Table 30: Trends in anticoagulant medication utilization as per patients’ sex
Month
Frequency
in male
patients
Cost
Frequency
in female
patients
Cost
January 271 $11,203.52 319 $19,968.23
June 222 $12,773.13 304 $19,230.51
July 266 $16,685.28 383 $20,282.57
September 466 $28,061.88 652 $41,968.25
October 583 $36,278.42 727 $47,321.07
November 666 $31,412.59 1,003 $53,868.90
Table 31 shows similar stratification data for patients using pain medications. The patient’s
sex data was missing for certain prescriptions in the month of January (0.5%) and June
(47.4%) and 25% of missing cost data for the prescriptions in November. The values for
pain medication categories were highest for both females and males than other medication

73
categories. Female patients had a higher pain medication utilization. Opioid agonist was
found to be the most commonly used class in both males and females. In June and July,
opioid combinations used in females were found to form a significant part of the
expenditure of the therapeutic class ($113,121.07 and $126,663.8). In November, female
patients were found to be administered the highest in the number of pain medications such
as non-steroidal anti-inflammatory drugs (805; 3%), opioid combinations (2,235; 0.9%),
and opioid agonists (17,745; 68%).
Table 31: Trends in pain medication utilization as per patients’ sex
Month
Frequency
in male
patients
Cost
Frequency
in female
patients
Cost
January 9,362 $161,651.25 13,171 $223,667.19
June 5,715 $113,121.07 8,355 $154,670.72
July 6,425 $126,663.8 9,469 $171,739.3
September 11,252 $215,511.2 16,315 $291,263.9
October 13,754 $258,542.05 18,865 $293,689.88
November 15,344 $239,861.60 22,117 $310,604.4
Table 32 demonstrates trends in all the therapeutic classes of pulmonary medication
utilization as per the patient’s sex. The patient’s sex data was missing for certain
prescriptions in January (1.5%) and June (47.2%). The missing cost data (9.18%) in the
month of November shows the cost values lower compared to the frequency in use of
medications. Sympathomimetics were highly used in both males and females; it constituted
the high expenditure in all the months.

74
Table 32: Trends in pulmonary medication utilization as per patients’ sex
Month
Frequency
in male
patients
Cost
Frequency
in female
patients
Cost
January 1,050 $52,288.81 1,322 $55,591.52
June 824 $33,681.99 1,062 $43,822.29
July 940 $41,184.77 1,284 $59,066.00
September 1,755 $79,736.37 2,213 $100,913.4
October 1,954 $88,792.66 2,725 $114,032.46
November 2,388 $87,879.98 3,072 $112,550.92
2. The difference in mean cost per patients across individual months and all the
months
Table 33 and Table 34 demonstrate the differences in mean cost per male and female
patients across three medical categories. The mean cost per patient values for June
differentiate from the other months due to significant missing gender data that was 50% of
the total anticoagulant medications, 47.4% of the total pain medications and 47% of the
total pulmonary medications. The mean of the cost per patients were found to be higher for
June, September, and November because of comparatively higher proportions of opioid
partial agonist prescriptions in the particular month’s pharmacy claims data.

75
Table 33. Differences in per male patient mean cost across three medical categories
SD: standard deviation
Table 34: Differences in per female patient mean cost across three medical categories
SD: standard deviation
g. Research question 3
The utilization of the medications was calculated using the quantity and strength data of
the prescriptions available in the database. The quantity data for the prescriptions was
Month
Mean cost per
male patients
(anticoagulants)
+/- SD
Mean cost
per male
patients
(pain)
+/-SD
Mean cost
per male
patients
(pulmonary)
+/-SD
January $48.16 $86.54 $15.12 $8.60 $66.95 $73.05
June $58.05 $104.54 $51.75 $112.45 $58.15 $78.50
July $61.00 $98.47 $14.71 $12.73 $66.82 $70.49
September $58.70 $91.12 $40.35 $60.57 $60.72 $65.65
October $63.44 $86.021 $14.63 $8.28 $74.84 $66.66
November $50.27 $64.92 $39.00 $81.56 $61.48 $64.50
Month
Mean cost per
female patients
(anticoagulants)
+/- SD
Mean cost
per female
patients
(pain)
+/- SD
Mean cost
per female
patients
(pulmonary)
+/- SD
January $60.80 $93.66 $25.39 $27.83 $68.28 $82.54
June $60.50 $103.33 $48.54 $78.06 $53.94 $66.62
July $57.40 $94.65 $19.55 $16.10 $72.02 $79.92
September $60.00 $98.76 $30.94 $44.31 $74.80 $67.15
October $63.60 $83.40 $11.00 $9.21 $66.31 $53.69
November $57.70 $66.50 $37.27 $91.24 $71.31 $74.75

76
missing for the months of January, June and July. Therefore, DDD values was calculated
for prescriptions in the months of September, October and November.
Consumption level of anticoagulants varied during the months of September, October, and
November (Table 35). Heparin was highly consumed in all the three months (638 DDDs
dispensed, 627 DDDs and 22,095 DDDs). Warfarin consumption level was found to be
stable in the months of October (43 DDDs) and November (40 DDDs). The overall pattern
in use of anticoagulants as per the PBM data was not found to be consistent.
Table 35. Total anticoagulant medication DDDs dispensed
Generic long names September October November
apixaban 85.03 40.00 41.80
heparin 637.73 627.93 22,095.17
warfarin 7.00 43.00 39.75
cilostazol 4.75 1.45 1.50
ticagrelor - 1.50 2.10
rivaroxaban 28.25 15.45 16.88
prasugrel 0.50 - 0.50
clopidogrel 128.13 68.00 76.96
enoxaparin 147.00 248.22
160.00
In the case of pain medications, consumption level was seen uniform for acetaminophen
and morphine (Table 36). Hydromorphone utilization uniformly increased from 517 DDDs
in September to 1209 DDDs in November. Overall consumption of the following
medications, ibuprofen (193 DDDs), methadone (741 DDDs), Oxycodone (628 DDDs)
and tramadol (338 DDDs), was found to higher in September. The consumption of fentanyl
was lowest in October (5 DDDs) and November (0.005 DDD) and highest in September
(6346 DDDs).

77
Table 36. Total pain medication DDDs dispensed
Generic long names September October November
APAP 626.92 489.44 520.70
diclofenac 10.10 0.206 0.173
ibuprofen 193.00 98.40 91.30
indomethacin 3.01 1.12 23.6
ketorolac 1.80 0.42 -
meloxicam 104.15 61.52 51.36
celecoxib 36.92 15.22 17.80
piroxicam - - 0.5
oxaprozine 13.33 - 0.66
etodolac 3.00 - 1.00
hydromorphone 517.22 1052.4 1209.50
methadone 741.70 368.45 337.60
morphine 1934.32 1095.10 1111.72
oxycodone 628.25 355.87 362.23
tramadol 337.64 136.36 178.11
buprenorphine 1.67 0.043 0.003
codeine 6.31 1.06 0.75
fentanyl 6345.84 5.46 0.01
Table 37 shows the comparable values of consumption for the pulmonary medications
across the three months. Pulmonary medication like albuterol, fluticasone, ipratropium,
theophylline was most commonly consumed in all the three months.

78
Table 37. Total pulmonary medication DDDs dispensed
Generic long names September October November
albuterol 27,534.50 13,822.40 14,793.50
budesonide 71.00 34.35 53.00
beclomethasone - 0.10 -
fluticasone 5.40 4.17 2.00
ipratropium 575.72 217.97 242.56
tiotropium 51.00 0.03 18.00
theophylline 17.13 7.31 7.10
phenylephrine 8.42 4.21 0.30
pseudoephedrine 966.70 564.51 866.60
79
CHAPTER 5: DISCUSSION
I. Key findings
The current study described the prevalence of pharmacists’ methadone recommendation
and medication utilization in hospice and palliative care settings. Phase I of the study
prospectively evaluated the rate of pharmacists’ methadone recommendations and their
acceptance at DeltaCareRx’s facilities. The second phase of the study retrospectively
assessed the frequency and expenditure of utilizing pain, pulmonary, and anticoagulants
medication at various DeltaCareRx client sites. The primary focus of the research study
was identifying potential ways for cost optimization at various client sites of DeltaCareRx.
The results of the systematic literature review conducted in this study support the
recommendation made by ASHP of involving clinical pharmacist in hospice and palliative
care multidisciplinary team. Although the objective of the study was assessing medication
utilization including methadone at DeltaCareRx’s client sites the current literature review
answers a slightly different research question. A pilot systematic review was conducted in
the initial stages of the study to lay the foundation of methadone use in hospice and
palliative care settings and its cost-effectiveness properties. The literature review consisted
of key terms such as methadone, hospice care setting, cost, and clinical benefits. However,
the preliminary search strategy failed to generate a higher number of evidence articles for
the hypothesis. The archived articles demonstrated the benefits of using methadone in all
types of pain, cancer-related pain, and its cost-effectiveness as compared to other
opioids.
45,77,78
The increasing expenditure and resources used in hospice and palliative care requires cost
savings to be generated at the hospice provider sites.
79
Few of the suggested ways include
the use of PBM services to secure lower for the prescription drugs, leveraging pharmacist
role in the multidisciplinary team, ensure adherence to the formulary, and serving patients
with cost-effective medications to achieve desirable outcomes.
80
Cost analysis in end-of-
life care is challenging due to difficulties quantifying the quality of life concept in patients
treated for their terminal illness. The need for cost saving in this setting requires
80
implications of various ways for cost containment.
21
Therefore, the study explores the
prevalence of cost-effective methadone medication and the overall frequency, expenditure,
and consumption of medications at the hospice and palliative care DeltaCareRx sites. The
methadone use results provide evidence of popularity in using the cost and clinically
effective medication for pain management.
81
The results of evaluating frequency and
expenditure of the most population medication categories pain, pulmonary, and
anticoagulants provide a head start to develop cost containment strategies at DeltaCareRx
client sites.
The results of phase one included the prevalence of methadone recommendation and its
acceptance in patients admitted at DeltaCareRx’s hospice and palliative care sites. The
pharmacist data collection tool #1 identified patients’ demographic and clinical
characteristics. The majority of patients opted for home hospice type of care and observed
experiencing both nociceptive and neuropathic pain. The overall prevalence and
acceptance of methadone recommendations were too low.
ASHP endorsed methadone for its use in pain relief as an effective medication use in
hospice and palliative care.
82
Methadone use for pain relief suggests constant monitoring
of patients and titration of doses frequently.
39
Current study provides potential reasons for
the low use of methadone in hospice and palliative care settings. The literature identifies
two conditions for low methadone use in patients with pain, which are QTc prolongation
and respiratory disorders.
83
In the current study, QTc prolongation was one of the
conditions found to be common among patients. In alignment with the literature, this can
be one of the potential reasons for the low prevalence and acceptance of methadone
recommendation.
The low acceptance rates of pharmacist’s recommendations also raise a concern regarding
awareness of methadone use for pain management. Hawely et al. explore the barriers to
continuing methadone prescription for pain management. Despite patients’ willingness to
receive methadone, there were barriers to receiving the treatment. The low popularity and
knowledge about the use of methadone among healthcare professionals contributed to its
81
low accessibility.
84
According to the literature, methadone is a potential treatment for
neuropathic pain because it is an N-methyl-D-aspartate (NMD) receptor antagonist and
prevents monoamine reuptake.
85
Results in the current study supports the effectiveness of
methadone in this type of pain. The accepted methadone recommendation was most
commonly accepted in patients with neuropathic pain (4 out of 8; 50%).
The Medicare billing policy changes encourage hospice cost-sharing for medication
utilization. Various measures are taken to promote the optimization of cost containment
and quality improvement for hospice providers. One such way is episode-based payment
models, which “gives health care providers a spending target for most types of care
provided during a clinical episode (e.g., six months of chemotherapy, an inpatient
admission or outpatient procedure plus most other care provided in the subsequent 90
days). If total spending is less than the target, Medicare pays providers a bonus; if total
spending is more than the target, Medicare recoups money from providers.”
86
In order to start developing cost-saving strategies for DeltaCareRx’s hospice providers, it
was essential to study the overall utilization of the most common medication categories in
hospice and palliative care settings. PBM claims data obtained from DeltaCareRx was
analyzed for this purpose. Overall, the frequency of pain medication use was comparatively
higher than that of pulmonary and anticoagulant medication categories. Opioid agonists
were most frequently used across the months. According to literature, opioids are prevalent
in use at hospice and palliative care sites due to pain being one of the important symptoms
experienced by these patients.
87
The key findings of the analysis demonstrate the frequency in the use of therapeutic classes,
their total and average monthly expenditure. It displays the overall expenditure between
various classes of individual medication categories. The findings can assist in designing
and executing a cost-saving strategy for each category. The known clinically effective but
cost-effective medications can be used more than the expensive ones from particular
categories. For example, the total DDDs of morphine medication dispensed is uniformly
higher in all the months. In terms of consumption of cost-saving medications like
82
methadone was found to be as much as other opioid medications. Overall, it displays a
comparable use of this medication among the pain medications at different hospice and
palliative care sites of DeltaCareRx.
In November, the frequency in use of pulmonary medications was the highest (5,460), and
due to missing cost data (9.18%), the total expenditure ($200,430.81) did not align with
the frequency. The average costs for pulmonary medications per male patients and female
patients across all the months was found to be $64.82 and $67.77. Sympathomimetics (like
albuterol, ipratropium, ipratropium bromide, arformoterol) was the most commonly used
therapeutic class. The expenditure of sympathomimetics was also found higher in all the
months. The average cost of the overall class in all the months was between the range of
($31-$38). This implies that the therapeutic class used in this class does not increase the
expenditure of the overall medication category.
Coumarin anticoagulants (warfarin sodium), had a uniform frequency in use across all the
months (January – 32%, June – 32.41%, July – 38.21%, September – 38%, October –
36.80%, November – 37.44%). The average costs for anticoagulant medications per male
patients and female patients across all the months was found to be $56.60 and $60.00.
Among all the therapeutic classes in the anticoagulant medication category, direct factor
Xa medications (like rivaroxaban and apixaban) were found to have the highest monthly
mean in all the months. Therefore, the formulary at DeltaCareRx can include cost-effective
direct factor Xa medications to increase cost savings at the client sites.
The frequency in use of opioid agonists (like morphine, fentanyl, hydromorphone,
oxycodone, tramadol, methadone) in all the six months was high (January – 72.61%, June
– 69.40%, July – 69.70%, September – 70.20%, October – 71.16%, November – 68.35%).
The overall frequency of pain medication was higher, but the per male and female patient
cost was not found to be that higher ($29.30 and $28.77). The higher prevalence of pain
symptoms among hospice and palliative care patients increases the overall use of these
drugs, increasing the total expenditure. The use of opioid partial agonists (like
buprenorphine) varied throughout the months. The higher cost of the medication in this
83
therapeutic class skewed the overall monthly mean cost for September, October, and
November. In terms of the pain medication category, any cost-effective medication used
in any therapeutic class will help in overall cost optimization at DeltaCareRx sites. The
results from the phase I of the study can be useful in road mapping an increase in the use
of methadone at the client sites to increase cost savings.
The prescription claims data obtained from DeltaCareRx identifies the cost savings
associated with methadone use at client sites. Methadone belongs to the opioid agonist
therapeutic class. In the combined data set of all the months, the overall frequency and
expenditure of methadone was found to be 10,993 and $110,376.49. The overall frequency
and expenditure of all other opioid agonists was found to be 48,305 and $135,79,26.494.
Therefore, the average expenditure of using methadone ($10) was lower compared to all
other opioid agonists ($28).
The key finding of the analysis evaluating medication utilization as per sex of the patient
demonstrated higher use of pain medication among female patients. In alignment with the
evidence available in the literature that women patients have the higher chances of
receiving pain medications. There are various explanation for this bais in use of pain
management medications such as high incidence of osteoporosis among women, biological
factors, higher adverse events of analgesic use in men compared to women and at time
phycians’s gender also influences their clinical judgemnet of medication prescription to
their male or female patients.
88,89
Evaluation of medication consumption was evaluated using DDD values of individual
medications in different months. The advantage of using the DDD methodology is that the
utilization of the medications can be compared across different months in a standardized
manner. The DDD values vary throughout due to differences in the consumption of
medications with specific strength and quantity. This can be explained with an example
such as warfarin consumption in September (7 DDD), October (42 DDD), and November
(39.95 DDD). The consumption of warfarin tablets with a 7.5 mg strength was higher in
October and November than in September.
84
Heparin and heparinoid-like agents such as enoxaparin were the most commonly utilized
anticoagulant medications in all the three months. The most commonly utilized direct
factor Xa inhibitor was apixaban and rivaroxaban. The utilization of opioid agonists was
found to be consistent in all three months, which was followed by APAP. The utilization
of different NSAIDs varied across the three months. Albuterol had higher consumption
values in all three months.
The major disadvantage of using DDD methodology is the difference between the
prescribed daily dose and WHO recommended DDD. Another limitation is that the DDD
values do not account for the potency of the drugs but depends on the frequency in use of
the dose of each drug.
90
Also, the database used for this study consisted of missing quantity
values for the prescriptions. This influences the DDD values acquired for a particular
medication. Additionally, the DDD values for combinations products other than products
listed in Appendix 4 was not available on the WHO DDD website. The database consisted
majorly of these combination products whose consumption cannot be studied due to the
unavailability of DDD values.
II. Limitations and future considerations
The study consisted of some limitations that may have impacted the results and are
important to consider for a clear interpretation of the study results.
a. Clinical outcomes of methadone use
The study evaluates the prevalence and acceptance of methadone recommendations. As a
part of future research avenues the accepted methadone recommendations can be followed
up for clinical outcomes. The clinical benefit of methadone use can be studied by following
the accepted patients for methadone use. A clinical trial study in patients with chronic and
opioid dependence when treated with methadone demonstrated improvement in pain
compared to buprenorphine.
91
Methadone has clinical advantages of relieving chronic pain,
longer half-life, safety in use despite renal and liver disease, and no active metabolites.
39
85
These treatment benefits can be identified by following the patients for their improvement
in pain management.
b. Generalizability
The data for phase one was collected from selective sites of DeltaCareRx. The geographical
location of the selected sites was not available to the researchers. The collected data was
not from all the sites of DeltaCareRx. This bias the study results. The methadone
recommendation might be low only at DeltaCareRx sites compared to other hospice and
palliative care settings. A comparative study between DeltaCareRx sites and non
DeltaCareRx hospice providers can help understand these differences in methadone use.
c. Limitation of using PBM claims database
The PBM claims database did not have any lab values such as patient’s pain scores, FEV1
values, partial thromboplastin time (PTT), etc. of the patients. Therefore, the clinical
benefit of using the medications was not quantified. The differences in higher and lower
consumption levels of various medications could have been aligned with the patients'
disease condition's incidence.
d. Impact of missing data
The inclusion of data from all the months of 2019 will help evaluate the trends in the
utilization of the medications over a year. The results based on the individual month data
are standalone and cannot be extrapolated to the use of medications in the whole year. Also,
geographical differences in the frequency of medication use and its costs can be valuable
to study. The facility-based evaluation will allow exploring differences in the utilization of
medications as per their geography.
III. Study implications and conclusion
Overall, the study provides evidence on the use of the pharmacist role and medication
utilization in hospice and palliative care settings. As per ASHP inclusion of pharmacists
does have a positive impact on the multidisciplinary hospice team. In this study, it was seen
86
that the pharmacists’ recommendation helped in increasing the probability of the use of
cost-effective treatments like methadone. The use of methadone in hospice and palliative
care setting is still a topic of discussion. The results of this study support the evidence of
its low popularity of use due to various reasons. The study provides a starting point in
understating the prevalence of methadone use in a real-world setting. The findings from
this research have implicated on importance of methadone use at DeltaCareRx sites and
how can the staff be trained on its use. The frequency and monthly average cost results will
help to develop a roadmap of increasing the use of cost-effective medications. The
formulary provided to the hospice sites by DeltaCareRx may include medications that were
most frequently used and less costly.

87
REFERENCES
1. Medicare-hospice-benefits. In. CMS: Centers for Medicare and Mediciad
services; 2019.
2. WHO. WHO defination of palliative care.
https://www.who.int/cancer/palliative/definition/en/
. Accessed November 4,
2019.
3. Palliative Care vs. Hospice Care. Centers for Medicare and Medicaid services.
https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-
Prevention/Medicaid-Integrity-Education/Downloads/infograph-PalliativeCare-
[June-2015].pdf. Published 2015. Accessed November 4, 2019.
4. Facts and figures hospice care in America.
https://legacy.nhpco.org/sites/default/files/public/Statistics_Research/2017_Facts_
Figures.pdf. Published 2017. Accessed November 28, 2019.
5. NHPCO facts and figures National Hospice and Palliative Care Organization
https://www.nhpco.org/wp-
content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Published 2018.
Accessed November 28, 2019.
6. America’s Care of Serious Illness. Center to Advance Palliative Care A STATE-
BY-STATE REPORT CARD ON ACCESS TO PALLIATIVE CARE IN OUR
NATION’S HOSPITALS Web site. Published 2019. Accessed November 18,
2019.
7. Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The
Growth of Palliative Care in U.S. Hospitals: A Status Report. J Palliat Med.
2016;19(1):8-15.
8. Care CtAPCaNP. America's care of serious illness https://reportcard.capc.org/
.
Accessed 05/08, 2020.
9. Quality of Life Matters: End of life care news and clinical findings for physicians.
NewsLine Web site.
https://www.wregional.com/Uploads/Public/Documents/Hospice/Hospice_QOLM
_18-3_newsletter.pdf. Accessed December 23, 2019.
10. Jennifer S. Temel MD, Joseph A. Greer, Ph.D., Alona Muzikansky, M.A.,, Emily
R. Gallagher RN, Sonal Admane, M.B., B.S., M.P.H.,, Vicki A. Jackson MD,
M.P.H., Constance M. Dahlin, A.P.N.,, Craig D. Blinderman MD, Juliet Jacobsen,
M.D., William F. Pirl, M. Early Palliative Care for Patients with Metastatic Non–
Small-Cell Lung Cancer. The New England journal of Medicine. 2010.
11. Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of
Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III
Randomized Controlled Trial. J Clin Oncol. 2015;33(13):1438-1445.
12. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for
patients with advanced cancer: a cluster-randomised controlled trial. The Lancet.
2014;383(9930):1721-1730.
13. Dalal S, Bruera E. End‐of‐Life Care Matters: Palliative Cancer Care Results in
Better Care and Lower Costs. The Oncologist. 2017;22(4):361-368.
14. Medciare Payment Policy: reprot to Congress. March 2019.
15. Hospice Costs & End-of-Life Options.
https://www.debt.org/medical/hospice-
costs/. Accessed December 10, 2019.

88
16. Commision MPA. Report to Congress: Medicare Payment Policy. CMS
http://www.medpac.gov/docs/default-
source/reports/mar19_medpac_entirereport_sec.pdf. Published 2019. Accessed
December 22, 2019.
17. Medicare Hospice Payment Reform: A Review of the Literature.
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/Hospice/Downloads/HPR-Lit-Review-Update-Report-.pdf. Updated
May 4, 2015. Accessed December 22, 2019.
18. Services CfMaM. MedicareHospicePaymentReformLiteratureReview2013. Abt
Associates Inc. . Published 2013. Accessed 05/27, 2020.
19. Field MJ, Cassel CK. Approaching death. [electronic resource] : improving care
at the end of life. National Academy Press; 1997.
20. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare Cost at End of Life. Am J
Hosp Palliat Care. 2019;36(8):705-710.
21. Ozanne KBaE. Importance of Costs and Cost Effectiveness of Palliative Care
2017;13(5).
22. Kelley AS, Deb P, Du Q, Aldridge Carlson MD, Morrison RS. Hospice
enrollment saves money for Medicare and improves care quality across a number
of different lengths-of-stay. Health Aff (Millwood). 2013;32(3):552-561.
23. Cynthia G. Tudor PD, Director Medicare Drug Benefit and C & D Data Group,
Laurence Wilson, Director Chronic Care Policy Group, Mark Majestic, Director
Medicare Program Integrity Group All Part D Plan Sponsors and Medicare
Hospice Providers. In: Centers for Medicare and Medicaid Services; 2013.
24. Role of Pharmacist in Palliative care. Oncology Nursing Society Country of
Publication: United States. 2015 Vol. 30 (2),:pp. 33.
25. Revisions to Chapter 2, “The Certification Process,” Sections 2080 – 2089. In: &
DoH, (DHHS) HS, & CfM, (CMS) MS, eds. Pub. 100-07 State Operations
Provider Certification: CMS; October 1, 2010.
26. ASHP Statement on the Pharmacist’s Role. 2002.
27. Herndon CM, Nee D, Atayee RS, et al. Ashp guidelines on the pharmacist's role
in palliative and hospice care. American Journal of Health-System Pharmacy.
2016;73(17):1351-1367.
28. Jimmie P. Leleszi D, Jeanne G. Lewandowski, MD Pain Management in End-of-
Life Care. The Journal of the American Osteopathic Association. March 2005;Vol
105(No 3).
29. Clinical Practice Guidelines for Quality Palliative Care, 4th edition. Published
2018. Accessed January 17, 2020.
30. National Hospice and Palliative Care Organization https://www.nhpco.org/
.
Accessed December 1, 2020.
31. Bennett MI, Kaasa S, Barke A, et al. The IASP classification of chronic pain for
ICD-11: chronic cancer-related pain. Pain. 2019;160(1):38-44.
32. (WHO) WHo. ICD-11. https://www.who.int/classifications/icd/en/
. Accessed
05/08, 2020.
33. WHO. Palliative Care: symptom management and end-of-life care. Integrated
Management of Adolescent and Adult Illness Web site. Published 2004.
Accessed January 17, 2020.

89
34. Klein C, Lang U, Bukki J, Sittl R, Ostgathe C. Pain Management and Symptom-
Oriented Drug Therapy in Palliative Care. Breast Care (Basel). 2011;6(1):27-34.
35. Blondell MA, MD; and Wisniewski, PharmD. Pharmacologic Therapy for Acute
Pain. Published 2013. Accessed January 17, 2020.
36. Groninger MV, MD. Pharmacologic Management of Pain at the End of life.
Published 2014. Updated July 1, 2014. Accessed January 17, 2020.
37. Care IAfHaP. World Health Organization Essential Medication list Palliative
care.
https://www.who.int/selection_medicines/committees/expert/19/applications/Palli
ativeCare_8_A_R.pdf. Published 2013. Accessed January 20, 2020.
38. Medscape. Methadone, Pain Management, and Palliative Care.
https://www.medscape.org/viewarticle/550895
. Accessed.
39. John F. Manfredonia D. Prescribing Methadone for Pain Management in End-of-
Life Care. 2005;105(3).
40. John F. Manfredonia D. Using Methadone to Control Pain in Patients During
Final Stages of Life. The Journal of the American Osteopathic Association.
2007;Vol 107(No 6).
41. Douglas J. Weschules P, * Jill A. McMath, PharmD,* Rollin Gallagher, MD,
MPH,*† Calvin J. Alt, RPh,* and Calvin H. Knowlton, RPh, MDiv, PhD*
Methadone and the Hospice Patient: Prescribing Trends in the Home-Care
Setting. 2003;4.
42. Watson CP. Methadone for neuropathic pain: a new use for an old drug? The
Canadian journal of neurological sciences Le journal canadien des sciences
neurologiques. 2005;32(3):271-272.
43. Brown R, Kraus C, Fleming M, Reddy S. Methadone: applied pharmacology and
use as adjunctive treatment in chronic pain. Postgrad Med J. 2004;80(949):654-
659.
44. Chou R, Cruciani RA, Fiellin DA, et al. Methadone safety: a clinical practice
guideline from the American Pain Society and College on Problems of Drug
Dependence, in collaboration with the Heart Rhythm Society. J Pain.
2014;15(4):321-337.
45. McPherson ML, Walker KA, Davis MP, et al. Safe and Appropriate Use of
Methadone in Hospice and Palliative Care: Expert Consensus White Paper. J Pain
Symptom Manage. 2019;57(3):635-645 e634.
46. George PP, Molina JA, Cheah J, Chan SC, Lim BP. The evolving role of the
community pharmacist in chronic disease management - a literature review. Ann
Acad Med Singapore. 2010;39(11):861-867.
47. Pharmacist-managed pain clinic at a Veterans Affairs medical center. Am J
Health-Syst Pharm. 2004;Vol 61.
48. Hadi MA, Alldred DP, Briggs M, Munyombwe T, Closs SJ. Effectiveness of
pharmacist-led medication review in chronic pain management: systematic review
and meta-analysis. Clin J Pain. 2014;30(11):1006-1014.
49. DeltaCareRx. https://www.deltacarerx.com/
. Accessed February 2, 2020.
50. Blouin RA, Adams ML. The Role of the Pharmacist in Health Care: Expanding
and Evolving. N C Med J. 2017;78(3):165-167.
90
51. Richter C. Implementation of a Clinical Pharmacist Service in the Hospice
Setting: Financial and Clinical Impacts. J Pain Palliat Care Pharmacother.
2019:1-4.
52. Valgus J, Jarr S, Schwartz R, Rice M, Bernard SA. Pharmacist-led,
interdisciplinary model for delivery of supportive care in the ambulatory cancer
clinic setting. Journal of Oncology Practice. 2010;6(6):e1-e4.
53. Bennett MI, Bagnall AM, Raine G, et al. Educational interventions by
pharmacists to patients with chronic pain: systematic review and meta-analysis.
Clin J Pain. 2011;27(7):623-630.
54. Selçuk AA. A Guide for Systematic Reviews: PRISMA. Turk Arch
Otorhinolaryngol. 2019;57(1):57-58.
55. Mancini R. Implementing a Standardized Pharmacist Assessment and Evaluating
the Role of a Pharmacist in a Multidisciplinary Supportive Oncology Clinic.
Journal of Supportive Oncology. 2012;10(3):99-106.
56. Atayee RS, Sam AM, Edmonds KP. Patterns of Palliative Care Pharmacist
Interventions and Outcomes as Part of Inpatient Palliative Care Consult Service.
Journal of Palliative Medicine. 2018;21(12):1761-1767.
57. Naidu D, Jones K, Kanyer D, Hausdorff J. Palliative care pharmacist
interventions in a community hospital. Am J Health Syst Pharm.
2018;75(13):933-936.
58. Ma JD, Tran V, Chan C, Mitchell WM, Atayee RS. Retrospective analysis of
pharmacist interventions in an ambulatory palliative care practice. Journal of
Oncology Pharmacy Practice. 2016;22(6):757-765.
59. Yamada M, Matsumura C, Jimaru Y, Ueno R, Takahashi K, Yano Y. Effect of
continuous pharmacist interventions on pain control and side effect management
in outpatients with cancer receiving opioid treatments. Biological and
Pharmaceutical Bulletin. 2018;41(6):858-863.
60. Ise Y, Morita T, Katayama S, Kizawa Y. The activity of palliative care team
pharmacists in designated cancer hospitals: A nationwide survey in Japan.
Journal of Pain and Symptom Management. 2014;47(3):588-593.
61. Chen J, Lu XY, Wang WJ, et al. Impact of a clinical pharmacist-led guidance
team on cancer pain therapy in China: a prospective multicenter cohort study. J
Pain Symptom Manage. 2014;48(4):500-509.
62. Pawłowska I, Pawłowski L, Lichodziejewska-Niemierko M. The role of a
pharmacist in a hospice: A nationwide survey among hospice directors,
pharmacists and physicians. European Journal of Hospital Pharmacy.
2016;23(2):106-112.
63. Edwards Z, Bennett MI, Blenkinsopp A. A community pharmacist medicines
optimisation service for patients with advanced cancer pain: a proof of concept
study. International Journal of Clinical Pharmacy. 2019.
64. Wilby KJ, Mohamad AA, Alyafei SA. Evaluation of clinical pharmacy services
offered for palliative care patients in Qatar. Journal of Pain and Palliative Care
Pharmacotherapy. 2014;28(3):212-215.
65. Geum MJ, Ahn JH, Kim JS, et al. Interprofessional Collaboration Between a
Multidisciplinary Palliative Care Team and the Team Pharmacist on Pain

91
Management. American Journal of Hospice and Palliative Medicine.
2019;36(7):616-622.
66. Lee J, McPherson ML. Outcomes of recommendations by hospice pharmacists.
Am J Health Syst Pharm. 2006;63(22):2235-2239.
67. Wilson S, Wahler R, Brown J, Doloresco F, Monte SV. Impact of pharmacist
intervention on clinical outcomes in the palliative care setting. Am J Hosp Palliat
Care. 2011;28(5):316-320.
68. JAMES D. TOOMBS MD, and LEE A. KRAL, PHARM.D. Methadone
Treatment for Pain States. American Family Physician 2005;71.
69. Methadone Dosing Recommendations for the Treatment of Chronic_Pain.
Published 2016. Accessed March 2, 2020.
70. Association ACP. American Chronic Pain Association Published 2008. Accessed
2019, September
71. Joan Harrold MD, M.P.H.,1 ELIZABETH RICKERSON, B.A.,2 JANET T.
CARROLL, M.S.N., R.N., C.H.P.N.,3 JENNIFER MCGRATH,4 KNASHAWN
MORALES, Sc.D.,5 JENNIFER KAPO, M.D.,6 and DAVID CASARETT, M.D.,
M.A.7. Is the Palliative Performance Scale a Useful Predictor of Mortality in a
Heterogeneous Hospice Population? 2005;8.
72. Palliative performance scale. Accessed 05/01, 2020.
73. Prevention CfDCa. Calculating_total_daily_dose. Accessed 05/18, 2020.
74. WHO. WHO Collaboration Center for Drug Statistics Methodology
https://www.whocc.no/ddd/definition_and_general_considera/
. Accessed 06/08,
2020.
75. WHO. The Anatomical Therapeutic Chemical Classification System with Defined
Daily Doses (ATC/DDD. https://www.who.int/classifications/atcddd/en/
.
Accessed August, 18, 2020.
76. WHO. WHO Collaboration Centre for Drugs Statistics Methodology
https://www.whocc.no/atc_ddd_index/
. Accessed 06/08, 2020.
77. Mercadante S, Bruera E. Methadone as a First-Line Opioid in Cancer Pain
Management: A Systematic Review. J Pain Symptom Manage. 2018;55(3):998-
1003.
78. Hanna V, Senderovich H. Methadone in Pain Management: A Systematic
Review. J Pain. 2020.
79. Marilyn J. Field and Christine K. Cassel ECoCatEoL, Institute of Medicine
Approaching Death: Improving Care at the End of Life.
80. Parker J. Experts Share Strategies to Reduce Hospice Medication Costs. Hospice
news Web site.
https://hospicenews.com/2019/07/07/experts-share-strategies-to-
reduce-hospice-medication-costs%EF%BB%BF/. Published 2019. Accessed
December 7, 2020.
81. Chary S. Methadone for pain management Past, present and future. Indian
Journal of Palliative Care. 2018;24(5):6-9.
82. ASHP guidelines on pharmacist role in hospice and palliative care. 2016.
83. Palat G, Chary S. Practical Guide for Using Methadone in Pain and Palliative
Care Practice. Indian journal of palliative care. 2018;24(Suppl 1):S21-S29.
84. Hawley P, Liebscher R, Wilford J. Continuing methadone for pain in palliative
care. Pain Res Manag. 2013;18(2):83-86.
92
85. Gagnon B, Almahrezi A, Schreier G. Methadone in the Treatment of Neuropathic
Pain. Pain Research and Management. 2003;8:236718.
86. Commission MPA. Healthcare Spending and the Medicare Program. Published
2020. Accessed 9, August, 2020.
87. Rome RB, Luminais HH, Bourgeois DA, Blais CM. The role of palliative care at
the end of life. Ochsner J. 2011;11(4):348-352.
88. Holdcroft JRA. Gender differences and pain medication. Women's Health
2009;5(1):79-88.
89. Carol S Weisse P, Paul C Sorum, MD, PhD, Kafi N Sanders, BS, and Beth L
Syat, BS. Do Gender and Race Affect Decisions About Pain Management? J Gen
Intern Med. 2001:211-217.
90. Franco De Conno CRaCBRaPCOU, National Cancer Institute of Milano, Milan.
Opioid purchases and expenditure in nine western European countries: 'Are we
killing off morphine?'. Palliative Medicine. 2005.
91. Neumann AM, Blondell RD, Jaanimägi U, et al. A preliminary study comparing
methadone and buprenorphine in patients with chronic pain and coexistent opioid
addiction. J Addict Dis. 2013;32(1):68-78.

93
APPENDICES
Appendix 1: Instrument #1 – Pharmacist Data Collection tool
DeltaCare patient ID: ________________ Date of admission: ________________
Instrument #1 – Pharmacist Data Collection
Duquesne study ID: _______________
Type of hospice: Home Nursing home Assisted living Inpatient
Terminal indications/diagnoses: ______________________________________
Type of pain (select all that apply): Nociceptive Neuropathic
Current pain medication regimen (medication/dose/schedule):
________________________________________________________________________
________________________________________________________________________
Pain intensity at admission (on a scale of 1 to 10): _______
Palliative prognosis score at admission: _______
Which of the following potential indications for methadone use are present? (select all that apply)
Neuropathic pain Morphine allergy High opioid tolerance Refractory to other opioids
Severe renal impairment
Other:
_____________________________________________________________
Which of the following contraindications/precautions for methadone are present? (select all that apply)
Clinically unstable Limited prognosis (<5 days) QTc prolongation/structural heart disease
Severe liver impairment Obstructive sleep apnea Substance use disorder Electrolyte
abnormalities
High fall potential Use of other long-acting CNS depressant included in current
medication regimen
Drug interactions (if so, list which one(s)): ________________________________
Other: _____________________________________________________________
Was a recommendation for methadone provided? Yes No
If yes, what recommendation was provided:
Switch to methadone as maintenance treatment
Addition of methadone as adjunctive/adjuvant treatment
Discontinue methadone previously prescribed
Other: ______________________________________

94
Appendix 2: Instrument #2 – Researcher Data Collection tool
DeltaCare patient ID: ________________
Instrument #2 – Researcher Data Collection
Duquesne study ID: _________________
Age (in years): _______________ Sex (M/F): ___________ Race/ethnicity: ___________
Height: ___________Weight: ___________
Allergies: ______________________________________________
Days since hospice admission: ________
Comorbidities: ________________________________________________________________________
________________________________________________________________________
PEG tube (Y/N): ___________Dysphagia (Y/N): ___________
Nutritional status: ___________________________
Renal function: ___________ Hepatic function: ___________
Pain medications the patient trialed prior to admission:
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Morphine milligram equivalents (MME) patient was on prior to admission: ___________
Pain control prior to admission: Mild Moderate Severe
Morphine milligram equivalents (MME) patient on currently: ________________
Current pain control: Mild Moderate Severe
Current palliative prognosis score: _______________
Was the pharmacist recommendation for methadone accepted? Yes No NA
If yes, what day of the week was methadone recommended? _______________
If yes, how many days after admission was recommendation implemented? ___________
If yes, was the dose/frequency recommended implemented? Yes No
Calculations for MME:

95
Appendix 3: Examples of categorization of variables
Terminal indication/diagnosis
Indication/ diagnosis category (1=cancer,
2=dementia, 3=cardiovascular, 4=respiratory,
5=liver, 6=kidney, 7=neurodegenerative, 8=other)
Sepsis
8
Alzheimer's
2
ESRD
6
Parkinson's
7
Alzheimer's
2
Cardiac arrhythmia
3
Amyloidosis
8
Pain medication
Med category1 (1=opioid, 2=NSAID,
3=APAP, 4= gabapentinoid, 5=other)
Morphine
1
APAP
3
Pregabalin
4
Fentanyl
1
Oxycodone
1
Methyl Salicylate
5
Ibuprofen
2
Hydrocodone/APAP
1, 3
Allergies
Allergy category (0=none,
1=opioid, 2=antibiotic,
3=topical, 4=other)
PCN
2
codeine, PCN, oxycodone
1, 2
morphine, amlodipine
1, 4
Crestor, sulfa
2, 4
Adhesive tape, nickel, morphine,
aluminum, ASA, azithromycin, loratadine
1, 2, 3, 4
NKA
0

96
Comorbidities
Comorbidity category (1=cancer,
2=dementia, 3=cardiovascular,
4=respiratory, 5=liver, 6=kidney,
7=endocrine, 8=psychiatric,
9=GI, 10=neurological,
11=other)
a fib, multivalvular regurgitation, CKD, hypoxemia
3, 4, 6
cervicalgia, carpal tunnel, oxygen dependent,
underweight, low back pain, anxiety, GERD, chronic
laryngitis, emphysema, hernia, HTN, resp failure
3, 4, 8, 9, 11
HTN, COPD, dementia, DM2, hypothyroid
2, 3, 4, 7
DVT, PE, HTN, cerebral atherosclerosis
3, 10
clotting disorder, ascites, OA, osteopenia
3, 5, 11
CKD stage 3, HTN, TIA
3, 6, 10

97
Appendix 4: List of DDD values
Medications with DDD values
Inhal: Inhalation, O: Oral route of administration, P: Parenteral route of administration, N: Nasal route of
administration, R: Rectal route of administration
Pain
NSAIDS
DDD
Route of administration
celecoxib
0.2 g
O
diclofenac
0.1 g
O, P, R
etodolac
0.4 g
O
ibuprofen
1.2 g
O
indomethacin
0.1 g
O
ketorolac tromethamine
30 mg
O, P
naproxen
1.2 g
O
naproxen sodium
0.5 g
O
piroxicam
20 mg
O
meloxicam
15 mg
O, P, R
sulindac
0.4 g
O, P, R
Analgesics
DDD
Route of administration
APAP
3 g
O
etodolac
0.4 g
O
acetylsalicylic acid
3 g
1 g
O
P
Opioids
DDD
Route of administration
buprenorphine
1.2 g
In the data 15mcg/hr (patch)
codeine sulfate
0.1 g
fentanyl
0.6 g
1.2 g
N, SL
TD
hydromorphone HCl
20 mg
4 mg
4 mg
O
P
R
methadone HCl
25 mg
O, P
morphine sulfate
0.1 g
30 mg
30 mg
O
P
R
oxycodone
75 mg
30 mg
O
P
tapentadol HCl
0.4 g
O
tramadol HCl
0.3 g
O, P, R

98
Pulmonary
DDD
Route of administration
budesonide
0.8 mg
0.8 mg
1.5 mg
Ihal. Aerosol
Inhal. Powder
Inhal. Solution
fluticasone propionate
0.2 mg
N
mometasone furoate
0.2 mg
N
ipratropium bromide
0.12 mg
0.3 mg
0.3 mg
Inhal aerosol
Inhal powder
Inhal sol
ipratropium bromide
0.24mg
N
tiotropium bromide
10 mcg
Inhal powder
umeclidinium bromide
55 mcg
Inhal powder
oxymetazoline HCl
0.4 mg
N
salbutamol (albuterol)
0.8 mg
10 mg
Inhal aerosol, powder
Inhal solution
terbutaline sulfate
15 mg
O, P
theophylline anhydrous
0.4 g
O, P, R
tiotropium bromide
10 mcg
5 mcg
Inhal powder
Inhal solution
pseudoephedrine
0.24 g
O
phenylephrine
40 mg
O
beclomethasone dipropionate
0.4 mg
N
Anticoagulants
DDD
Route of administration
apixaban
10 mg
O
cilostazol
0.2 g
O
clopidogrel bisulfate
75 mg
O
enoxaparin sodium
2 TU (time unit)
P
prasugrel HCl
10 mg
O
rivaroxaban
20 mg
O
ticagrelor
0.18 g
O
warfarin sodium
7.5 mg
O, P
heparin
10 TU
P

99
Medications without DDD values
Medications
albuterol sulfate
arformoterol tartrate
azelastine HCl/fluticasone propionate
albuterol sulfate
budesonide/formoterol fumarate
fluticasone furoate/umeclidinium bromide/vilanterol trifenat
fluticasone propionate/salmeterol xinafoate
formoterol fumarate
ipratropium bromide
ipratropium bromide/albuterol sulfate
levalbuterol HCl
levalbuterol tartrate
mometasone furoate
mometasone furoate/formoterol fumarate
terbutaline sulfate
theophylline anhydrous
tiotropium bromide
tiotropium bromide/olodaterol HCl
umeclidinium bromide
umeclidinium bromide/vilanterol trifenatate
