
163
Universal breastfeeding is recommended by the American
College of Obstetricians and Gynecologists (ACOG), the World
Health Organization (WHO), the United Nations Children’s
Fund (UNICEF), the American Academy of Pediatrics (AAP),
and the Special Supplemental Nutrition Program for Women,
Infants, and Children (WIC), but recommendations alone are
not sucient to promote breastfeeding. It is the responsibility
of every physician to recommend and promote breastfeeding
enthusiastically and support the breastfeeding mother- infant
dyad with informed, applicable, evidence- based medicine. is
is especially true in obstetrics, where a physician’s advice can
immediately inuence a woman’s informed decision concerning
breastfeeding and create or diminish barriers to successful
breastfeeding.
Benefits of Breastfeeding
Breastfeeding provides signicant benets for both the mother
and the infant. A number of these benets are documented
in an evidence- based analysis in the Agency for Healthcare
Research and Quality (AHRQ) report on breastfeeding
in developed countries.
1,2
ACOG and other groups have
championed the benets of breastfeeding and the use of human
milk.
3- 6
e benets are so signicant that the AAP and ACOG
recommend exclusive breastfeeding for the rst 6 months of life
and continued breastfeeding through 12 months or more.
7
e
WHO recommends that mothers initiate breastfeeding within 1
hour of birth and provide exclusive breastfeeding for the rst 6
months of life to achieve optimal infant growth, development,
and health; subsequently, to meet their evolving nutritional
requirements, infants should receive nutritionally adequate and
safe complementary foods, while continuing to be breastfed for
up to 2 years or beyond.
8
Breast milk is species specic, made uniquely for the human
infant.
9
Protein in breast milk is readily digested and is present
in amounts that can be handled by the developing kidney.
Various minerals (e.g., iron) and nutrients exist in a form
and in conjunction with other components that make them
easily absorbed to meet infants’ needs during periods of rapid
growth.
9,10
Cholesterol and docosahexaenoic acid have been
shown to play a role in central nervous system development
and may contribute to the enhanced intelligence quotient
measurements reported in breastfed infants.
11- 14
Protection against infections, including otitis media, croup,
pneumonia, and gastrointestinal infections, is mediated by the
over 50 immunologically active components found in breast
milk.
9,15- 17
ese immunologically active components include
viable functioning cells (T and B lymphocytes, macrophages),
T cell–secreted products, immunoglobulins (especially secretory
immunoglobulin A [IgA]), carrier proteins such as lactoferrin
and transferrin, enzymes (lysozyme and lipoprotein lipase),
and nonspecic factors such as complement, bidus factor,
gangliosides, oligosaccharides, and nucleotides. Other immune
factors in breast milk include hormones, hormone- like factors,
and growth factors that contribute to the normal maturation of
the mucosal barrier of the respiratory and gastrointestinal tracts
as well as the developing infant’s immune system. Breast milk
is a very dynamic uid, varying with the mother- infant dyad’s
environment and needs, especially in the face of infection or
stress (providing, e.g., leukocytes, nucleotides, oligosaccharides,
secretory IgA, interleukin, interferon, and cytokines).
16- 21
ere
is also evidence that breastfeeding provides protection against
some noninfectious illnesses such as asthma, eczema, childhood
lymphoma, insulin- dependent childhood- onset diabetes, and
obesity
16,22- 27
in children who are exclusively breastfed for the
rst 4 to 6 months of life. A cohort study of infants in Australia
28
and a meta- analysis
29
showed lower odds of developing type 1
diabetes and type 2 diabetes, respectively.
Cognitive and psychological benets for breastfed infants
have been suggested, including those for developmental
performance,
30
visual acuity,
31- 33
school performance,
34
and performance on standardized and intelligence quotient
tests.
35
More recent articles continue to support the impact
of breastfeeding on intellectual development while fostering
debate over the relative contributions of nutrition, genetics,
and environment to the intellectual development of infants and
the possible inuence on the child’s or adult’s future cognitive
abilities as measured by intelligence quotient testing.
13,36,37
e
psychological benets are more dicult to measure but are
well described by Newton and Newton
38
and, indeed, by most
mothers who have successfully breastfed their infants.
39
One
of the most consistent ndings of exclusive breastfeeding is its
inuence on later intelligence, with a few test points’ advantage
to the breastfed infant.
13
Reports questioning this eect have
been based on heterogenous denitions of breastfeeding (any
breastfeeding, not exclusive breastfeeding) and may not have
controlled for all potential confounders.
40
Potential benets to the mother in the short term
include improved postpartum recovery,
41
a decreased risk
of postpartum hemorrhage,
42
and prolonged amenorrhea
in mothers who exclusively or predominantly breastfeed in
the rst 6 months postpartum, which may increase spacing
between births.
43
ere are data supporting the psychological
benets of breastfeeding for the mother, but there are also
some equivocal studies. e relationship between breastfeeding
and postpartum depression is complicated. Prenatal and
11
The Breast and the Physiology of
Lactation
ADETOLA LOUIS- JACQUES, MD | ROBERT M. LAWRENCE, MD | RUTH A. LAWRENCE,
MD
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
164
postpartum depression are associated with early cessation of
breastfeeding.
44
Among women without a prenatal diagnosis
of depression, high positive emotions during infant feeding at
2 months were associated with lower depression and anxiety
symptoms at 2, 6, and 12 months.
45
In contrast, breastfeeding
worries, lower breastfeeding self- ecacy, negative breastfeeding
attitudes, breastfeeding challenges, and pain have increased risk
of developing postpartum depression.
44
Long- term benets of lactation include a reduced incidence
of metabolic syndrome, hypertension, type 2 diabetes mellitus,
and breast and ovarian cancers.
1,4,6,46- 49
Bone metabolism
changes during pregnancy and lactation to meet the needs of the
mother and infant. Specically, during lactation, maternal bone
resorption occurs to meet the demand for calcium, although
these bone losses are reversed over time.
50
Feltner and colleagues
reviewed the risk of fractures rather than the risk of bone mass
loss.
49
ey identied that many variables may contribute
to the risk of fractures (age, hormone replacement therapy,
physical activity, parity, and body mass index [BMI]) related
to lactation. ey concluded that no studies demonstrated a
signicant association between breastfeeding and fracture. Most
of the studies described a lower odds of fracture with greater
breastfeeding duration that was not statistically signicant.
49
Increasing number of pregnancies, longer oral contraceptive use,
and increasing duration of lactation are all protective against
ovarian cancer.
51- 53
e incidence of breast cancer is lower among
women who have nursed.
54,55
Newer data show an association
between breastfeeding and lower rates of diabetes,
56,57
and for
women with gestational diabetes who breastfeed there is lower
risk of developing type 2 diabetes in the postpartum period.
58
Endometrial and thyroid cancers have also been reported to
be lower in breastfeeding mothers.
59,60
Other benets include
lower cost of providing adequate infant nutrition and lower
environmental costs: 6 months of breastfeeding saves between 95
and 153 kg of CO
2
compared with formula feeding.
61,62
ere is a dose- response relationship between the amount
of human milk received by an infant and the benets or
immunologic protection gained. e health benets to
the mother from breastfeeding also relate to the dose and
duration of breastfeeding. e relative “dose” of breastfeeding
has been dened in terms of exclusivity versus the amount of
supplementation (Table 11.1).
63
e WHO provides a specic
denition of exclusive breastfeeding: “Exclusive breastfeeding
means that the infant receives only breast milk. No other liquids
or solids are given – not even water – with the exception of oral
rehydration solution, or drops/syrups of vitamins, minerals or
medicines.”
64
e importance of this dose- response relationship
is emphasized in the AAP’s and ACOG’s recommendation for
exclusive breastfeeding in the rst 6 months of life and the
AHRQ report’s analysis of the benets of breastfeeding relative
to measured durations of breastfeeding.
1- 4,6- 8
It is essential that a discussion of the benets of breastfeeding
for families (fathers and partners included) be presented
alongside any potential risks or contraindications. e
benets of breastfeeding are tremendous, and the risks and
contraindications are few. Summarized here and in Table
11.2 are the conditions in which the risks of breastfeeding or
providing expressed mother’s own milk to infants may outweigh
its benets.
65
•
Women who take illicit drugs, abuse legal substances, or
do not control their alcohol intake and are not in stable
substance abuse treatment.
66,67
Various groups (AAP,
ABM, ACOG, NIH/LactMed, WHO) dene abuse, use
disorders, and excess consumption/lack of control of
intake based on the specic substances.
6,68- 70
•
A woman who has an infant with classic galactosemia,
because both human and cow’s milk exacerbate the con-
dition. A lactose- free formula is recommended for these
infants. In milder forms of galactosemia, partial breast-
feeding is possible.
6
•
Women who are infected with human immunodeciency
virus or human T- cell leukemia virus type I (see Maternal
Infections During Breastfeeding in this chapter).
•
Women who have active untreated tuberculosis. Because
of the increased risk of airborne transmission associ-
ated with the close contact that is typical of breastfeed-
ing, women with active tuberculosis should not feed their
infant by any method until treatment is initiated. However,
infected women can provide their pumped milk to their
infants (see later).
•
Women who are known or suspected to be infected with
Ebola virus, Marburg virus, Lassa virus, or dengue virus,
when a safe alternative food source is readily available (see
later).
•
Women who take certain medications (see Medications
While Breastfeeding, later).
Medical situations that indicate a potential risk from
breastfeeding must be weighed against the potential benets for
both mother and infant.
Some of the contraindications may be permanent or
temporary. For those infections with predominantly airborne
or contact precautions, expressed milk may still be given to
Breastfeeding Definitions
Definition
Amount of Supplementation
Full breastfeeding Exclusive human breast milk only Infant ingests no other nutrients, supplements, or liquids
Almost exclusive No milk other than human milk; only minimal amounts of other substances
such as water, juice, tea, or vitamins
Partial breastfeeding High partial Nearly all feeds are human milk (at least 80%)
Medium partial A moderate amount of feeds are breast milk, in combination with other
nutrient foods and nonhuman milk (20%–80% of nutritional intake is
human breast milk)
Low partial Almost no feeds are breast milk (less than 20% of intake is breast milk)
Token breastfeeding Breastfeeding primarily for comfort; nonnutritive, for short periods of time,
or infrequent
Never breastfed Infant never ingested any human milk
Modified from Labbok M, Krasovec K. Toward consistency in breastfeeding definitions. Stud Fam Plan. 1990;21(4):226–230.
TABLE
11.1
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 165
the infant. When mothers need to temporarily discontinue
breastfeeding, instructions should be provided on when and
how to resume breastfeeding, and lactation support should be
provided to help with maintenance of their supply.
ROLE OF THE OBSTETRICIAN IN PROMOTING
BREASTFEEDING
Obstetricians have many responsibilities for breastfeeding,
including the following:
•
Enthusiastically promoting and supporting breastfeed-
ing, based on the published literature of its benets advo-
cated by the major pediatric, obstetric, and women’s health
organizations.
4- 6,71,72
•
Imparting clinical information to the lactating mother
about the physiology of lactogenesis and lactation, before
and aer the birth.
73
•
Developing and supporting hospital policies that facilitate
breastfeeding and actively remove any barriers to it.
•
Supporting community eorts to provide women with
adequate information to make an informed decision about
breastfeeding, including links to community breastfeeding
resources.
•
Providing balanced anticipatory guidance and lactation
support to mothers and families regarding potential con-
cerns during labor, delivery, the postpartum period, and
breastfeeding (e.g., antenatal consultation with a breastfeed-
ing medicine specialist or lactation specialist for anticipated
needs such as suspected fetal cle palate, multiple gestation,
prematurity, and prior breast reduction surgery).
•
Actively assessing women for potential breastfeeding chal-
lenges, especially those with high- risk pregnancies. Be
prepared to fully evaluate and manage breastfeeding dif-
culties mothers encounter, personally or with the assis-
tance of a breastfeeding medicine specialist or lactation
specialist. Enabling breastfeeding in this at- risk popula-
tion may ameliorate cardiometabolic disease trajectories
in the mother and child dyads.
•
Providing additional breastfeeding support for mothers
at increased risk of low or insucient milk production or
other breastfeeding challenges.
74
•
Proactively providing equitable lactation care address-
ing potential social challenges to initiating and continuing
breastfeeding through the individual attention of the obste-
trician and the coordinated and collaborative eorts of insti-
tutional and community- based lactation support teams.
5
•
Fostering a general acceptance of breastfeeding by promot-
ing a normative portrayal of breastfeeding and supporting
the provision of sucient time and facilities in the workplace.
•
Performing breast examinations before and aer the birth
and emphasizing lactation as the primary function of the
breast.
•
Participating in breastfeeding education in medical and
other health profession schools.
72
•
Supporting breastfeeding within their own medical facili-
ties by instituting the “Ten Steps to Successful Breastfeed-
ing” as outlined by UNICEF/WHO
75
(Box 11.1).
e mother’s plan for infant feeding should be addressed early
in prenatal care, with counseling, a medical history focused on
breast health and breastfeeding, and a physical examination of
the breast. An outline for breastfeeding promotion in the prenatal
setting is provided in the Academy of Breastfeeding Medicine’s
Clinical Protocol #19.
76
Counseling can be modeled aer “e
Best Start ree- Step Counseling Strategy”
77
as suggested by
Lazarov and Evans.
78
is strategy advises beginning with open-
ended questions about breastfeeding. An acknowledgment that
feelings of doubt about the ability to breastfeed successfully are
normal is a good place to begin. Education about breastfeeding
then continues with discussion of how others have dealt with
these concerns. is conversation will elucidate much about the
woman’s knowledge of breastfeeding, her previous experiences
with breastfeeding, and her own attitudes and those of the
mother’s partner, the extended family, and other potentially
supportive persons in the mother’s life. To be respectfully
inclusive of all parents/families and avoid one example of
discrimination and inequity as experienced by the LGBTQ+
community, arming health care should begin with arming
names, pronouns, and consideration of the experiences and
opinions of both parents. e Academy of Breastfeeding
Medicine (ABM) provides some guides for providing arming
lactation care to LGBTQ+ patients and families.
79
To support breastfeeding optimally, the concerns of family
and friends must be addressed actively to foster needed support
Contraindications to Breastfeeding and or
Feeding of Breast Milk
Mothers should NOT
breastfeed or feed
expressed breast
milk to their infants
• Classic galactosemia in the infant
• Mother actively using illicit street
drug, such as PCP or cocaine
• Mother infected with HIV,
a
human
T- cell lymphotropic virus type I or
type II
• Mother with confirmed or
suspected Ebola virus disease
Mothers should
temporarily NOT
breastfeed or feed
expressed breast
milk to their infants
• Mother is infected with untreated
brucellosis
• Mother is taking certain
medications; e.g., certain
chemotherapies
• Mother is undergoing diagnostic
imaging with radiopharmaceuticals
• Mother has an active herpes
simplex virus infection with lesions
present on the breast (transmission)
• May feed or provide expressed
milk from the unaffected breast
provided the lesions on the
affected breast are covered
• May resume feeding or providing
expressed milk from the affected
breast once the lesions have
resolved
Mothers should
temporarily NOT
breastfeed, but CAN
feed expressed
breast milk
• Mother has untreated, active
tuberculosis
• May resume breastfeeding after
2 weeks of appropriate treatment
and no longer contagious
• Mother has active varicella that
developed within 5 days prior or 2
days after delivery
a
HIV recommendation only applies to the specific countries that
have recommended this as a component of their national efforts at
perinatal HIV transmission prevention (e.g., United States, Canada,
United Kingdom, and Italy, among others).
Data from Centers for Disease Control and Prevention.
Contraindications to Breastfeeding or Feeding Expressed Breast
Milk to Infants. Updated 2018. https://www.cdc.gov/breastfeeding/
breastfeeding- special- circumstances/contraindications- to-
breastfeeding.html.
TABLE
11.2
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
166
on many levels. Misconceptions and potential barriers must be
identied and reasonable solutions developed in partnership
with the woman. ese oen include feelings of responsibility
for every unexplained problem the infant displays; conicts
among a woman’s several roles as mother, sexual partner, and
worker outside the home; and, most commonly, a greater time
commitment and fatigue than was expected. It is important
to address these and other questions repeatedly throughout
pregnancy and not just in the immediate postpartum period,
working closely with the infant’s pediatrician.
5,74
Dr. Alison
Stuebe, a maternal- fetal medicine expert and a member of
the Academy of Breastfeeding Medicine, utilizes open- ended
questions following a format recommended by Duggan
and Street that encompasses relational functions (fostering
healing and validating and responding to patient emotions) of
provider- patient communication and “task- driven” functions
(exchanging and managing information, making treatment
decisions, enabling patient self- management, and managing
uncertainty) to approach maternal and familial recurring issues
and concerns regarding breastfeeding.
80,81
It is important to be familiar with and in communication
with members of the medical team who support breastfeeding
throughout the community including lactation consultants,
pediatric practices, and support groups. Specic codes from
the International Classication of Diseases, 10th Revision
(ICD- 10) commonly used for breastfeeding care and breast
abnormalities are listed by ACOG to facilitate billing for
the time required for informed medical care and eective
communication.
82
Examination of the Breast
e medical history related to the breasts should include their
development, previous experience with breastfeeding, systemic
illnesses, infections, breast surgery or trauma, medications,
allergies, self–breast examinations and ndings, and any
anatomic or physical concerns the mother has about her breasts.
e breast examination at prenatal and postpartum visits should
include careful inspection and palpation. Inspection of the breasts
is most eective in the sitting position, rst with the arms overhead
and then with the hands on the hips. Skin changes, distortions in
shape or contour, and the form and size of the areola and nipple
should be noted. Palpation can begin in the sitting position, looking
for axillary and supraclavicular adenopathy. Palpation in the supine
position is easier for the complete examination of the breast and
surrounding anterolateral chest wall. Size, shape, consistency,
masses, scars, tenderness, and any abnormalities can be noted
in both descriptive and picture form for future comparison.
Serial examinations should document maturational changes of
pregnancy (size, shape, fullness, enlargement of areola) and nipple
position (inversion or eversion).
e changes in the breast during pregnancy provide
important prognostic data regarding successful breastfeeding.
With the increased frequency of cosmetic breast surgery, it
is important to be aware of the nature of any surgery and to
examine carefully for the location of the surgical scars. Many
women successfully breastfeed aer surgery for benign breast
disease, breast augmentation, or breast reduction. However,
a periareolar incision or “nipple translocation technique” for
breast reduction can damage nerves and ducts, making this
more dicult. Nipple piercing is another increasingly common
procedure, aer which breastfeeding can be successful with the
jewelry removed. Such surgeries do not preclude successful
breastfeeding but rather remind us that additional early support
should be provided to these mothers from physicians, nurses,
lactation consultants, and peer support groups.
Perinatal Period
e obstetrician can make important contributions to successful
breastfeeding through the conduct of the labor, delivery, and
puerperium. A stressful or exhausting labor and delivery has
been shown to aect lactation adversely.
83
A safe delivery for both
mother and infant is, of course, the most important outcome.
During the delivery and aerward, any medications used should be
compatible with breastfeeding and not interfere with the bonding
and rst feeding. Immediate skin- to- skin contact between mother
and infant and a rst feeding within 1 hour of delivery are probably
the most important intrapartum steps to increase the likelihood of
successful breastfeeding. Having the infant in the mother’s room,
feeding on demand, and early breastfeeding support (including
teaching appropriate techniques) within the rst 24 to 36 hours
can also help. Supplementation should be avoided unless medically
indicated and ordered by the pediatrician.
For the breastfeeding woman, medication choices are
very important (see Medications While Breastfeeding in this
chapter). Most women and many health professionals assume
that no medication can be safely administered to a lactating
woman, but the number of contraindicated drugs is in fact quite
small. Before assuming a medication is unsafe, expert advice
should be consulted, available in texts, websites, or through
drug information telephone services. (e.g., LactMed, Infant Risk
Center, MotherToBaby Call Center).
69,84,85
CRITICAL MANAGEMENT PROCEDURES
1a. Comply fully with the International Code of Marketing of
Breast- milk Substitutes and relevant World Health Assembly
resolutions.
1b. Have a written infant feeding policy that is routinely com-
municated to staff and parents.
1c. Establish ongoing monitoring and data- management systems.
2. Ensure that staff have sufficient knowledge, competence,
and skills to support breastfeeding.
KEY CLINICAL PRACTICES
3. Discuss the importance and management of breastfeeding
with pregnant women and their families.
4. Facilitate immediate and uninterrupted skin- to- skin contact
and support mothers to initiate breastfeeding as soon as
possible after birth.
5. Support mothers to initiate and maintain breastfeeding and
manage common difficulties.
6. Do not provide breastfed newborns any food or fluids other
than breast milk, unless medically indicated.
7. Enable mothers and their infants to remain together and to
practice rooming- in 24 hours a day.
8. Support mothers to recognize and respond to their infants’
cues for feeding.
9. Counsel mothers on the use and risks of feeding bottles,
teats, and pacifiers.
10. Coordinate discharge so that parents and their infants have
timely access to ongoing support and care.
From UNICEF/WHO. The ten steps to successful breastfeeding.
https://www.who.int/activities/promoting- baby- friendly- hospitals/
ten- steps- to- successful- breastfeeding. Accessed January 28, 2021.
BOX 11.1 THE TEN STEPS TO SUCCESSFUL
BREASTFEEDING
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.
11 The Breast and the Physiology of Lactation 167
Early follow- up (2 to 4 days aer discharge) with the infant’s
health provider should be arranged for all breastfeeding
mothers. Continued support of breastfeeding for the mother
should occur through the 6- week postpartum visit. Discussions
about breastfeeding should cover techniques to ensure adequate
emptying of the breast as well as problems such as nipple soreness
or trauma, plugged duct (in the form of a small lump), mastitis,
breast abscess, breast masses, and bloody nipple discharge, all
of which can usually be treated without stopping breastfeeding.
The Breast
To fully understand the process of lactation, one needs to
understand the anatomy and physiology of the breast as it applies
to this function. e human mammary gland is the only organ
that does not contain all of the rudimentary tissues at birth.
It experiences dramatic changes in size, shape, and function
from birth through menarche, pregnancy, and lactation and
ultimately during involution. e three major phases of growth
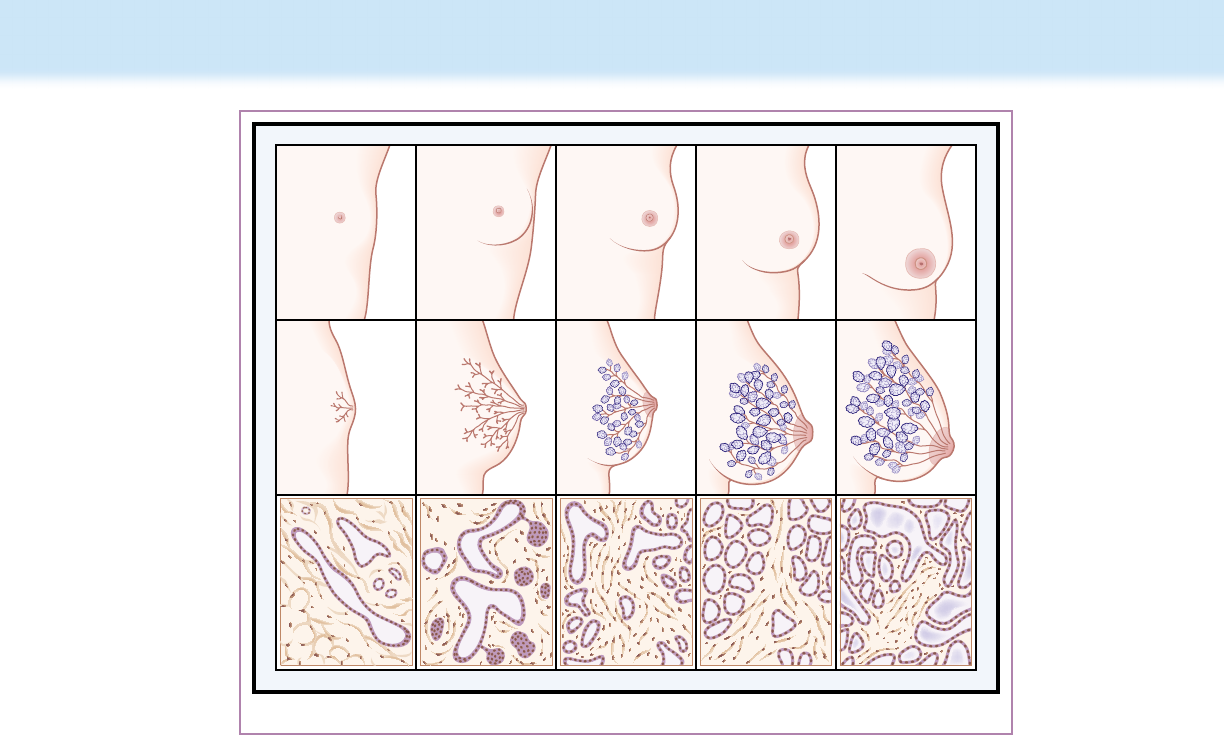
and development before pregnancy and lactation occur in utero,
during the rst 2 years of life, and at puberty (Fig. 11.1 and Table
11.3).
EMBRYONIC DEVELOPMENT
e milk streak appears in the fourth week of gestation when
the embryo is approximately 2.5 mm long. It becomes the milk
line, or milk ridge, during the h week of gestation (2.5 to 5.5
mm). e mammary gland itself begins to develop at 6 weeks
of embryonic life, and proliferation of the milk ducts continues
throughout embryonic growth and again in pregnancy and
lactation. e process of forming the nipple in the human
embryo begins with a thickened, raised area of ectoderm in the
region of the future gland by the fourth week of pregnancy. is
thickened ectoderm becomes depressed into the underlying
mesoderm and thus the surface of the mammary area soon
becomes at and nally sinks below the level of the surrounding
epidermis. e mesoderm that is in contact with the ingrowth
of the ectoderm is compressed, and its elements become
arranged in concentric layers that at a later stage give rise to the
gland’s stroma. By dividing and branching, the ingrowing mass
of ectodermal cells gives rise to the future lobes and lobules, and
much later to the alveoli.
By 16 weeks’ gestation in the fetus, the branching stage has
produced 15 to 25 epithelial strips that represent the future
secretory alveoli. By 28 weeks’ gestation, placental sex hormones
enter the fetal circulation and induce canalization in the fetal
mammary tissue. e lactiferous ducts and their branches are
developed from outgrowth in the lumen. ey open into a
shallow epidermal depression known as the mammary pit. e
pit becomes elevated because of mesenchymal proliferation,
forming the nipple and areola. An inverted nipple is the failure
of this pit to elevate.
86
At 32 weeks’ gestation, the lumen has
formed in the branching system, and by term there are 4 to 18
ABCDE
Figure 11.1 Female breast from infancy to lactation, with corresponding duct structure and tissue cross sections. (A–C) Gradual development
of the well- differentiated ductular and peripheral lobular- alveolar system. (D) Ductular sprouting and intensified peripheral lobular- alveolar develop-
ment in pregnancy. Glandular luminal cells begin actively synthesizing milk fat and proteins near term; only small amounts are released into the lumen.
(E) With postpartum withdrawal of luteal and placental sex steroids and placental lactogen, prolactin is able to induce full secretory activity of alveolar
cells and release of milk into alveoli and smaller ducts. (From Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession. 7th ed.
St Louis, MO: Mosby; 2010:43.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
168
mammary ducts that form the fetal mammary gland.
87
Fig. 11.2
shows the hormonal regulation of mammary development in
the mouse.
e nipple, areola, and breast bud are important landmarks
for the determination of gestational age in the newborn. At 40
weeks, the nipple and areola are clearly seen and the breast bud
is up to 1.0 cm in diameter. In the rst weeks aer delivery,
the breast bud is visible and palpable; however, the gland then
regresses to a quiescent stage as maternal hormones in the
infant diminish. Aer this, the gland grows only in proportion
to the rest of the body until puberty.
PUBERTAL DEVELOPMENT
With the onset of puberty in the female, further growth of
the breast occurs, and the areolae enlarge and become more
pigmented. e further development of the breast involves two
distinct processes: organogenesis and milk production. e
ductal and lobular growth is organogenesis, and this is initiated
before and throughout puberty, resulting in the growth of breast
parenchyma with its surrounding fat pad. e formation of
alveolar buds begins within 1 to 2 years of the onset of menses
and continues for several years, producing alveolar lobes. is
menarchial stimulus begins with the extension of the ductal tree
and the generation of its branching pattern. e existing ducts
elongate. e ducts can develop bulbous terminal end buds that
are the forerunners of alveoli. e formation of the alveolar bud
begins within 1 to 2 years of the onset of menses. During this
ductal growth, the alveoli enlarge and the nipple and areola
become more pigmented. is growth involves an increase in
connective tissue, adipose tissue, and vascular channels and is
stimulated by estrogen and progesterone released by the ovary.
88
Stages of Mammary Development
Developmental Stage Hormonal Regulation Local Factors Description
Embryogenesis ? Fat pad necessary for
ductal extension
Epithelial bud develops in 18- to 19- week-
old fetus, extending short distance into
mammary fat pad with blind ducts that
become canalized; some milk secretion may
be present at birth
Mammogenesis Anatomic development
Puberty
Before onset of
menses
Estrogen, GH IGF- I, hGF, TGF- β;
others?
Ductal extension into mammary fat pad;
branching morphogenesis
After onset of
menses
Estrogen, progesterone; PRL? Lobular development with formation of
terminal duct lobular unit
Pregnancy Progesterone, PRL, hPL HER; others? Alveolus formation; partial cellular differentiation
Lactogenesis Progesterone withdrawal, PRL,
glucocorticoid
Not known Onset of milk secretion
Stage I: midpregnancy
Stage II: parturition
Lactation PRL, oxytocin FIL Ongoing milk secretion
Involution PRL withdrawal, alpha-
lactalbumin dimer
Milk stasis; FIL? Alveolar epithelium undergoes apoptosis and
remodeling; gland reverts to prepregnant
state
FIL, Feedback inhibition of lactation; GH, growth hormone; HER, heregulin; hGF, human growth factor; hPL, human placental lactogen; IGF- I,
insulin- like growth factor I; PRL, prolactin; TGF- β, transforming growth factor- β.
Modified from Neville MC. Mammary gland biology and lactation: a short course. Presented at: International Society for Research on Human Milk
and Lactation annual meeting; October 1997; Plymouth, MA.
TABLE
11.3
Adipose
stroma
Adipose
stroma
Te rminal
end bud
PRL
progesterone
GH,
estradiol
(IGF-I, HGF/SF,
TGF-β)
Ductal elongation
branching patterning
Epithelial
bud
Formation of
alveolar bud
(HER)
Figure 11.2 Schema for hormonal regulation of mammary development in the mouse. GH, Growth hormone; HER, heregulin; HGF/SF, human
growth factor/secretory factor; IGF- I, insulin- like growth factor I; PRL, prolactin; TGF- β, transforming growth factor- β. (From Neville MC. Mammary
gland biology and lactation: a short course. Presented at: International Society for Research on Human Milk and Lactation annual meeting; October
1997; Plymouth, MA.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 169
During the menstrual cycle, there continues to be cyclic
microscopic proliferation and regression of ductal breast tissue.
e breast continues to enlarge slightly with further division of
the ductal system until about the age of 28 unless pregnancy
intervenes.
THE MATURE BREAST
e mature breast is located in the supercial fascia between
the second and sixth intercostal cartilages and is supercial to
the pectoralis muscle. It measures 10 to 12 cm in diameter. It
is located horizontally from the parasternal to the midaxillary
line. e central thickness of the gland is 5 to 7 cm. In the
nonpregnant state, the breast weighs on average 200 g. During
pregnancy, however, the size and weight increase to about 400 to
600 g, and to 600 to 800 g during lactation. Early in pregnancy
there is a signicant increase in ductal expansion and branching
attributed to estrogen. Lobular formation increases due to
prolactin, progesterone, and chorionic gonadotropin. By the
third month of gestation, secretory material like colostrum is
present in acini. Prolactin stimulates the secretion of colostrum
in the second trimester, but production of milk prior to delivery
is limited by the presence of progesterone. A projection of
mammary tissue into the axilla is known as the tail of Spence
and is connected to the central duct system. e breast is usually
dome shaped or conic, becoming more hemispheric in the adult
and pendulous in the older parous woman.
ABNORMALITIES
In some women, mammary tissue develops at other sites in the
galactic band. is is referred to as hypermastia, which is the
presence of accessory mammary glands that are phylogenic
remnants. ese remnants may include accessory nipples or
accessory gland tissue located anywhere along the milk line.
From 2% to 6% of women have hypermastia. ese remnants
remain quiet until pregnancy, when they may respond to the
hormonal milieu by enlarging and even secreting milk during
lactation. If le unstimulated, they will regress aer the birth.
Major glandular tissue in the axilla may pose a cosmetic or
management problem if the tissue enlarges signicantly during
pregnancy and lactation, secreting milk. It is distinct from the
tail of Spence.
Other abnormalities include amastia (absence of the breast
or nipple), amazia, hyperadenia, hypoplasia, polythelia, and
symmastia (Box 11.2). Abnormalities of the kidneys have been
associated with polythelia. Other variations include hyperplasia
or hypoplasia in various combinations, as listed in Box 11.3.
Gigantomastia is the excessive enlargement of the breasts
in pregnancy and lactation, sometimes to life- threatening
proportions. is enlargement may occur with the rst or any
pregnancy and may not recur. e enlargement recedes but
rarely back to original size.
9
Breastfeeding has been successful
in some cases of gigantomastia with appropriate professional
support. In extreme cases, gigantomastia may require heroic
measures, including emergency mastectomy.
Mothers with congenital abnormalities of the breast may
wish to breastfeed. Not all abnormalities or variations preclude
breastfeeding, and the decision is made on a case- by- case basis.
NIPPLE AND AREOLA
e skin of the breast includes the nipple and areola and the thin,
exible, elastic skin that covers the body of the breast. e nipple
is a conic elevation in the center of the areola at the level of about
the fourth intercostal space, just below the midline of the breast.
e nipple contains smooth muscle bers and is richly innervated
with sensory and pain bers. It has a verrucous surface and has
sebaceous and apocrine sweat glands but not hair.
e areola surrounds the nipple and is also slightly pigmented
and becomes deeply pigmented during pregnancy and lactation.
e average diameter is 15 to 16 mm, but the range may exceed
5 cm during pregnancy. e sensory innervation is less than that
of the nipple. e nipple and areola are very elastic and elongate
into a teat when drawn into the mouth by the suckling infant.
e surface of the areola contains Montgomery glands, which
hypertrophy during pregnancy and lactation and resemble
vesicles. During lactation, they secrete a sebaceous material to
lubricate the nipple and areola and protect the tissue while the
infant suckles. ese glands atrophy aer weaning and are not
visible to the naked eye except during pregnancy or lactation.
Each nipple contains 4 to 18 lactiferous ducts, of which 5 to
8 are main ducts surrounded by bromuscular tissue.
89
ese
ducts end as small orices at the tip of the nipple from which the
milk ows. e corpus mammae is an orderly conglomeration
of a number of independent glands known as lobes. e
morphology of the gland includes parenchyma that contains the
ductular- lobular- alveolar structures. It also includes the stroma,
which is composed of connective tissue, fat tissue, blood vessels,
nerves, and lymphatics.
Accessory breast: Any tissue outside the two major glands
Amastia: Congenital absence of breast or nipple
Amazia: Nipple without breast tissue
Hyperadenia: Mammary tissue without nipple
Hypoplasia: Underdevelopment of breast
Polythelia: Supernumerary nipple(s) (also hyperthelia)
Symmastia: Webbing between breasts
From Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the
Medical Profession. 8th ed. St Louis, MO: Mosby; 2015:39.
BOX 11.2 BREAST ABNORMALITIES
HYPOPLASIA
Unilateral hypoplasia, contralateral breast normal
Unilateral hypoplasia, contralateral breast hyperplasia
Unilateral hypoplasia of breast, thorax, and pectoral muscles
(Poland syndrome)
Bilateral hypoplasia with asymmetry
HYPERPLASIA
Unilateral hyperplasia, contralateral breast normal
Bilateral hyperplasia with asymmetry
ACQUIRED ABNORMALITIES
Caused by trauma, burns, radiation treatment for hemangioma
or intrathoracic disease, chest tube insertion in infancy, and
preadolescent biopsy
From Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the
Medical Profession. 8th ed. St Louis, MO: Mosby; 2015:40.
BOX 11.3 TYPES OF BREAST HYPOPLASIA,
HYPERPLASIA, AND ACQUIRED
ABNORMALITIES
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
170
e mass of breast tissue consists of tubuloalveolar glands
embedded in adipose tissue, which gives the gland its smooth,
rounded contour. e mammary fat pad is essential for the
proliferation and dierentiation of the ductal arborization (Fig.
11.3). Each lobe is separated from the others by connective
tissue and opens into a duct that opens into the nipple. e
extension of ducts is orderly and protected by an inhibitory
zone into which other ducts cannot penetrate.
90
Blood is supplied to the breast from branches of the
intercostal arteries and perforating branches of the internal
thoracic artery. e main blood supply comes from the internal
mammary artery and the lateral thoracic artery. e venous
supply parallels the arterial supply.
Lymphatic drainage has been thoroughly studied by
researchers of breast cancer. e main drainage is to axillary
nodes and the parasternal nodes along the thoracic artery
within the thorax. e lymphatics of the breast originate in
lymph capillaries of the mammary connective tissue and drain
through the deep substance of the breast.
e breast is innervated from the branches of the fourth,
h, and sixth intercostal nerves. e sensory innervation of
the nipple and areola is extensive and includes both autonomic
and sensory nerves. e innervation of the corpus mammae
is meager by comparison and is predominantly autonomic.
Neither parasympathetic nor cholinergic bers supply any part
of the breast. e eerent nerves are sympathetic adrenergic.
Most of the mammary nerves follow the arteries. A few bers
course along the walls of the ducts. ey may be sensory bers
that sense milk pressure. No innervation has been identied to
supply the myoepithelial cells. e conclusion is that secretory
activities of the acinar epithelium of the ducts depend on
hormonal stimulation, such as by oxytocin.
When sensory bers are stimulated, the release of
adenohypophyseal prolactin and neurohypophyseal oxytocin
occurs. e areola is most sensitive to the stimulus of suckling
and the nipple the least; the skin of the breast is intermediate.
e large number of dermal nerve endings results in high
responsiveness to suckling. Pain bers are more numerous in the
nipple, with few in the areola. All cutaneous nerves run radially
toward the nipple. Breast nerves can inuence the mammary
blood supply and therefore also inuence the transport of
oxytocin and prolactin to the myoepithelial cells and the lacteal
cells, respectively.
MAMMARY GLAND IN PREGNANCY
During the rst trimester, rapid growth and branching from the
terminal duct system into the adipose tissue is stimulated by the
changing levels of circulating hormones. As epithelial structures
proliferate, adipose tissue decreases. ere is increased
inltration of the interstitial tissue with lymphatics, plasma
cells, and eosinophils. By the third trimester, parenchymal
cell growth slows and alveoli become distended with early
colostrum. Alveolar proliferation is extensive.
e lactating mammary gland has many alveoli that are
made up of cuboidal, epithelial, and myoepithelial cells. Little
connective tissue separates the alveoli. Lipid droplets are visible
in the cells. By complex interplay of the nervous system and
endocrine factors (progesterone, estrogen, thyroid, insulin,
and growth factors), the mammary gland begins to function
(lactogenesis stage I) and other hormones establish the milk
secretion and maintain it (lactogenesis stage II).
Human prolactin has a signicant role in both pregnancy
and lactation. e levels are high during pregnancy, but the
inuence of prolactin on the breast itself is inhibited by a
hormone produced by the placenta, originally referred to as
prolactin- inhibiting hormone but believed to be progesterone.
Physiology of Lactation
LACTOGENESIS
Lactation is the physiologic completion of the reproductive
cycle. e human infant is the most immature and dependent
of all mammals except for marsupials, and thus the breast
provides the most physiologically appropriate nutrients
required by the human infant at birth. roughout pregnancy,
the breast develops and prepares to take over the role of fully
nourishing the infant when the placenta is expelled. e breast
is prepared for full lactation aer 16 weeks’ gestation. e
physiologic adaptation of the mammary gland to its role in
infant survival is a complex process, only the outline of which
is discussed here. ere are a number of complete reviews of
the newer scientic studies on the physiology of lactation.
89- 91
Hormonal control of lactation can be described in relationship
to the ve major changes in the development of the mammary
gland: embryogenesis, mammogenesis or mammary growth,
lactogenesis or initiation of milk secretion, lactation or full milk
secretion, and involution (see Table 11.3). Detailed explanation
of mammary growth is beyond the scope of this discussion. e
two most important hormones involved in lactation itself are
prolactin and oxytocin, and these are described with respect to
their impact on lactogenesis.
Lactogenesis is the initiation of milk secretion, beginning
with the changes in the mammary epithelium in early pregnancy
and progressing to full lactation. Stage I lactogenesis occurs
Mammary
lobus containing
several lobuli
Mammary
lobulus containing
10 to 100 alveoli
Areola
mammae
Montgomery
glands
Mammary
fat
Connective
tissue septa
Figure 11.3 Morphology of the mature breast. Diagrammatic dis-
section reveals mammary fat and duct system. (Modified from Lawrence
RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession.
9th ed. St Louis, MO: Mosby; 2021:45.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 171
during pregnancy and is achieved when the gland is suciently
dierentiated to secrete milk. It is prevented from doing so by
high circulating plasma concentrations of progesterone.
92
Stage
II is the onset of copious milk secretion associated with delivery
of infant and the placenta.
90
e progesterone level decreases
sharply, by 10- fold in the rst 4 days. is is accompanied by
the programmed transformation of the mammary epithelium.
93
By day 5, the infant has 500 to 750 mL of milk available (Fig.
11.4). e changes in milk composition that occur in the rst
10 postpartum days should be viewed as part of a continuum in
which the rapid changes of the rst 4 days are followed by slower
changes in various components of milk throughout lactation.
90
A change in permeability of the paracellular pathways results
in a shi from high concentrations of sodium, chloride, and
the protective immunoglobulins and lactoferrin, little lactose,
and no casein in colostrum to increasing amounts of all milk
components.
94
Lactogenesis stage II results in an increase of milk from 100
mL in the rst 24 hours to large volumes (500 to 750 mL/day)
by day 4 or 5, gradually leveling o at 600 to 700 mL/day by
day 8.
95
ese volume changes are associated with a decrease in
sodium and chloride concentration and an increase in lactose
concentration. e production of lactose drives the production
of milk. e early changes in sodium and chloride are a function
of the closure of the tight junctions that block the paracellular
pathway.
96- 98
Secretory IgA and lactoferrin represent 10% by
weight of the milk produced in the rst 48 hours, and although
their amounts remain the same, the increased volume of milk
produced decreases their concentration. At 8 days, secretory
IgA and lactoferrin are 1% by weight and 2 to 3 g/day.
99
At 36 postpartum hours (in multiparas) and at up to 72
hours (in primiparas), milk production increases 10- fold (from
50 to 500 mL/day). Women refer to this as their milk “coming
in.” It reects a massive increase in synthesis and secretion of
the components of mature milk, including lactose, protein, and
lipid.
96
During pregnancy, hormones maintain the pregnancy and
produce mammary tissue that is prepared to produce milk but
does not do so. Progesterone, prolactin, and possibly placental
lactogen are credited with the development of the alveoli.
Progesterone has been identied as the major inhibitor of milk
production during pregnancy.
100
Prolactin levels in pregnancy
are greater than 200 ng/mL. Apparently, the continued high
level of prolactin and a decrease in progesterone are necessary
for stage II lactogenesis aer parturition.
100
e placenta is
the main source of progesterone in pregnancy. Aer the birth,
progesterone receptors are lost in the human breast and estrogen
levels drop precipitously.
In addition to prolactin, insulin and corticoids are essential
to milk synthesis.
101
Delayed lactogenesis is seen in women who
had retained placenta, cesarean section, diabetes, and stress
during delivery.
95,101- 103
Many factors contribute to delayed
onset of lactogenesis in women with gestational diabetes,
including prepregnancy obesity, older maternal age, and
insulin requirement for gestational diabetes.
104- 106
In the 1940s,
Jackson rst noted that stressful labors inuenced the early
breastfeeding experience in the rooming- in unit.
107
Stress alone
may be the trigger for delayed lactogenesis in the conditions
other than retained placenta.
83,108
It has been observed that
high sodium levels in early milk samples are seen in pregnancy,
mastitis, involution (weaning), premature birth, and inhibition
of prolactin secretion by bromocriptine. ese observations
suggest that junctional closure depends on adequate suckling
or eective milk removal in the rst 3 postpartum days. e
signicance of having a high sodium concentration in breast
milk requires further study.
94
If milk removal does not begin by 72 hours, the changes in
milk composition associated with lactogenesis are reversed and
the probability that lactation will be successful decreases.
109
us clinical eorts that facilitate early suckling by the newborn
enhance the probability of lactation success. Early stimulation of
the breast by pumping before 72 postpartum hours is essential
when the infant is unable to nurse directly. Initiation of milk
expression within the rst 6 hours aer birth has resulted in
increased milk volume, greater colostrum production, and
sustained milk volumes.
110
is knowledge is important to
reinforce with mothers, families, and sta to encourage and
facilitate early expression of milk by mothers of premature
infants or when infants and mothers are separated for any
reason.
LET- DOWN (EJECTION) REFLEX
An eective let- down reex is key to successful lactation. is
reex, also known as the ejection reex, was rst described
in humans by Peterson and Ludwick in 1942
111
and was later
demonstrated clinically by Newton and Newton to be caused by
the release of oxytocin by the pituitary.
38
Since that time, many
renements in the understanding of the process of milk ejection
have been published,
112- 114
but the fundamental principles are
unchanged (Fig. 11.5). A mother may produce milk, but if it is
not excreted, further production is eventually suppressed. e
reex is a complex function that depends on hormones, nerves,
and glandular response and can be inhibited most easily by
psychological inuences.
38
Oxytocin is the hormone responsible for stimulating the
myoepithelial cells to contract and eject the milk from the ductal
system. e ducts begin at the alveoli, which are surrounded by
a basket- like structure of myoepithelial cells that also surround
the ducts all the way to the nipple. When the infant stimulates
the breast by suckling, impulses sent to the central nervous
system and to the posterior pituitary result in the release of
oxytocin, which is then carried by the bloodstream to the
800
600
mL/day
400
200
0
02 46 8
Postpartum day
Figure 11.4 Milk volumes during first postpartum week. Mean val-
ues from 12 multiparous White women who test- weighed their infants
before and after every feeding for the first 7 postpartum days. (Redrawn
from Neville MC, Keller RP, Seacat J, et al. Studies in human lactation:
milk volumes in lactating women during the onset of lactation and full
lactation. Am J Clin Nutr. 1988;48:1375–1386.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
172
myoepithelial cells. is is a neuroendocrine reex. Oxytocin
release can also be stimulated by other pathways of sight, sound,
and smell that represent the infant. Oxytocin also stimulates
the myoepithelial cells in the uterus, which are very sensitive
to oxytocin during parturition and for a week or so aer the
birth. is causes the uterus to contract, decreases blood loss,
and hastens postpartum involution. e uterus of a mother who
breastfeeds returns to a prepregnant state more rapidly. e
uterine cramping experienced while breastfeeding is a result of
this stimulus (see Fig. 11.6).
Newton and Newton
38
demonstrated that pain and stress
interfered with the let- down reex because it interfered with
oxytocin release. In their experimental model, they stimulated
stress with pain, loud noises, or pressure to solve mathematical
problems. In other species, oxytocin release has been shown to
stimulate mothering behaviors.
115
Levels of adrenocorticotropin
and plasma cortisol are decreased in lactating women compared
with nonlactating women in response to stress.
Prolactin is central to the production of milk and regulates
the rate of synthesis. Its release depends on the suckling of the
Afferent arcs
Suckling
Oxytocin
Oxytocin
Prolactin
Direct
reflex arc
Cervical
dilation
Hypothalamus
Prolactin-inhibiting factor
Pituitary
Figure 11.5 Neuroendocrine control of milk ejection. (Modified from Vorherr H. The Breast: Morphology, Physiology and Lactation. New York,
NY: Academic Press; 1974.)
Oxytocin
Uterus
Pituitary gland
Myoepithelial
cell
Lacteal cell
Prolactin
Hypothalamus
Figure 11.6 Diagram of let- down (ejection) reflex arc. When the infant suckles the breast, mechanoreceptors in the nipple and areola are stimu-
lated, which sends a stimulus along nerve pathways to the hypothalamus, which stimulates the posterior pituitary gland to release oxytocin. Oxytocin
is carried via the bloodstream to the breast and uterus. Oxytocin stimulates myoepithelial cells in the breast to contract and eject milk from the alveo-
lus. Prolactin is responsible for milk production in the lacteal cells lining the alveolus. Prolactin is secreted by the anterior pituitary gland in response
to suckling. Stress (e.g., pain, anxiety) can inhibit the let- down reflex. Seeing or hearing the cry of the infant can stimulate the release of oxytocin but
not prolactin. (From Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession. 7th ed. St Louis, MO: Mosby; 2010:259.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 173
infant or the stimulation of the nipple by mechanical pumping
or manual expression. Prolactin is also released through a
neuroendocrine reex. Its inuence is modied, however,
by the actual release of milk from the alveoli. Local factors in
the ductal system or in the accumulated milk can inhibit milk
release and thus inhibit further milk production. Prolactin is
not released as a result of sound, sight, or smell of the infant, as
is the case with oxytocin, but only by suckling (Fig. 11.7).
INITIATION OF LACTATION
Although breastfeeding is a natural process in postpartum
women, it is a learned skill, not a reex. Because the incidence
of breastfeeding in developed countries dropped to about
10% in the 1950s and 1960s, there are few experienced role
models available to support, encourage, and assist new mothers
in feeding their infants at the breast. In the late 1940s, Edith
Jackson at Yale, in cooperation with Herbert oms, established
the rst rooming- in unit in the United States, introduced
“childbirth without fear,” and reestablished breastfeeding as
the norm for mothers and infants at the Yale New Haven
Hospital.
116
Obstetric and pediatric residents were well schooled
in the practical aspects of breastfeeding and human lactation.
Jackson and her pediatric colleagues published the classic article
on the management of breastfeeding,
117
on which decades of
publications, both lay and professional, were based.
e obstetrician and pediatrician have become more involved
in the decision to breastfeed and in the practical management of
the mother- infant dyad. Medical schools are gradually adding
breastfeeding and lactation to their curriculum. Although it is not
the physician’s role to put the infant to the breast, it is important
to understand the process, to recognize problems, and to know
how to solve them. Breastfeeding support is a team eort in
which the physician works with many health care professionals,
including nurses, midwives, doulas, and dietitians, to provide
complete care to the perinatal patient. Lactation specialists
may be nurses, dietitians, nonmedical individuals with special
training, or physicians with specialty designation. e physician
should be sure that consultants are licensed and board certied
by the International Board of Lactation Consultant Examiners
and that other collaborating physicians are recognized as fellows
of the Academy of Breastfeeding Medicine.
Except in extreme cases, breast size does not inuence milk
production. Augmentation mammoplasty does not interfere
with lactation unless a periareolar incision was made and
nerves were interrupted. If augmentation was done for cosmetic
enhancement, the tissue should function well, but if there was
little or no palpable breast tissue before surgery, lactation may
be improbable.
Reduction mammoplasty is more invasive surgery, and
lactation results depend on the technique used. If many ducts
were severed and the nipple and areola transplanted, lactation
is interfered with. However, if the nipple and areola remained
intact on a pedicle of ducts, lactation could be successful. Other
incisions (e.g., for lump removal) should be discussed but
usually do not interfere with lactation.
During pregnancy, the obstetrician should document the
changes in the breasts in response to pregnancy, when the
nipple and areola should become more pigmented and enlarged
and the breast should enlarge one- half cup size or more. Lack of
breast changes should also be communicated to the pediatrician,
because this represents a risk for early failure to thrive in the
infant because of insucient milk supply. A breast examination
should be conducted late in the pregnancy to check for any new
ndings of masses, lumps, discharge, or pain. Berens described
the role of the obstetrician throughout pregnancy in detail.
118
Initiating Breastfeeding
e ideal time to initiate breastfeeding is immediately aer birth
(the Baby- Friendly Initiative recommends within 1 hour of birth).
When le on the mother’s abdomen to explore, the unmedicated
newborn will move toward the breast, latch on, and begin suckling.
is usually takes 20 to 30 minutes if unassisted.
119
is has been
described as initiating breastfeeding by breast crawl.
120
e infant
is ready to feed and has been sucking in utero since about 14 weeks’
gestation, consuming amniotic uid daily (about 1 g protein/kg
of fetal weight is received daily from amniotic uid). e infant
at 28 weeks’ gestation has already developed coordinated rooting,
suckling, and swallowing necessary for breastfeeding. e ability to
coordinate suck and swallow while bottle feeding does not occur
until 34 weeks.
Holmes and coworkers outlined recommendations for
peripartum breastfeeding management for the healthy mother
and infant at term.
121
Shortly aer delivery, the mother should
be oered the opportunity to breastfeed and should be assisted
to assume a comfortable position, usually lying on her side. e
infant can be placed beside her, tummy to tummy, facing the
breast. e mother should support her breast with her hand,
keeping her ngers behind the areola so the infant can latch
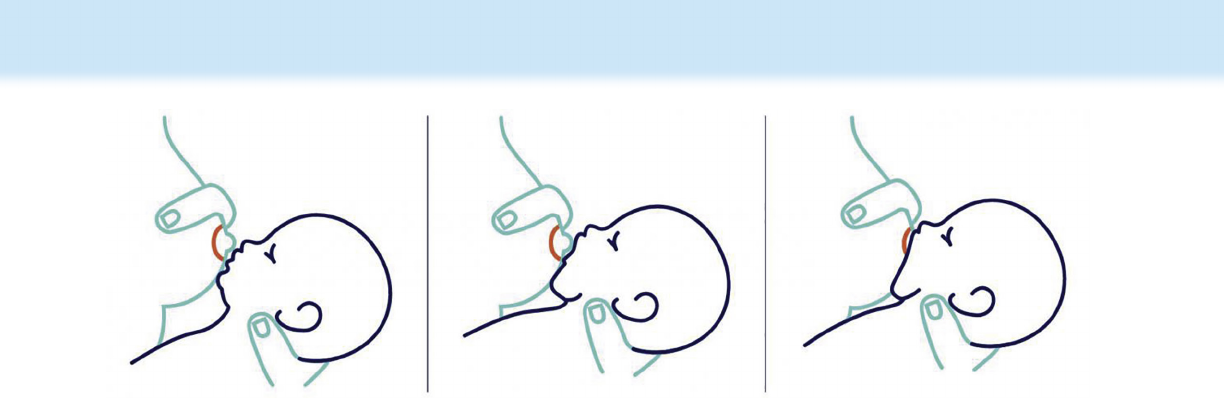
on. e mother should stroke the center of the infant’s lower
lip with the breast (see Fig. 11.8).
122
e infant should open the
mouth wide, extend the tongue, and draw the nipple and areola
into the mouth to form a teat. e teat is compressed against the
palate by the tongue, and the gums and lips form a seal with the
60 30 030
20
30
40
50
100
200
300
400
500
1000
2000
Play with
Infant
Milk
Let-
Down
Nursing
60 90
Time in minutes
120 150
Prolactin ng/mL
180
Figure 11.7 Plasma prolactin stimulation. Plasma prolactin levels
were measured by radioimmunoassay before, during, and after a period
of nursing in three mothers between 22 and 26 days after the birth. The
levels rose with suckling but not with infant contact only. (Modified from
Josimovich JB, Reynolds M, Cobo E. Lactogenic hormones, fetal nutri-
tion, and lactation. In: Josimovich JB, Reynolds M, Cobo E, eds. Prob-
lems of Human Reproduction. Vol 2. New York, NY: John Wiley & Sons;
1974:1.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.
PART 1 Scientific Basis of Perinatal Biology
174
breast. It is the peristaltic motion of the tongue that stimulates
the let- down reex. e continued peristaltic motion travels
to the posterior tongue, the pharynx, and down the esophagus
as one coordinated motion so that swallowing is automatically
coordinated with suckling during breastfeeding.
Ultrasound imaging of milk ejection in the breasts of
lactating women has provided a more detailed description
of the process compared with the traditional serial sampling
of plasma oxytocin levels and measurements of intraductal
pressure. A signicant increase in milk- duct diameter can be
observed during milk ejection. Multiple milk ejections occur
during the process and are correlated with milk ow and with
the changes in milk- duct diameter, although they are not sensed
by the mother.
114,123
e number of milk ejections inuences
the amount of milk available to the infant.
Sucking an articial nipple is a quite dierent tongue motion
that is not coordinated with swallow. A newborn should
not be given a bottle to test his or her feeding ability before
breastfeeding. It is wise to avoid all articial nipples (bottles
or paciers) in the early weeks of breastfeeding. If, for medical
reasons, the infant requires donor human milk or a breast milk
substitute, it can be given by medicine cup (cup feeding), spoon,
dropper, or supplemental feeder.
124- 126
e initial contact may be limited to exploration of the
breast by the infant, with licking and nuzzling of the nipple,
or the infant may latch on and suck for minutes. Timing is not
necessary because the infant will interrupt him- or herself. In
the rst hour aer birth, the term unmedicated infant will be
quietly alert. It is an opportunity for the mother, father/partner,
and infant to get acquainted.
Ideally, mother and infant recover in the same room together.
e infant is fed on awakening, and the mother learns the early
signs of hunger. Crying is a late sign of hunger in the infant.
e mother also learns about caring for her healthy term infant.
ere should be no schedules and no intervention unless an
infant does not feed for over 6 hours. A normal feeding pattern
for breastfeeding in early infancy is 8 to 12 feeds every 24 hours
until satiety. e nursing sta and lactation consultants ensure
that the infant latches on well and the mother’s questions are
answered. Breastfeeding should not hurt; when it does, the
process should be observed and adjusted. e obstetrician
should be involved if needed in the evaluation of breast pain and
can review frequent and eective feeding prior to the mother’s
discharge. e physician should observe a feeding as part of
the infant’s discharge examination. If there is persistent breast
or nipple pain with breastfeeding additional evaluation will be
needed as outlined by the Academy of Breastfeeding Medicine’s
Clinical Protocol #26: Persistent Pain with Breastfeeding or
ACOG Committee #820 on Breastfeeding Challenges.
127
e
mother should be aware of the milk letting down by tingling
in the breast or dripping from the opposite breast. During the
feeding session the infant should be observed in coordinated
sucking, swallowing, and breathing to eect adequate milk
transfer while breastfeeding. Breastfeeding infants use primarily
negative pressure to remove milk from the breast. Vacuum is
created by the downward movement of the anterior portion of
the tongue parallel to the hard palate. e infant does not suck-
swallow- breathe in a 1:1:1 ratio but in variable combinations of
the three actions during breastfeeding.
128
e infant’s weight is measured daily and again just before
discharge. A weight loss of greater than 5% in the rst 48
hours should be assessed by checking the feeding process
and reviewing voidings and stoolings. Maximum weight loss
should not exceed 7% in a breastfed infant by 72 hours. e
weight should plateau aer 72 to 96 hours and start to rise with
adequate feedings. Birth weight should be regained by 7 days
or, at the latest, 10 days by a breastfeeding infant. Flaherman
and colleagues have developed nomograms to track such weight
loss aer vaginal or cesarean births. ere is also an online tool
for calculating percent weight loss over time since birth at Penn
State Health, e NEWT Newborn Weight Tool.
129,130
A healthy infant voids at least once and stools at least once
in the rst 24 hours, both at least twice in the second 24 hours,
and both at least three times in the third 24 hours. From then on
voiding should occur at least six times daily. An infant should
stool at least once (and preferably three times) every day in the
rst month of life. Aer 3 to 4 months of age, a perfectly healthy
breastfed infant may go a week without stooling and then pass
a so yellow stool, but this should not occur under 1 month of
age.
Early discharge from the hospital has increased the need for
newborn care visits within a few days aer discharge and as
required thereaer at 2- to 4- week intervals for assessment. A
follow- up visit at 48 to 72 hours aer discharge, or at 3 to 5 days
of age for infants discharged at 48 hours or less, is recommended
for reassessment of latch, ecacy of feeding, feeding pattern(8
Figure 11.8 Getting a good latch. (a) Tickle the baby’s lips with your nipple to encourage him or her to open wide, (b) pull your baby close so
that the baby’s chin and lower jaw move in to your breast, and (c) watch the baby’s lower lip and aim it as far from the base of the nipple as possible
so that the baby takes a large mouthful of breast. (From Office on Women’s Health in the US Department of Health and Human Services. Getting a
Good Latch. https://www.womenshealth.gov/breastfeeding/learning- breastfeed/getting- good- latch. Updated August 28, 2018. Accessed January
30, 2021.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 175
to 12 feeds every 24 hours), hydration status, and frequency of
urination and stooling.
6,8
e transition from hospital to home is a stressful time for all
families, but when an infant is premature, small for gestational
age, or even near term, transition can be extremely dicult.
e stress can be reduced by appropriate detailed discharge
planning. e presence of eective, coordinated sucking,
swallowing, and breathing should be pointed out to the parents
and documented in the chart. Mothers/parents should receive
adequate assistance in the days before discharge; positioning,
latch, and milk transfer should be perfected, or gavage feeding,
supplementation, and fortication should be managed by the
parents prior to discharge. e AAP Committee on Fetus and
Newborn has delineated the following three competencies of
infants that are recognized as essential before hospital discharge
of the preterm infant: the ability to maintain body temperature
in a home environment, suciently mature respiratory control,
and oral feeding with or without gavage feeding sucient to
support appropriate growth.
131,132
Issues in the Postpartum Period
BREAST ENGORGEMENT AND NIPPLE
TENDERNESS
A little engorgement of the breast in the rst 24 hours is
physiologically normal as the vascular supply shis from the
once- gravid uterus to the breasts. Absence of any engorgement,
such as absence of breast growth during pregnancy, is cause
for concern. Not only is excess engorgement painful but the
increased vascular pressure compresses the alveoli and ducts
and interferes with milk production and release.
74,133
Prevention
of excessive engorgement is the best treatment and involves the
following: (1) wearing a well- tting nursing brassiere, even
before the breasts are engorged, and around the clock; (2)
frequent feedings for the infant, being sure to balance the use
of both breasts; (3) gentle massage and soening of the areola
before oering the breast to the infant, so that proper latch- on
can be accomplished; (4) if necessary, applying cold packs or
cold compresses aer a feeding; and (5) taking acetaminophen
or ibuprofen, which may be safely used by the mother for
discomfort. erapeutic breast massage during lactation is a
recently studied intervention that is being recommended.
134
Peak engorgement usually occurs between 72 and 96
postpartum hours when the mother has arrived home and is on
her own. At the peak of discomfort, standing in a warm shower
to let milk drip or applying warm compresses before pumping
to relieve the pressure and stimulate ow provides relief before
the phenomenon subsides. Although a variety of remedies
have been recommended for engorgement, there is insucient
published evidence to recommend one individual treatment
over others.
135- 137
Sore nipples are a common complaint when early lactation
has gone unassisted. It should not hurt to breastfeed. When it
does hurt, the infant should be taken o the breast by breaking
the suction with a nger and reattaching the infant carefully,
following the steps previously described. e major cause of sore
nipples is inadequate latch- on. It is not caused by breastfeeding
too long or too frequently. A newborn usually feeds about every
2 hours in the rst few weeks of life. Persistent sore nipples,
cracks, or oozing may require the assistance of a licensed certied
lactation consultant or breastfeeding medicine specialist who
can take the time and has the experience to work with the
mother to identify the cause, determine eective treatment, and
assist the dyad in maintaining pain- free breastfeeding.
127,138
A clinically dened phenomenon has recently been described
in which breastfeeding mothers become acutely saddened,
tearful, and depressed with milk ejection. is picture has been
described as a dysphoric milk- ejection reex (D- MER). A clinical
case study and a descriptive study by Ureno and colleagues have
related this picture to a severe drop in maternal dopamine and
described ameliorating factors including distraction, chocolate
ice cream, herbals, smoking cigarettes, pseudoephedrine, and
bupropion.
139,140
Management of this condition should begin
with “normalization,” as the experience is oen accompanied
by signicant maternal guilt for feeling negatively toward the
supposedly positive experience of bonding, and can include
various distraction interventions. e physician should assist
the mother in deciding whether continued breastfeeding or
weaning is best for her and her child.
FAILING MILK SUPPLY
Many misconceptions lead to the impression that a failing milk
supply is a common occurrence. Many women discontinue
breastfeeding before 3 postpartum months, believing their milk
is diminishing because their breasts are no longer engorged. A
very common question for all breastfeeding mothers is “Am I
making enough milk?” ere is a legitimate question regarding
whether this is just the mother’s perception or actual insucient
milk production for appropriate infant growth.
141
Once supply
and demand have equilibrated and the breast makes what the
infant needs, the breasts are so and do not constantly drip. e
emptying time of the stomach of the infant fed human milk is
90 minutes, that of the infant fed formula is 3 to 4 hours, and
that of the infant fed cow’s milk is 6 hours. Continuing to feed
every 3 hours is a testimony to the breast milk’s digestibility, not
its inadequacy. Weight gain in the infant is the better barometer
of success. ACOG and AAP recommend that exclusive
breastfeeding should continue for 6 months, with continued
breastfeeding while adding weaning foods for the next 6 months
and then as long thereaer as mutually desired by mother and
child.
74,142
Genuine failure to produce enough milk may result from
infant causes, such as increased need, increased uid losses,
or lack of adequate suckling, or from maternal causes, such as
failure to let down or failure of production. Each case should be
carefully reviewed because most situations are remediable. ere
is a long list of conditions associated with low or insucient milk
supply in ACOG’s Committee Opinion #820 on Breastfeeding
Challenges.
74
Ideally, both the obstetrician and the pediatrician
are experienced in lactation management or have a sta member
who is. Working together with a licensed certied lactation
consultant, the issues can be resolved, and breastfeeding can
continue successfully. ere are common situations where the
evaluation of breastfeeding is appropriate but supplementation
with breast milk substitutes is not. Donor human milk should
be considered if it is available. ere are few situations when
supplementation in healthy term infants is indicated, and
some examples include asymptomatic hypoglycemia, signs
and symptoms indicative of inadequate milk intake (especially
infant weight loss or insucient gain), hyperbilirubinemia,
or the infant is ill, necessitating select macronutrients. Some
maternal indications for supplementation may include delayed
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
176
secretory activation (with inadequate intake by the infant),
primary glandular insuciency, breast pathology or previous
surgery resulting in poor milk production, situations requiring
temporary cessation of breastfeeding (maternal chemotherapy),
and intolerable breast pain during feedings unimproved by
interventions.
143
In certain situations, galactogogues may be
recommended to maintain the milk supply. Published reviews
of the use of galactogogues are available.
144- 148
BREASTFEEDING AFTER PREMATURE OR
MULTIPLE BIRTHS
Human milk is benecial in the management of the premature
infant, according to the policy statement of the AAP.
6
is can be
mother’s own milk (MOM) or donor human milk (DHM).
149,150
e benets include infection protection; improvement
in gastrointestinal function, digestion, and absorption of
nutrients; and improvement in neurodevelopmental outcome.
e psychological well- being of the mother is enhanced when
she provides her milk for her compromised infant.
151
Meeting
the intrauterine rates of growth and nutrient accretion requires
attention. Although human milk satises these needs for larger
premature infants, it can be carefully supplemented for smaller
infants and still preserve the benets of human milk. Factors
related to the infants, the mothers, and clinical practice clearly
aect exclusive breastfeeding of premature infants.
152- 155
ere is
a commercial product created with 100% human milk developed
to enhance mother’s milk and replace supplementation made
with cow’s milk.
156
Twins and triplets present problems of time management for
the mother.
157
e mother will make enough milk, as supply will
meet demand. Twins learn quickly to nurse simultaneously and
will continue to do so for months or years. Breastfeeding ensures
a mother’s interaction with her infants. Helpful friends and
relatives can perform the other household duties. e mother
can also provide enough milk for triplets. Some mothers prefer
to nurse two at a feeding, giving the third a bottle but rotating
the three, feeding by feeding. Any breast milk is valuable in
this situation. Mothers of singleton infants may need help, but
mothers of multiples especially need help. It may be necessary
for the physician to prescribe help and careful attention to
proper rest. Mothers have also breastfed quadruplets and higher
numbers of infants. e mother can nurse several infants at
each feeding and rotate bottle feeding. Exclusive breastfeeding
of quadruplets for the rst year has been reported by Berlin.
158
Contraception
Open- ended questioning is appropriate to support maternal and
familial decision making about breastfeeding, sexuality, birth
spacing, and contraception. Appropriate information sharing
should include (1) there are improved maternal and infant
outcomes in subsequent pregnancies if the interpregnancy
interval is greater than 18 months, (2) exclusive and frequent
breastfeeding can be highly eective in birth spacing, and
(3) there are a number of options for contraception which
are both eective and safe during breastfeeding.
159
e
Centers for Disease Control and Prevention (CDC) and
WHO Medical Eligibility Criteria for Contraception include
the lactational amenorrhea method for birth spacing. e
published risk of pregnancy is less than 2% when women
meet three situational variables: amenorrhea, fully or nearly
fully breastfeeding with frequent feedings and no periods of
>4–6 hours between breastfeeds, and less than 6 months since
the most recent delivery.
160,161
Women should be aware that
these variables need to be met to provide protection against
pregnancy. Hormonal methods of contraception are oered
over nonhormonal/barrier methods due to their higher ecacy.
Estrogen or combination products are usually avoided before
6 weeks postpartum. Progestin- only contraceptives, such as
the mini- pill tablet, injectable medroxyprogesterone acetate
(Depo- Provera), and levonorgestrel implants, are the hormonal
methods of choice in this period. ere remain theoretical
concerns regarding whether exogenous progesterone disrupts
milk synthesis; however, some limited studies present data
that progestin- only methods are safe even in the rst 6 weeks
postpartum. is theoretical risk of diminished breastfeeding
with immediate postpartum progestin- only contraception
should be communicated to mothers and families to promote
and support autonomous informed decision making.
162
Recommendations for addressing contraception during
lactation are available from the Academy of Breastfeeding
Medicine and from ACOG.
163,164
ACOG maintains an online
resource for patients as well.
165
Maternal Infections During
Breastfeeding
Although oen a mother is concerned about the risk to a
breastfeeding infant when she has an infectious illness, maternal
infection is not a contraindication to breastfeeding in most
cases. Proscribing breastfeeding out of fear of infection deprives
infants of signicant immunologic, nutritional, and emotional
benets of breastfeeding when they are most needed.
9
e decision- making process to breastfeed despite maternal
infection should involve discussion of the usual route of
infection transmission, reasonable infection control precautions,
potential severity of infection in the infant or child, medications
to treat the mother that are compatible with breastfeeding, the
potential of prophylaxis for the infant, the protective eect of
breast milk, and the acceptability of using expressed breast milk
temporarily. e discussion should involve the mother and
family, weighing the known and potential risks of the infection
against the known benets of breastfeeding.
9
For example, diphtheria and active pulmonary tuberculosis
in the mother are commonly transmitted via the respiratory
route, so contact between infant and mother should be
proscribed regardless of how the infant is being fed. Diphtheria
and tuberculosis are not transmitted in the milk, except in the
case of cutaneous diphtheria involving the nipple and breast
or tuberculosis mastitis. As long as there are no lesions on the
breast, expressed breast milk can be given to the infant during the
initial treatment of the mother (probable infectious periods are
5 days for diphtheria and 14 days or until the sputum is negative
for acid- fast tuberculous bacilli). Prophylactic antibiotics for the
infant are appropriate in each case—penicillin or erythromycin
for diphtheria and isoniazid for tuberculosis.
9,166,167
Brucellosis is known to be transmitted via unpasteurized
milk of animals. ere are case reports of transmission of
brucella from mothers to infants via breast milk, but human-
to- human transmission is rare overall. In the case of suspected
maternal brucellosis, rst conrm the diagnosis by culture and
serology and then treat the mother during pregnancy between
12 and 36 weeks of gestation with rifampicin and trimethoprim/
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 177
sulfamethoxazole to avoid disrupting organogenesis prior to 12
weeks or causing neonatal kernicterus aer 36 weeks. If the
mother has been eectively treated the infant can breastfeed,
or the infant can be given rifampicin beginning at birth with
trimethoprim/sulfamethoxazole added aer 4 weeks of age as
prophylaxis while continuing breastfeeding.
168
In certain highly contagious and serious infections such as
the hemorrhagic fevers—specically with Ebola, Marburg, or
Lassa virus—the risk of transmission from any contact with the
infected mother is high. e CDC and WHO recommend that, if
there are safe alternatives to breastfeeding and care of the infant
by another unexposed, uninfected adult, then mothers with
probable or conrmed Ebola virus disease should be separated
from the infant until recovery.
169,170
A safe, feasible alternative
source of feeding is essential before considering withholding
breast milk. If feasible, pasteurized mother’s milk (via routine
pasteurization, Pretoria pasteurization, or ash heating) may be
utilized in special circumstances when alternative feeding is not
available.
170,171
ere have been few case reports on transmission of dengue
virus or other aviviruses to infants via breast milk. It has been
recommended to suspend breastfeeding once the diagnosis is
made in a mother, maintain the milk supply by pumping and
discarding the milk, and reinitiate breastfeeding when the
mother is well. is may not be feasible if an alternative source
of infant feeding is not available.
172,173
Possible West Nile virus (WNV) transmission to an infant
through breastfeeding has been reported,
174
but the data on this
infection in pregnant or breastfeeding women and their infants
are limited. Hinckley and coworkers reported 10 instances of
maternal or infant WNV- related illness while breastfeeding. In
ve cases, the transmission of WNV through breast milk could
not be conrmed or ruled out, and in the other ve cases, there
was no evidence of vertical transmission. ey concluded that
the information they presented does not support a change in
breastfeeding practices aer infection with WNV, and that more
information is needed.
175
In the case of infections at specic sites in the mother, the
management varies with the specic etiologic organism. For
example, with mastitis, continued eective milk removal
is the essential rst step along with supportive measures
(rest, adequate uids and nutrition, and breast massage) and
analgesia. If the obstruction to milk ow and inammation
progress in the next 24 hours, antibiotics are indicated. Empiric
antibiotics for acute lactational infectious mastitis include
dicloxacillin, ucloxacillin, or a rst- generation cephalosporin.
For methicillin- resistant Staphylococcus aureus coverage,
local resistance patterns should be reviewed; clindamycin,
trimethoprim/sulfamethoxazole, or vancomycin are options.
Alternative therapy for penicillin allergy includes erythromycin,
clindamycin, or trimethoprim/sulfamethoxazole. Close follow-
up for improvement of symptoms and for potential abscess
formation are appropriate. Physical exam should be adequate
to diagnose an abscess, but an ultrasound can help on occasion
and can guide aspiration/drainage.
176
Cytomegalovirus
(CMV) is transmitted from the mother to the infant via breast
milk.
177- 179
In the full- term well infant this does not produce
disease in the infant and is considered “natural immunization.”
ere is considerable debate concerning the risks of CMV
infection in premature or very- low- birth- weight infants. ere
are conicting data regarding the rates of symptomatic disease
in CMV acquired via breast milk in premature infants and
the occurrence of long- term sequelae in these infants.
178,180
e risk of transmission and long- term sequelae may be more
signicant for extremely premature infants less than 28 weeks’
gestational age. Because of the concern for signicant short-
term and possible long- term eects of postnatal infection with
CMV via breast milk, the Red Book Committee of the AAP has
issued some guidance. ey recommend considering screening
mothers of premature infants less than 32 weeks’ gestational
age and short- term pasteurization of breast milk prior to
administration to the infant.
181
Use of CMV- negative donor
human milk is another alternative. Across nurseries worldwide
there is no consensus practice because pasteurization also
inactivates many beneciary components of fresh breast milk.
ere is ongoing research on ash heating, freezing, and
irradiation for inactivation of CMV in breast milk without
inactivation of various biologically active components. Until
there is a broadly accepted consensus opinion, the conservative
approach would be to follow the AAP recommendation from
the Red Book Committee.
In mothers with hepatitis, identication of the etiologic
agent is required before the appropriate management can be
determined. Before the etiologic agent is identied, care must
include precautions for all potential organisms. Consultation
with an infectious disease specialist is oen appropriate.
For hepatitis A virus, infection in the newborn or young
infant is uncommon and not associated with severe illness.
Breastfeeding can continue, and if the diagnosis is made within
2 to 3 weeks of the infant’s initial exposure to the infected
mother, immune serum globulin and hepatitis A virus vaccine
given simultaneously can decrease infection in the infant. With
hepatitis B virus, the risk of chronic infection and its serious
complications are high (up to 90%) when infection occurs
perinatally or in early infancy. Hepatitis B immune globulin
and hepatitis B virus vaccine given simultaneously prevent
hepatitis B virus transmission in over 95% of cases, regardless of
whether the infant is fed by breast or bottle. erefore it is very
appropriate to continue breastfeeding along with provision of
eective immune therapy.
181
With hepatitis C virus (HCV) the rate of mother- to- child
transmission (MTCT) is about 6%. Several cohort studies
suggest that most infants acquire HCV infection in utero or in
the peripartum period. HCV has been detected in colostrum
and breast milk at low levels. Large cohort studies, including
1854 mother- infant pairs, have not shown a signicant
dierence in acquisition of HCV infection between breastfed
and formula- fed infants.
182
Guidelines from the CDC and AAP
state that maternal HCV infection is not a contraindication
to breastfeeding.
183
HCV and human immunodeciency
virus (HIV) coinfection in the mother is a contraindication
to breastfeeding in high- income countries. Because HCV
is a blood- borne virus, the CDC recommends avoiding
breastfeeding from the aected breast, at least temporarily, if an
HCV- infected mother experiences nipples that are cracked or
bleeding.
183
No clear data indicate HCV transmission via breast milk
in HIV- negative mothers.
184
ere is evidence of inactivation
of HCV by breast milk.
185
However, given the multiple issues
involved—low risk of HCV transmission via breast milk,
increased risk of transmission in association with HIV infection
or high levels of HCV RNA in maternal serum, lack of eective
preventive treatments (vaccines or immune serum globulin), and
the risk of chronic HCV infection and serious liver disease—it
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
178
is essential to educate the parents about the possible low risks of
continued breastfeeding and the many benets of breastfeeding
in order to facilitate autonomous informed decision making.
Maternal retroviral infection and breastfeeding is a highly
controversial issue that continues to be evaluated and debated.
HIV type 1 (HIV- 1) is transmissible via breastfeeding and can
signicantly increase the risk of HIV infection in infants born
to HIV- positive mothers. One meta- analysis of ve studies
of infants born to HIV- infected mothers reported the risk of
HIV transmission to infants strictly from breastfeeding as
14% (95% condence interval [CI], 7% to 22%).
186
Among
the many concerns about HIV and breastfeeding are the risk
of transmission related to the duration of breastfeeding, the
relative risks of exclusive versus nonexclusive breastfeeding, the
risk of mortality and morbidity resulting from other infections
and malnutrition associated with not breastfeeding, the
signicance of HIV viral loads and CD4
+
T- cell counts in the
mother relative to transmission from breast milk, the potential
protective eects of breast milk against HIV infection, and the
degree to which antiretroviral therapy for the mother or infant
will be protective against HIV infection. Social issues involved
in this debate include the right of the mother to make choices
for herself and her infant, the social stigma of not breastfeeding
in certain cultures and communities, and the possibility that
breastfeeding rates in HIV- negative mothers will be adversely
aected by the advice given to HIV- positive mothers. In many
countries, neither choice is optimal: breastfeeding risks HIV
infection in the infant, but not breastfeeding increases the risks
of other infections and malnutrition. e lack of adequate data
from controlled trials about the various factors contributing
to infection adds to the diculty of making straightforward
recommendations applicable to diverse situations around
the world. In the United States, it is appropriate to advise no
breastfeeding for infants of HIV- infected mothers to decrease
the risk of HIV transmission to the infant.
187
In resource- poor settings, more recent review of the
available data on providing antiretroviral prophylaxis to either
the mother or the infant during pregnancy and lactation has
led to new recommendations from the WHO.
188,189
ese
include specic recommendations for the use of antiretroviral
therapy in women who need treatment for their own health, the
continuation of which beyond the postpartum period provides
additional protection against MTCT at the same time as allowing
the other benets to the infant from breastfeeding. e criteria
for initiating antiretroviral therapy for pregnant women are the
same as those for nonpregnant women. All HIV- infected women
who are not in need of antiretroviral therapy for their own health
should receive an eective antiretroviral prophylaxis regimen to
prevent MTCT. Eective antiretroviral therapy for the mother
can be continued through the period of lactation to protect the
infant. Additionally, the infant can receive antiretroviral therapy
to prevent against HIV infection via breastfeeding. HIV- infected
women should still receive education concerning the choices of
infant feeding, with emphasis on the benets of breastfeeding
in combination with antiretroviral prophylaxis provided to the
mother or infant for the duration of breast milk consumption.
Education, support, and medical care throughout lactation for
both mother and infant should be provided to achieve the goals
of 100% prevention of MTCT, optimal maternal health and
survival, and long- term infant health and survival.
ere are limited reports that deal with the risk of HIV
type 2 (HIV- 2) transmission via breastfeeding. Studies suggest
that HIV- 2 transmission via breast milk is less common than
HIV- 1 transmission.
190
However, until adequate information
is available, it is appropriate to use the same guidelines as for
HIV- 1.
Transmission of human T- cell leukemia virus type I
(HTLV- I) infection is associated with breastfeeding, although
short- term breastfeeding (less than 6 months) may pose no
greater risk than the risk for formula- fed infants.
191- 193
In
Japan, where high rates of infection with this virus occur,
proscription of breastfeeding is common. In the United
States, when the mother has documented HTLV- I infection,
it is appropriate to discuss the options, risks, and benets of
breastfeeding and to consider short- term breastfeeding. ere
are many uncertainties concerning HTLV type II, related to the
diseases associated with infection and to whether transmission
occurs via breast milk. Here again, it is appropriate to discuss
the available data with the parents and to include an infectious
disease consultant in the discussion to facilitate an autonomous
informed decision.
9
Complications of the Breast
ere are a variety of conditions that can manifest as palpable
breast masses during lactation. Some are lactation- specic and
some non- lactation- specic (see Table 11.4). Some of these
lactation- specic masses are more likely to require imaging
for diagnosis and management, such as galactocele, phlegmon,
abscess, lactating adenoma, and lactiferous sinus, among other
less common masses.
74,194,195
ACCESSORY BREAST TISSUE
Accessory tissue occurs in approximately 2% to 6% of women.
It oen rst manifests during the breast enlargement during
pregnancy. e tissue is frequently in the axilla and bilateral in
approximately one- third of women. Its presence can be acutely
obvious during engorgement of the breast.
PLUGGED DUCTS
Tender lumps in the breast in a mother who is otherwise well
are probably caused by plugging of a collecting duct. e best
treatment is to continue nursing while manually massaging
the area to initiate and ensure complete drainage. Holding
the infant in a dierent position may encourage ow, as may
application of hot packs before a feeding. If repeated plugging
occurs, a check should be made for possible obstruction from
a brassiere strap or other external forces. Some women can
actually see small plugs ejected when they massage. For some,
reducing polyunsaturated fats in the diet and adding lecithin
provides relief.
GALACTOCELE
Milk- retention cysts are uncommon and are usually associated
with lactation. e swelling is smooth, rounded, and
nontender. e cyst may be aspirated to conrm the diagnosis
and to avoid surgery, but it will ll up again. e cyst can be
removed with local anesthesia without interruption of the
breastfeeding routine. e diagnosis can also be conrmed by
ultrasound, by which the cyst and milk look similar but tumor
is distinguishable.
9
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 179
MASTITIS
Mastitis is an infectious process in the breast producing
localized tenderness, redness, and heat, together with systemic
symptoms of a ulike illness with fever and malaise. It can be
distinguished from engorgement and plugged duct (Table 11.5).
Usually a red, tender, hot, swollen, wedge- shaped area of the
breast is visible, and it corresponds to a lobe (Fig. 11.9). e
common organisms are Staphylococcus aureus, Escherichia coli,
and, rarely, Streptococcus. An approach to managing mastitis
in the breastfeeding mother is outlined by the Academy of
Breastfeeding Medicine Protocol Committee; the major points
of management are as follows:
•
Breastfeeding should continue on both breasts, optimizing
eective milk removal.
•
Analgesia is appropriate and necessary to facilitate let-
down and eective milk removal.
•
Supportive measures, including rest, uids, adequate
nutrition, heat for let- down and cold for pain aer milk
expression, and home care support for the infant and the
mother are the rst line of management.
•
If the mastitis is not improving with conservative support-
ive measures, antibiotics appropriate to the probable cause
and relevant sensitivities should be prescribed.
•
Antibiotics are recommended by most practitioners for
10 to 14 days, but there are no controlled trials for the
eective duration of antibiotics. e antibiotic should be
safe for the infant (see Mastitis under Maternal Infec-
tions).
e most common cause of recurrent mastitis is delayed or
inadequate treatment of the initial disease. Mastitis can evolve
into formation of a phlegmon and/or an abscess, sometimes
necessitating drainage and/or imaging. In most situations of
mastitis, laboratory or diagnostic procedures are unnecessary.
Recommended exceptions to this include no response to
antibiotic therapy within 2 days, recurrence of mastitis,
hospital- acquired mastitis, medication allergies to the standard
antibiotics for mastitis, and severe cases of mastitis.
176
On
recurrence, cultures of a midstream ow of milk should be sent
and antibiotics chosen according to the results.
LACTATING ADENOMAS
ese are benign painless masses most frequently observed in
the upper outer quadrant of the breast. ey seem to present
spontaneously, are presumed to be due to hormonal secretion,
and oen resolve aer lactation ends. Biopsy is sometimes
Breast Masses
Lactation- Specific Clinical Findings Non- lactation- Specific Clinical Findings
Accessory breast tissue Common in axilla, bilateral in one-
third of cases, noticed most often
during pregnancy enlargement
Fibroadenoma Mobile rubbery smooth mass, asymptomatic
or tender
Plugged ducts Occur in areas of milk stasis Phyllodes tumor Fibroepithelial lesion similar to fibroadenoma
Galactocele Common, “milk retention cyst,”
fluid filled
Cysts With fibrocystic disease or simple or complex
Phlegmon Poorly defined collection of fluid Intramammary lymph
nodes
Uncommonly palpated but usually identified
by the patient
Abscess Usually well- defined, inflamed fluid
collection
Fat necrosis Irregular palpable mass, tender or not
Lactating adenoma Rubbery or firm mass, fairly well-
circumscribed
Hematoma Tender mass, usually + history of obvious
trauma
Lactiferous sinuses Subareolar mass Periductal mastitis Squamous metaplasia of lactiferous ducts,
most commonly in smokers
Idiopathic
granulomatous
mastitis
Evolving inflammatory swellings
Breast cancer Variable presentations
Data from Mitchell KB, Johnson HM. Breast conditions in the breastfeeding mother. In: Lawrence RA, Lawrence RM, eds. Breastfeeding: A Guide
for the Medical Profession. 9th ed. St. Louis, MO: Elsevier; 2021 [chapter 16]; and Mitchell KB, Johnson HM, Eglash A, Academy of Breastfeeding
Medicine Clinical Protocol #30: Breast Masses, Breast Complaints, and Diagnostic Breast Imaging in the Lactating Woman. Breastfeed
Med. 2019;14(4):208–214. https://doi.org/10.1089/bfm.2019.29124.kjm.
TABLE
11.4
Comparison of Engorgement, Plugged Duct, and Mastitis
Onset Site
Symptom
Swelling and Heat Pain Body Temperature
Systemic
Symptoms
Engorgement Gradual,
immediately
after birth
Bilateral Generalized Generalized <38.4°C (101°F) Feels well
Plugged duct Gradual, after
feedings
Unilateral May shift; little or no
heat
Mild but
localized
<38.4°C Feels well
Mastitis Sudden, after
10 days
Usually unilateral Localized, red, hot,
swollen
Intense but
localized
>38.4°C Flulike
symptoms
From Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession. 8th ed. St Louis, MO: Mosby; 2015:568.
TABLE
11.5
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
180
recommended to conrm the diagnosis but breastfeeding can
continue.
LACTIFEROUS SINUSES
ese are relatively “dilated” lactiferous sinuses palpable beneath
the areola. ey are more noticeable in breastfeeding women
than in those who have stopped breastfeeding.
CANDIDIASIS OF NIPPLE AND BREAST
Candidiasis of the breast is frequently overdiagnosed because
there are several causes for the breast pain that is described by
mothers as feeling like “a stab with a hot poker.” On examination,
there may be little to see except a pinkish hue or slight darkening
to the nipple and central areola. Rarely are white plaques seen
on the nipple. If the mother has a history of vaginal candidiasis,
the infant’s mouth may have become colonized, and this could
have resulted in inoculation of the nipples. e infant should
also be examined for both thrush and diaper rash and treated
simultaneously with the mother for a full 2 weeks. Nystatin
ointment is applied aer each feeding to nipples and areolae,
and the infant receives nystatin drops orally to the oral mucous
membranes aer each feeding. For a recurrent episode, the
mother can be treated with 200 mg oral uconazole systemically
once daily for 7 to 14 days. e infant can be given 6 mg/kg
on day 1 and then 3 mg/kg per dose every 24 hours orally.
Paciers and bottle nipples that are put in the mouth should
be discarded and new ones sterilized daily. Persistent thrush
requires a complete evaluation of the mother and may require
treatment for vaginal thrush, decreased sugar in the diet, and
colonization with lactobacillus by capsule or yogurt. Persistent
pain (longer than 2 weeks) with breastfeeding is an important
situation necessitating evaluation and management. Berens and
colleagues provide an approach to management and detailed
discussion of potential causes of persistent pain.
127
Medications While Breastfeeding
Questions about medication during breastfeeding are very
common. e transfer of maternal drugs to the infant during
lactation is dierent from transfer to the fetus during pregnancy.
Although it is almost always better to breastfeed, the mother
must weigh the potential benets and risks of a medication for
herself and her infant against the substantial benet of being
breastfed for the infant. e risk- benet ratio diers for each
drug and clinical setting. Both scientic information and
experienced clinical judgment are required to assess the risks
and benets and to determine the therapeutic choice.
e AAP Committee on Drugs has published a list of
commonly used drugs and chemicals that may transfer into
human milk.
196
e categories from the AAP list
196
are as
follows:
1. Cytotoxic drugs that may interfere with cellular metabo-
lism of the nursing infant
2. Drugs of abuse for which adverse eects on the infant
during breastfeeding have been reported
3. Radioactive compounds that require temporary cessation
of breastfeeding
4. Drugs for which the eect on nursing infants is unknown
but that may be of concern—for example, bromocriptine,
ergotamine compounds, and lithium
5. Drugs that have been associated with signicant eects
on some nursing infants and should be given to nursing
mothers with caution
6. Maternal medication usually compatible with breastfeed-
ing
7. Food and environmental agents that might have an eect
on the breastfeeding infant
ere are various printed and online resources which
can be consulted concerning the use of medications in
lactation.
69,84,85,197
A readily available and frequently updated
handbook by omas Hale, Medications and Mothers’ Milk,
provides a scale that is roughly the reverse of the long-
established classication system developed by the AAP.
198
e
basic denitions from Hale’s text are as follows:
•
L1: safest
•
L2: safer
•
L3: moderately safe
•
L4: possibly hazardous
•
L5: contraindicated
A lack of information about a drug does not necessarily require
cessation of breastfeeding. Understanding the pharmacology
of a drug, the dosing schedule, and the stage of growth and
development of the infant inform the decision about whether it
would aect the infant. Characteristics of the drug that inuence
its passage into milk include the size of the molecule, its solubility
in lipid or water, whether it binds to protein, the pH, and the
diusion rates (Box 11.4). e route of administration inuences
the blood levels and therefore the milk levels. Passive diusion is
the principal transport mechanism. How the drug is metabolized
inuences whether it is present in the milk in its active form or as
an inactive metabolite (Fig. 11.10).
e infant’s ability to absorb, digest, metabolize, store, and
excrete a drug must be considered when choosing a medication
for a nursing mother. A drug that is not orally bioavailable will
not be absorbed from the milk by the infant. e ability to absorb
and metabolize a drug depends on the infant’s developmental
and chronologic age. An 18- month- old who nurses briey
about four times a day for comfort will get little medication, has
a substantial diet other than mother’s milk, and can metabolize
and excrete more eciently than a newborn. In the rst weeks
of life, the maturation or gestational age should be considered
when determining the safety of a medication, because the less
mature the infant is, the less mature are the liver and kidneys.
Figure 11.9 Mastitis of right breast, upper outer quadrant. (From
Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical
Profession. 7th ed. St. Louis, MO: Mosby; 2010:554.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 181
With the exception of radioactive compounds such as
iodine 131, there is no drug whose possible presence in the
milk would require immediate withholding of breastfeeding
because the physician does not know the data. e American
College of Radiology has produced a manual for contrast
agents; based on reports from their analysis of the data, less
than 1% of the maternal dose is excreted in the breast milk, and
less than 1% of the contrast in breast milk reaching the infant’s
gastrointestinal tract is absorbed.
199
Box 11.5 provides some
salient points for the use of radiologic and nuclear medicine
studies during lactation. erefore arbitrary interference with
breastfeeding until information can be obtained is not justied.
Ample references and information lines are available to resolve
the issue. For medications used once or for a short time (e.g.,
anesthesia), the time required for the drug to clear the mother’s
system and her milk can be determined. e mother can pump
and discard her milk for that period if deemed necessary using
evidence- based resources and return to breastfeeding (usually
in a few hours or days, not weeks).
MILK- TO- PLASMA RATIO
e milk- to- plasma ratio, a term applied to drugs being used
by a lactating mother, indicates the level of the drug in the milk
compared with the level in the plasma at a given time. e
dosage of the drug, including time and route of dosing, must
be known to interpret the milk- to- plasma ratio. If there is a
very low level in the plasma and the same very low level in the
milk, the ratio is close to or equal to 1. A ratio of 1 suggests
that the level is of concern; however, the actual level in milk is
low and therefore the risk to the infant is likely to be low. Most
drugs have a milk- to- plasma ratio of less than 1. It is important
to know peak plasma and peak milk levels, and peak plasma
and peak milk times, to make appropriate recommendations
to avoid feeding the infant when transfer of the drug would be
greatest.
ANALGESIA AND ANESTHESIA FOR THE
BREASTFEEDING MOTHER
e appropriate use of analgesia and anesthesia during labor and
delivery and in the peripartum period, as well as at other times
for the breastfeeding mother, is a skill that every obstetrician
should master. e use of pharmacologic agents for pain relief
during labor and in the postpartum period is appropriate and
may improve outcomes for the infant and mother. eir use
may inuence the course of labor, the neurobehavioral status
of the infant, and the initiation of breastfeeding. e eects
of such analgesic or anesthetic medications on lactation will
depend of various factors, such as the age and size of the infant,
the ability of the infant to clear the quantity of medication he
or she is exposed to, and the stage of lactation. Pain, suering,
fear, and anxiety during labor can aect delivery and have a
negative eect on breastfeeding. ese issues may necessitate
pharmacologic treatment, but continuous support in labor and
nonpharmacologic management of pain may decrease the need
for medications and facilitate early skin- to- skin contact and
initiation of breastfeeding. Appropriately referenced guidelines
for analgesia and anesthesia use in lactating women are available
from the Academy of Breastfeeding Medicine.
200
Box 11.6
presents a few salient points from these guidelines.
Ongoing Breastfeeding Support
The duration of lactation will vary significantly by mother-
infant dyad. Support should be given to every mother and
family to maintain her milk supply, continue breastfeeding,
and meet her breastfeeding goals. This is especially
important when the mother and infant are separated for
longer periods of time, as might occur with maternal
hospitalization, surgery, or return to work or school, some of
the more common examples of separation. One of the more
effective practices that can assist mothers in maintaining
their milk supply involves creating a “breastfeeding- friendly
physician’s office.”
201
Overall, this involves providing an
office environment that is supportive of breastfeeding, which
1. Mammary alveolar epithelium represents a lipid barrier with
water- filled pores and is most permeable for drugs during
the colostral phase of milk secretion (first postpartum week).
2. Drug excretion into milk depends on the drug’s degree of
ionization, molecular weight, solubility in fat and water, and
relationship of pH of plasma (7.4) to pH of milk (7.0).
3. Drugs enter mammary cells basally in the nonionized, non-
protein- bound form by diffusion or active transport.
4. Water- soluble drugs of molecular weight less than 200 pass
through water- filled membranous pores.
5. Drugs leave mammary alveolar cells by diffusion or active
transport.
6. Drugs may enter milk via spaces between mammary alveolar
cells.
7. Most ingested drugs appear in milk; drug amounts in milk
usually do not exceed 1% of ingested dosage, and levels in
the milk are independent of milk volume.
8. Drugs are bound much less to milk proteins than to plasma
proteins.
9. The drug- metabolizing capacity of mammary epithelium is
not understood.
Data from Lawrence RM, Lawrence RA. Medications, herbal prepara-
tions, and natural products in breast milk. In: Lawrence RA, Law-
rence RM. Breastfeeding: A Guide for the Medical Profession. 9th
ed. St. Louis, MO: Elsevier; 2021;332.
BOX 11.4 THE PASSAGE OF DRUGS INTO BREAST
MILK
Gut
Lungs
Skin
IM
IV
Storage
Excretion
Absorption Bloodstream
(bound and unbound)
Distribution to tissues
Membrane
Live
rF
atBrain Kidney Breast Bone
Metabolism
Interaction
Excretion
Excretion
Storage
Storage
Figure 11.10 Distribution pathways for drugs. Distribution path-
ways vary with the drug and are relevant to advising the lactating mother
about breastfeeding when drugs have been prescribed. IM, Intramus-
cular; IV, intravenous. (Modified from Rivera- Calimlim L. Distribution
pathways for drugs, once absorbed during lactation. Clin Perinatol.
1976;14:51.)
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
182
can include such specific practices as providing appropriate
written and audiovisual breastfeeding materials in the office,
providing a private lactation room, offering prenatal and
postnatal visits with an emphasis on breastfeeding, ensuring
access to a lactation consultant, and providing positive
feedback for breastfeeding from the staff and clinicians. Of
note, some of the same factors that contribute to successful
breastfeeding will facilitate the maintenance of the milk
supply during times of separation: early skin- to- skin contact
and suckling within the first hour of life, emphasis on early
feeding cues and correct technique, encouraging “instinctual
breastfeeding behaviors” for both the mother and infant,
202
and encouraging exclusive and unrestricted (on- demand)
breastfeeding. Mothers should be instructed on how to
express their milk, appropriately store it for home use, and
maintain lactation. Not every woman needs a pump. Every
woman should be trained how to manually express her breasts
before she leaves the hospital because this will facilitate her
managing common problems such as a plugged duct or
engorgement. Patient information on hand expression of
milk and a demonstration video are available online.
203,204
Hand pumps, battery- powered pumps, and electrical pumps
are available. The most effective expression of milk will
vary from woman to woman, but the expression should be
comfortable and meet the infant’s and mother’s needs.
As part of the normal discussion of ongoing lactation, the
mother should be asked about potential periods of separation
from her infant and in particular about her plans for return to
work or school as an introduction to the topics of human milk
storage for home use and the maintenance of lactation. Box
11.7 provides recommendations concerning human milk home
storage and use.
205
ONGOING BREASTFEEDING SUPPORT WITH
SOCIAL CHALLENGES
Substantial inequities exist in breastfeeding initiation and
duration by age, race/ethnicity, and socioeconomic status.
5
Reviewing CDC breastfeeding data by race/ethnicity, Black
non- Hispanic mothers had the lowest breastfeeding rates in
2017 across all parameters (Table 11.6).
206
Breastfeeding rates
assessing initiation, duration, and exclusivity were lower among
women who were less educated, were younger, were unmarried,
had lower household incomes, and received WIC. Obstetricians
1. Various organizations have made recommendations regard-
ing breast imaging in pregnant and lactating women (Ameri-
can College of Radiology [ACR], National Comprehensive
Cancer Network [NCCN], Journal of Radiographics, Academy
of Breastfeeding Medicine [ABM], among others).
2. Screening mammography should be done based on the
woman’s individual risk and expected duration of lactation.
Ultrasound can be used as a supplemental screening test with
mammography. Magenetic resonance imaging (MRI) can be
used in high- risk women breastfeeding for short periods of
time, 3 months after cessation of lactation.
3. Diagnostic breast imaging during lactation is the same as for
nonlactating women. Breast imaging by screening or diagnostic
mammography, ultrasound, or MRI with gadolinium- based intra-
venous contrast does not require interruption of breastfeeding.
4. “Pump and dump” after imaging studies is not appropriate.
No breastfeeding interruption is necessary for noncontrast
radiographs, nonvascular administration of iodinated con-
trast, computed tomography (CT) with iodinated intravenous
contrast, MRI with gadolinium- based intravenous contrast,
nuclear medicine imaging (positron emission tomography
[PET] scan or bone scan).
5. The calculated dose of an infant’s systemic exposure due to
a CT scan with iodinated intravenous contrast given to the
mother is <0.01% of the mother’s intravenous dose (less than
1 % of the dose administered to the mother reaches the breast
milk and <1% of the contrast ingested by the infant in the milk
is absorbed by the gastrointestinal tract). Similarly, the calcu-
lated dose of an infant’s systemic exposure when a mother un-
dergoes an MRI with gadolinium- based intravenous contrast
is <0.0004% of the mother’s dose (less than 0.04% of the ma-
ternal dose is excreted in breast milk and <1% of the ingested
contrast is absorbed form the infant’s gastrointestinal tract).
6. Thyroid imaging with I- 131 necessitates cessation of breast-
feeding for this child due to high gamma energy, high beta
emission, and long half- life (8.04 days). Thyroid imaging with
I- 123 is accompanied by recommendations for cessation
of breastfeeding varying up to 3 weeks (half- life 13 hours).
Technetium- 99m pertechnetate with its shorter half- life (6
hours) may necessitate interruption of breastfeeding for 4
hours up to 12 to 24 hours depending on the dose and the
recommendation of the local nuclear medicine specialist.
a
7. Renal imaging utilizing Tc- 99m DTPA, Tc- 99m MAG3, Tc- 99m
DMSA, or Tc- 99m glucoheptonate usually do not require ces-
sation of breastfeeding,
b
although the International Atomic
Energy Administration (IAEA) recommends withholding
breastfeeding for 4 hours for the possibility of any exter-
nal radiation and free Tc- 99m pertechnetate in the imaging
product.
b
8. Cardiac imaging with Tc- 99m Sestamibi or Tc- 99m Tetrofos-
min usually do not require cessation of breastfeeding.
b
Mul-
tigated acquisition scan (MUGA) with Tc- 99m labeled RBCs
can be used to assess left ventricular ejection fraction. No
interruption of breastfeeding is necessary for in vitro labeling,
but a 6- to 12- hour interruption is recommended for in vivo
labeling of the RBCs.
a,b
An echocardiogram is an alternative
method of assessing ventricular ejection fraction.
9. VQ scan is used to evaluate for pulmonary embolism. No
breastfeeding interruption is needed for the ventilation
agents of the scan, although a 12- hour interruption is recom-
mended for the perfusion agent Tc- 99m macroaggregated
albumin.
a
10. Mothers exposed to an accidental, unexpected radiation ex-
posure should be assessed by the institutional radiology safe-
ty officer (RSO), and recommendations can be sought from
other expert sources (Infantrisk.com or MotherToBaby.org).
a
Expressed milk obtained during that period of interruption of breastfeeding can be stored, refrigerated, and given to the infant later after 10
physical half- lives or ∼60 hours, which will lead to the elimination of the radiation.
b
Although the International Atomic Energy Administration (IAEA) recommends withholding breastfeeding for 4 hours for the possibility of any
external radiation and free Tc- 99m pertechnetate in the imaging product.
Data from Mitchell KB, Fleming MM, Anderson PO, Giesbrandt JG, and Academy of Breastfeeding Medicine. ABM Clinical Protocol #31: Radiol-
ogy and Nuclear Medicine Studies in Lactating Women. Breastfeed Med. 2019;14(5):290–294. https://doi.org/10.1089/bfm.2019.29128.kbm.
Epub 2019 May 20. Accessed January 9, 2021; and American College of Radiology Committee on Drugs and Contrast Media. American College
of Radiology Manual on Contrast Media. 2020. https://www.acr.org/- /media/ACR/files/clinical- resources/contrast_media.pdf Updated 2020.
Accessed January 31, 2021.
BOX 11.5 USE OF RADIOLOGY AND NUCLEAR MEDICINE STUDIES DURING LACTATION
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 183
1. Mothers who have received general anesthesia with healthy
term or older infants can generally resume breastfeeding as
soon as they are awake, stable, and alert.
2. Infants at risk for apnea, hypotension, or hypotonia may ben-
efit from a brief interruption of breastfeeding (6–12 hours) af-
ter maternal anesthesia. In this situation, mothers can express
and store milk in small amounts to be used when the infant is
older, or it can be mixed with fresh milk containing no medica-
tions to dilute the medications present.
3. The most concerning class of medications used for anesthesia
and analgesia in breastfeeding mothers is opioids, as these
medications transfer into breast milk and may cause infant se-
dation or apnea. Judicious use of opioids for short periods is
likely to be safe for most breastfeeding mothers and infants.
4. Drugs used for induction, such as propofol, midazolam,
etomidate, or thiopental, enter the milk compartment only
minimally due to their extraordinarily brief plasma distribu-
tion, and hence their transport to milk is low to nil.
5. Little has been reported on the use of anesthetic gases in
breastfeeding mothers, but they also have brief plasma distri-
bution phases and milk levels are likely to be nil.
6. Neuromuscular blocking agents are safe for the breastfeeding
infant, as they have low lipid solubility and are largely distribut-
ed in the extracellular fluid volume. There is little or no data on
the pharmacokinetics of these drugs in breast milk, but based
on their physical characteristics and their poor oral availability,
they are considered safe for use in the breastfeeding mother.
7. For women undergoing postpartum tubal ligation, interrupt-
ing breastfeeding is not indicated because the volume of co-
lostrum an infant would receive is small.
8. Women who receive single doses of medication for sedation
and analgesia for short procedures can breastfeed as soon as
they are awake and stable.
9. Local anesthetics such as lidocaine, bupivacaine, and ropi-
vacaine can be safely used in breastfeeding mothers. These
and other local anesthetics are poorly absorbed orally and the
large, polarized molecules do not easily transfer into milk.
10. Regional anesthesia (spinal, epidural, or peripheral nerve
block) should be considered whenever possible for intraop-
erative anesthesia or postoperative analgesia. It reduces the
need for intraoperative medications and may also decrease
the amount of pain medication needed postoperatively. This
may leave the mother more awake and alert in the immediate
postoperative period, and she will therefore be more likely to
resume breastfeeding sooner.
11. Antiemetics are used commonly in the perioperative period,
and most of these medications are considered safe during
breastfeeding. Ondansetron, dexamethasone, and metoclo-
pramide may be preferred because of their lack of sedating
side effects. Prochlorperazine, promethazine, and scopol-
amine are likely safe, but may lead to maternal sedation. Pro-
methazine and scopolamine have been noted to affect milk
supply if given repeatedly.
Modified from Reece- Stremtan S, Campos M, Kokajko L, and the Academy of Breastfeeding Medicine. ABM Clinical Protocol #15: Analgesia and
Anesthesia for the Breastfeeding Mother, Revised 2017. Breastfeeding Medicine. 2017;12(9):1–7. https://doi.org/10.1089/bfm.2017.29054.srt.
BOX 11.6 ANESTHESIA FOR SURGERY IN BREASTFEEDING MOTHERS
PREPARATION FOR HUMAN MILK STORAGE
1. Women should wash their hands before expressing breast milk
(using soap and water or a waterless hand cleanser).
2. Milk expression can be done by hand or by a pump. A va-
riety of pumps are available, and selection can be based on
cost, availability, frequency and duration of expression, time
constraints, and comfort, etc. Appropriate cleaning of hands
and cleaning of pump parts (per manufacturer’s instructions)
is adequate to limit milk contamination during expression and
storage. The CDC has guidelines for pump cleaning available
on their website.
a
3. Only food- grade plastic containers should be used for human
milk storage. There are a variety of studies concerning storage
containers and their effects on fat, carbohydrate, protein IgA,
and white blood cell content of the milk after storage. Glass
and polypropylene containers are frequently used. The plastic
bags used for human milk storage should be sturdy, easily and
effectively sealed, and readily labeled (name of child and date
of milk expression). The bags should be stored in an area of
the freezer to minimize damage to the bag. Containers made
with bisphenol A or S should be avoided due to the potential
effect the containers have on the milk during storage. Human
milk should not be stored in hospital plastic specimen storage
containers used for other bodily fluids.
4. Containers for human milk storage do not have to be sterilized.
Washing in hot soapy water with careful rinsing and thoroughly
drying (by air or paper towels) is sufficient. If soap is not readily
available or water is limited, boiling them in water and cool-
ing prior to use may be the preferred method of preparation.
Chemical disinfection is not recommended.
5. There is no need to discard the first few drops of milk at the
beginning of milk expression.
6. The mother’s breasts and nipples do not need to be washed
prior to milk expression.
MILK STORAGE GUIDELINES
Location of
Storage Temperature
Maximum
Recommended Storage
Duration
Room
temperature
16–29°C
(60–85°F)
4 hours optimal
6–8 hours acceptable
under very clean
conditions
Refrigerator ∼4°C (39.2°F) 4 days optimal
5–8 days under very
clean conditions
Freezer <−4°C (24.8°F) 6 months optimal
12 months acceptable
USING STORED HUMAN MILK
1. Fresh milk is better than frozen milk; use the oldest milk first
from the refrigerator or freezer.
2. Avoid using containers that have leaked or been rewarmed
and recooled or refrozen.
3. Stored milk may have altered smell and taste due to enzymatic
breakdown. This breakdown of fats can aid in digestion and is not
harmful; however, some infants may refuse to drink it. Milk that
appears stringy, foul, or purulent should not be fed to the infant.
4. The infant may drink the milk cool, at room temperature, or
warmed, and infants may demonstrate a preference.
5. Defrost the stored milk in the refrigerator overnight, in run-
ning warm water, or by setting it in a container of warm water.
Do not microwave the milk.
6. Previously frozen human milk, thawed in the refrigerator for 24
hours, should be used within a few hours at room temperature.
7. Avoid refreezing thawed human milk, due to lack of safety
information on its reuse.
BOX 11.7 INFORMATION ON STORAGE OF HUMAN MILK FOR HOME USE WITH FULL- TERM INFANTS
Continued
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

PART 1 Scientific Basis of Perinatal Biology
184
need to understand and address structural determinants of
health such as transportation, access to lactation/postpartum
support, and paid family leave in order to eliminate breastfeeding
inequities. For example, in 2012, 23% of women living in the
US returned to work as early as 10 days postpartum,
207
making
mental wellness, maternal- infant bonding, and establishment of
breastfeeding extremely challenging in that short time frame. To
address the health care inequities associated with breastfeeding,
the obstetrician should understand the varied causes of health
disparities and recognize that the solutions must occur at
the patient level, practitioner level, health care system level,
institution level, and at the level of policy and programs.
208,209
Antiracism health care is paramount to demolish the negative
impact of racism, not race, on health care outcomes.
e social challenges in health care and the threats to
equitable, nondiscriminatory health care facing mothers and
families extend into the lesbian, gay, bisexual, transgender,
queer/questioning, plus (+) (LGBTQ+) community.
79,210
Individuals from this community have experienced stigma,
misunderstanding, and inadequate care due to prejudice and
lack of understanding of individual needs. Here also there are
tremendous opportunities to provide arming, respectful
health care. ese can begin with the respectful use of language
and terms, education of health care providers, providing
gender- neutral bathrooms, displaying inclusive signage in
the health care facility, and understanding the signicance of
gender- arming medical and surgical treatments on pregnancy
and breastfeeding/chestfeeding. (For example, chestfeeding
8. It is reasonable to discard milk leftover from a feeding within
1–2 hours (based on limited information). Storing milk in small
volume increments (15, 20, or 60 mL) may limit wasting or
discarding unfed amounts of milk.
9. Expressed human milk does not require special handling (e.g.,
universal precautions) as is recommended for other bodily flu-
ids (such as blood, urine). It can be stored in any refrigerator
with other stored foods, labeled and dated, although moth-
ers may prefer to store their milk in a personal container.
10. Uncontaminated human milk naturally contains nonpatho-
genic bacteria, which is important to establishing normal neo-
natal intestinal flora. There is no evidence that stored milk
from a mother with breast pain due to infection needs to be
discarded.
a
Centers for Disease Control and Prevention. How to Keep Your Breast Pump Kit Clean: The Essentials. https://www.cdc.gov/healthywater/
hygiene/healthychildcare/infantfeeding/breastpump.html Reviewed July 8, 2020. Accessed January 9, 2021.
Data from Eglash A, Simon L, and the Academy of Breastfeeding Medicine Protocol Committee. ABM Clinical Protocol #8: Human Milk Storage
Information for Home Use for Full- Term Infants. Revised 2017. Breastfeeding Med. 2017;12(7):390–395. https://doi.org/10.1089/bfm.2017.29047.
aje.
BOX 11.7 INFORMATION ON STORAGE OF HUMAN MILK FOR HOME USE WITH FULL- TERM INFANTS—cont’d
Rates of Any and Exclusive Breastfeeding by Sociodemographic Status Among Children Born in 2017
ANY BREASTFEEDING (BF) (%) EXCLUSIVE BREASTFEEDING (EBF) (%)
Ever BF BF at 6 months BF at 12 months
EBF through 3
months
EBF through 6
months
RACE/ETHNICITY
Hispanic 84.1 55.4 33.9 41.5 21.5
Non- Hispanic White 86.7 61.9 38.2 52.4 28.7
Non- Hispanic Black 73.7 47.8 26.1 38.7 21.2
Non- Hispanic Asian 90.0 73.5 50.0 47.7 26.8
Non- Hispanic Hawaiian/Pacific Islander 85.2 NA NA NA NA
Non- Hispanic American Indian/Alaska Native 80.7 NA NA NA NA
2 or more races 83.7 56.5 31.0 43.9 26.6
MATERNAL EDUCATION
Less than high school 73.6 49.0 28.9 30.8 17.1
High school graduate 75.6 44.1 25.7 41.7 21.5
Some college/ technical school 84.7 53.6 31.1 45.4 23.3
College graduate 93.3 74.0 46.6 57.2 32.8
AGE
Younger than age 20 74.0 NA NA NA 18.7
20–29 82.4 50.9 28.6 45.4 24.2
30 or older 85.2 62.7 39.2 47.8 26.5
MARITAL STATUS
Married 89.7 68.2 43.7 53.7 30.6
Unmarried 75.1 42.5 21.8 36.1 17.6
RECEIVING WIC
Yes 77.0 45.4 24.8 37.3 19.0
No, but eligible 82.1 61.3 43.4 53.8 31.1
Ineligible 92.1 71.4 44.8 55.7 31.3
From Centers for Disease Control and Prevention. Rates of Any and Exclusive Breastfeeding by Socio- demographics among Children Born in 2017
[updated 08/01/2019]. https://www.cdc.gov/breastfeeding/data/nis_data/rates- any- exclusive- bf- socio- dem- 2017.htm.
TABLE
11.6
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

11 The Breast and the Physiology of Lactation 185
describes the action of a masculine- identied trans person in
feeding their baby at the chest with or without previous surgery
to aect existing mammary tissue.) Options regarding fertility
(childbearing) and contraception should be explored with
both parents, as should expressing or pumping breast milk,
inducing lactation, sharing of infant feeding, colactation of an
infant by two or more parents, bonding of the infant with each
parent, use of donor milk and informal milk sharing, and the
plans for parenting and support for the parents/family at home
with or without breastfeeding. Some of these opportunities for
creating and expanding respectful health care during pregnancy
and lactation are reviewed by the Academy of Breastfeeding
Medicine in their clinical protocol on lactation care for
LGBTQ+ patients.
79
ere remains a tremendous amount of
work for each of us and all of us to do in collaboration with
our patients to combat prejudices and institutional racism
and create equitable, respectful lactation care for all mothers,
partners, and all families.
Key Points
•
Mother’s milk is the most appropriate feeding for the
infant in the rst 6 months of life. It is ideal to continue
mother’s milk, while adding weaning foods, for the next 6
months and as long as mother and baby wish.
•
ere are very few true contraindications to the use of the
mother’s own milk for her infant. Maternal use of drugs
of abuse should be limited if possible and breastfeeding
continued considering the individual medications and
the actual maternal doses. Specic maternal infections
can be a contraindication to breastfeeding: HIV; HTLV-
I; Ebola, Marburg, Lassa, and dengue viruses; and active
pulmonary tuberculosis. Most maternal infections can be
treated without interrupting breastfeeding. If the infant
has the severe form of galactosemia, that can be a contra-
indication to breastfeeding.
•
e obstetrician plays an important role in support of
breastfeeding, beginning with the rst prenatal visit,
through pregnancy, at delivery, during the hospital stay,
and as long as the mother requires assistance.
•
A good understanding of the anatomy and physiology of
the breast and the physiology of lactation is essential to
guiding and assisting lactating mothers to achieve their
breastfeeding goals.
•
Establishing a breastfeeding- friendly oce and hospital
environment is essential to breastfeeding success.
•
Obstetricians, pediatricians, and lactation professionals
should work together to establish a knowledgeable and
skilled environment for successful breastfeeding. ere are
numerous current resources of information, readily avail-
able to practitioners, to optimize the care of breastfeeding
mothers and infants.
SUGGESTED READINGS
Briggs GG, Freeman RK, Towers CV, Forinash AB. Drugs in Pregnancy and Lac-
tation. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2017.
Hale TW. Medications and Mothers’ Milk. 17th ed. Amarillo, TX: Hale; 2017.
Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession.
9th ed. St. Louis: Mosby; 2021.
Family Larsson- Rosenquist Foundation. In: Breastfeeding and Breast Milk: From
Biochemistry to Impact. Stuttgart, Germany: ieme; 2018.
A full reference list is available online at ExpertConsult.com.
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

185.e1
REFERENCES
1. Ip S, Chung M, Raman G, et al. Breastfeeding
and maternal and infant health outcomes in
developed countries. Evid Rep Technol Assess
(Full Rep). 2007:1–186.
2. Ip S, Chung M, Raman G, Trikalinos TA, Lau
J. A summary of the Agency for Healthcare
Research and Quality’s evidence report on
breastfeeding in developed countries. Breast-
feed Med. 2009;4(suppl 1):S17–30.
3. Victora CG, Bahl R, Barros AJ, et al. Breast-
feeding in the 21st century: epidemiol-
ogy, mechanisms, and lifelong effect. Lancet.
2016;387:475–490.
4. American College of Obstetrics and Gynecol-
ogy: Committee Opinion No. 756. Optimizing
support for breastfeeding as part of obstetric
practice. Obstet Gynecol. 2018;132:e187–e196.
5. American College of Obstetrics and Gynecolo-
gy. Barriers to breastfeeding: supporting initia-
tion and continuation of breastfeeding: ACOG
Committee Opinion, Number 821. Obstet Gy-
necol. 2021;137:e54–e62.
6. Section on Breastfeeding, American Academy
of Pediatrics: Breastfeeding and the use of hu-
man milk. Pediatrics. 2012;129:e827–e841.
http://doi.org/10.1542/peds.2011- 3552. origi-
nally published online February 27, 2012.
7. Kramer MS, Kakuma R. Optimal duration of
exclusive breastfeeding. Cochrane Database
Syst Rev. 2012. 2012;Cd003517.
8. World Health Organization: Breastfeeding. ;
2018. https://www.who.int/news- room/facts-
in- pictures/detail/breastfeeding. [Accessed
January 28, 2021].
9. Lawrence RA, Lawrence RM. Breastfeeding:
A Guide for the Medical Profession. 9th ed. St.
Louis: Mosby; 2021.
10. Dewey KG. Nutrition, growth, and comple-
mentary feeding of the breastfed infant. Pedi-
atr Clin North Am. 2001;48:87–104.
11. SanGiovanni JP, Berkey CS, Dwyer JT, Colditz
GA. Dietary essential fatty acids, long- chain
polyunsaturated fatty acids, and visual resolu-
tion acuity in healthy fullterm infants: a system-
atic review. Early Hum Dev. 2000;57:165–188.
12. Horwood LJ, Darlow BA, Mogridge N. Breast
milk feeding and cognitive ability at 7- 8 years.
Arch Dis Child Fetal Neonatal Ed. 2001;84:F23–
F27.
13. Schack- Nielsen L, Michaelsen KF. Advances
in our understanding of the biology of human
milk and its effects on the offspring. J Nutr.
2007;137:503s–510s.
14. Horta BL, Loret de Mola C, Victora CG.
Breastfeeding and intelligence: a system-
atic review and meta- analysis. Acta Paediatr.
2015;104:14–19.
15. Li R, Dee D, Li CM, Hoffman HJ, Grummer-
Strawn LM. Breastfeeding and risk of infections
at 6 years. Pediatrics. 2014;134(suppl 1):S13–S20.
16. Butte NF, Goldblum RM, Fehl LM, et al. Daily
ingestion of immunologic components in hu-
man milk during the first four months of life.
Acta Paediatr Scand. 1984;73:296–301.
17. Quan R, Barness LA. Do infants need nu-
cleotide supplemented formula for opti-
mal nutrition? J Pediatr Gastroenterol Nutr.
1990;11:429–434.
18. Srivastava MD, Srivastava A, Brouhard B,
Saneto R, Groh- Wargo S, Kubit J. Cytokines
in human milk. Res Commun Mol Pathol Phar-
macol. 1996;93:263–287.
19. Garofalo R. Cytokines in human milk. J Pedi-
atr. 2010;156:S36–40.
20. Bode L. Human milk oligosaccharides: ev-
ery baby needs a sugar mama. Glycobiology.
2012;22:1147–1162.
21. Hassiotou F, Hepworth AR, Metzger P, et al.
Maternal and infant infections stimulate a
rapid leukocyte response in breastmilk. Clin
Transl Immunology. 2013;2:e3.
22. von Kries R, Koletzko B, Sauerwald T, et al.
Breast feeding and obesity: cross sectional
study. BMJ. 1999;319:147–150.
23. Pickering LK, Granoff DM, Erickson JR, et al.
Modulation of the immune system by human
milk and infant formula containing nucleo-
tides. Pediatrics. 1998;101:242–249.
24. Martin RM, Gunnell D, Owen CG, Smith GD.
Breast- feeding and childhood cancer: a sys-
tematic review with metaanalysis. Int J Cancer.
2005;117:1020–1031.
25. Mayer- Davis EJ, Rifas- Shiman SL, Zhou L, Hu
FB, Colditz GA, Gillman MW. Breast- feeding
and risk for childhood obesity: does maternal
diabetes or obesity status matter? Diabetes
Care. 2006;29:2231–2237.
26. Owen CG, Martin RM, Whincup PH, Smith
GD, Cook DG. Does breastfeeding influence
risk of type 2 diabetes in later life? A quantita-
tive analysis of published evidence. Am J Clin
Nutr. 2006;84:1043–1054.
27. Scholtens S, Gehring U, Brunekreef B, et al.
Breastfeeding, weight gain in infancy, and over-
weight at seven years of age: the prevention and
incidence of asthma and mite allergy birth co-
hort study. Am J Epidemiol. 2007;165:919–926.
28. Mamun AA, O’Callaghan MJ, Williams GM,
Najman JM, Callaway L, McIntyre HD. Breast-
feeding is protective to diabetes risk in young
adults: a longitudinal study. Acta Diabetol.
2015;52:837–844.
29. Horta BL, Loret de Mola C, Victora CG. Long-
term consequences of breastfeeding on cho-
lesterol, obesity, systolic blood pressure and
type 2 diabetes: a systematic review and meta-
analysis. Acta Paediatr. 2015;104:30–37.
30. Lucas A, Morley R, Cole TJ, Lister G, Leeson-
Payne C. Breast milk and subsequent intelli-
gence quotient in children born preterm. Lan-
cet. 1992;339:261–264.
31. Neuringer M. Infant vision and retinal func-
tion in studies of dietary long- chain polyun-
saturated fatty acids: methods, results, and im-
plications. Am J Clin Nutr. 2000;71:256s–267s.
32. Jørgensen MH, Hernell O, Lund P, Hølmer G,
Michaelsen KF. Visual acuity and erythrocyte
docosahexaenoic acid status in breast- fed and
formula- fed term infants during the first four
months of life. Lipids. 1996;31:99–105.
33. SanGiovanni JP, Parra- Cabrera S, Colditz GA,
Berkey CS, Dwyer JT. Meta- analysis of dietary
essential fatty acids and long- chain polyun-
saturated fatty acids as they relate to visual
resolution acuity in healthy preterm infants.
Pediatrics. 2000;105:1292–1298.
34. Horwood LJ, Fergusson DM. Breastfeeding
and later cognitive and academic outcomes.
Pediatrics. 1998;101:E9.
35. Lucas A, Morley R, Cole TJ. Randomised trial
of early diet in preterm babies and later intel-
ligence quotient. BMJ. 1998;317:1481–1487.
36. Jacobson SW, Chiodo LM, Jacobson JL.
Breastfeeding effects on intelligence quotient
in 4- and 11- year- old children. Pediatrics.
1999;103:e71.
37. Horta BL. Breastfeeding: investing in the fu-
ture. Breastfeed Med. 2019;14:S11–S12.
38. Newton N, Newton M. Psychologic aspects of
lactation. N Engl J Med. 1967;277:1179–1188.
39. Krol KM, Grossmann T. Psychological effects
of breastfeeding on children and mothers.
Bundesgesundheitsblatt Gesundheitsforschung
Gesundheitsschutz. 2018;61:977–985.
40. Guzzardi MA, Granziera F, Sanguinetti E,
Ditaranto F, Muratori F, Iozzo P. Exclusive
Breastfeeding predicts higher hearing-lan-
guage development in girls of preschool age.
Nutrients. 2020;12:2320–2332. https://doi.
org/10.3390/nu12082320. www.mdpi.com/
journal/nutrients.
41. Groer MW, Davis MW. Cytokines, infections,
stress, and dysphoric moods in breastfeeders
and formula feeders. J Obstet Gynecol Neonatal
Nurs. 2006;35:599–607.
42. Saxton A, Fahy K, Rolfe M, Skinner V, Hastie
C. Does skin- to- skin contact and breast feed-
ing at birth affect the rate of primary postpar-
tum haemorrhage: results of a cohort study.
Midwifery. 2015;31:1110–1117.
43. Chowdhury R, Sinha B, Sankar MJ, et al.
Breastfeeding and maternal health outcomes:
a systematic review and meta- analysis. Acta
Paediatr. 2015;104:96–113.
44. Dias CC, Figueiredo B. Breastfeeding and de-
pression: a systematic review of the literature. J
Aect Disord. 2015;171:142–154.
45. Wouk K, Gottfredson NC, Tucker C, et al.
Positive emotions during infant feeding and
postpartum mental health. J Womens Health
(Larchmt). 2019;28:194–202.
46. Gunderson EP, Jacobs Jr DR, Chiang V, et al.
Duration of lactation and incidence of the
metabolic syndrome in women of reproduc-
tive age according to gestational diabetes
mellitus status: a 20- year prospective study
in CARDIA (Coronary Artery Risk Develop-
ment in Young Adults). Diabetes. 2010;59:
495–504.
47. Ram KT, Bobby P, Hailpern SM, et al. Dura-
tion of lactation is associated with lower preva-
lence of the metabolic syndrome in midlife—
SWAN, the study of women’s health across the
nation. Am J Obstet Gynecol. 2008;198. 268.
e261- 266.
48. Bartick MC, Schwarz EB, Green BD, et al.
Suboptimal breastfeeding in the United States:
maternal and pediatric health outcomes and
costs. Matern Child Nutr. 2017;13.
49. Feltner C, Weber R, Stuebe A, Grodensky C,
Orr C, Viswanathan M. Breastfeeding Pro-
grams and Policies, Breastfeeding Uptake, and
Maternal Health Outcomes in Developed Coun-
tries. Rockville (MD): Agency for Healthcare
Research and Quality (US); 2018. https://doi.
org/10.23970/AHRQEPCCER210. Report
No.: 18- EHC014- EF.
50. Mori T, Ishii S, Greendale GA, et al. Parity, lac-
tation, bone strength, and 16- year fracture risk
in adult women: findings from the Study of
Women’s Health Across the Nation (SWAN).
Bone. 2015;73:160–166.
51. Whittemore AS. Characteristics relat-
ing to ovarian cancer risk: implications for
prevention and detection. Gynecol Oncol.
1994;55:S15–S19.
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

References
185.e2
52. John EM, Whittemore AS, Harris R, Itnyre
J. Characteristics relating to ovarian cancer
risk: collaborative analysis of seven U.S. case-
control studies. Epithelial ovarian cancer in
black women. Collaborative Ovarian Cancer
Group. J Natl Cancer Inst. 1993;85:142–147.
53. Rosenblatt KA, Thomas DB. Lactation and the
risk of epithelial ovarian cancer. The WHO Col-
laborative Study of Neoplasia and Steroid Con-
traceptives. Int J Epidemiol. 1993;22:192–197.
54. Newcomb PA, Storer BE, Longnecker MP, et al.
Lactation and a reduced risk of premenopausal
breast cancer. N Engl J Med. 1994;330:81–87.
55. Kim Y, Choi JY, Lee KM, et al. Dose- dependent
protective effect of breast- feeding against
breast cancer among ever- lactated women in
Korea. Eur J Cancer Prev. 2007;16:124–129.
56. Aune D, Norat T, Romundstad P, Vatten LJ.
Breastfeeding and the maternal risk of type
2 diabetes: a systematic review and dose-
response meta- analysis of cohort studies. Nutr
Metab Cardiovasc Dis. 2014;24:107–115.
57. Jäger S, Jacobs S, Kröger J, et al. Breast- feeding
and maternal risk of type 2 diabetes: a prospec-
tive study and meta- analysis. Diabetologia.
2014;57:1355–1365.
58. Gunderson EP, Hurston SR, Ning X, et al.
Lactation and progression to type 2 diabetes
mellitus after gestational diabetes mellitus:
a prospective cohort study. Ann Intern Med.
2015;163:889–898.
59. Jordan SJ, Na R, Johnatty SE, et al. Breastfeed-
ing and endometrial cancer risk: an analysis
from the Epidemiology of Endometrial Cancer
Consortium. Obstet Gynecol. 2017;129:1059–
1067.
60. Yi X, Zhu J, Zhu X, Liu GJ, Wu L. Breastfeed-
ing and thyroid cancer risk in women: a dose-
response meta- analysis of epidemiological
studies. Clin Nutr. 2016;35:1039–1046.
61. Karlsson JO, Garnett T, Rollins NC, Röös E.
The carbon footprint of breastmilk substitutes
in comparison with breastfeeding. J Clean
Prod. 2019;222:436–445.
62. Joffe N, Webster F, Shenker N. Support for
breastfeeding is an environmental impera-
tive. BMJ (Clinical research ed.). 2019;367:
l5646.
63. Labbok M, Krasovec K. Toward consistency
in breastfeeding definitions. Stud Fam Plann.
1990;21:226–230.
64. World Health Organization: E- library of evi-
dence for nutrition actions (eLENA). Exclu-
sive breastfeeding. http://www.who.int/elena/
titles/exclusive_breastfeeding/en/. [Accessed
January 23, 2017].
65. Centers for Disease Control and Prevention.
Contraindications to Breastfeeding or Feeding
Expressed Breast Milk to Infants; 2019. https://
www.cdc.gov/breastfeeding/breastfeeding-
special- circumstances/contraindications- to-
breastfeeding.html. [Accessed December 14,
2019].
66. Ryan SA, Ammerman SD, O’Connor ME.
Marijuana use during pregnancy and breast-
feeding: implications for neonatal and child-
hood outcomes. Pediatrics. 2018;142.
67. Reece- Stremtan S, Marinelli KA. ABM clinical
protocol #21: guidelines for breastfeeding and
substance use or substance use disorder, re-
vised 2015. Breastfeed Med. 2015;10:135–141.
68. D’Apolito K. Breastfeeding and substance
abuse. Clin Obstet Gynecol. 2013;56:202–211.
69. US National Library of Medicine: Toxnet:
Toxicology Data Network. LactMed: drugs
and lactation database. https://toxnet.nlm.nih.
gov/newtoxnet/lactmed.htm. [Accessed Feb-
ruary 1, 2021].
70. World Health Organization. Guidelines for
the identication and management of sub-
stance use and substance use disorders in preg-
nancy; 2014. file:///C:/Users/Rober/Down-
loads/9789241548731_eng.pdf. [Accessed
January 29, 2021].
71. Office of Women’s Health. U.S. Department
of Health and Human Services. Breastfeeding.
https://www.womenshealth.gov/breastfeeding
72. Lockwood CRL, Blackmon L, et al. Guidelines
for Perinatal Care. 6th ed. Washington DC:
American College of Obstetricians and Gy-
necologists, American Academy of Pediatrics;
2007.
73. Neville MC, Morton J, Umemura S, Lactogen-
esis. The transition from pregnancy to lacta-
tion. Pediatr Clin North Am. 2001;48:35–52.
74. American College of Obstetricians and Gy-
necologists. Breastfeeding challenges: ACOG
Committee Opinion Summary, Number 820.
Obstet & Gynecol. 2021;137(2):e42–e53.
75. UNICEF/ WHO. e ten steps to successful
breastfeeding. Revised BFHI Guidelines; 2018.
https://www.who.int/activities/promoting-
baby- friendly- hospitals/ten- steps- to- successful-
breastfeeding. [Accessed January 3, 2021].
76. Rosen- Carole C, Hartman S. ABM Clinical
Protocol #19: Breastfeeding promotion in the
prenatal setting, revision 2015. Breastfeed Med.
2015;10:451–457.
77. Community Health Training Institute: Breast-
feeding promotion in the prenatal setting.
https://hriainstitute.org/breastfeedingcme/
cme- 2/section/breastfeeding- promotion- in- the-
prenatal- setting. [Accessed February 1, 2021].
78. Lazarov M, Evans A. Breastfeeding: encour-
aging the best for low- income women. Zero to
ree; 2000.
79. Ferri RL, Rosen- Carole CB, Jackson J,
Carreno- Rijo E, Greenberg KB. The Acad-
emy of Breastfeeding Medicine: ABM Clinical
Protocol #33: lactation care for lesbian, gay,
bisexual, transgender, queer, questioning, plus
patients. Breastfeed Med. 2020;15:284–293.
80. Duggan A, Street Jr RL. Interpersonal commu-
nication in health and illness. Health Behavior:
eory, Research, and Practice. 5th ed. Hobo-
ken, NJ, US: Jossey- Bass/Wiley; 2015:243–267.
81. Stuebe A. Population Health and Informed
Feeding Decisions. In: Lawrence RA, Lawrence
RM, eds. Breastfeeding a Guide for the Medical
Profession. St. Louis, MO: Elsevier; 2021.
82. American College of Obstetricians and Gyne-
cologists. Breastfeeding coding for obstetricians-
gynecologists; 2016. https://www.acog.org/
About- ACOG/ACOG- Departments/Toolkits-
for- Health- Care- Providers/Breastfeeding-
Toolkit/Breastfeeding- Coding. [Accessed Feb-
ruary 1, 2021].
83. Dewey KG. Maternal and fetal stress are asso-
ciated with impaired lactogenesis in humans. J
Nutr. 2001;131:3012s–3015s.
84. Infant Risk Center at Texas Tech University
Health Center: Infant Risk Call Center. Call
Center: 1 (806) 352- 2519. Available at: https://
www.infantrisk.com/
85. Organization of Teratology Information Spe-
cialists (OTIS): Mother to Baby Call Center.
Call Center: 1 (866) 626- 6847. Available at:
https://mothertobaby.org/
86. Bland K, Romrell L. Congenital and acquired
disturbances of breast development and
growth. In: Bland KI, Copeland III EM, eds.
e Breast: Comprehensive Management of
Benign and Malignant Diseases. Philadelphia:
WB Saunders; 1991.
87. Neville MC. Mammary gland biology and lac-
tation: a short course. International Society for
Research on Human Milk and Lactation annual
meeting; 1997. Plymouth, MA.
88. Osbourn MP. Breast development and anato-
my. In: Harris JR, Lippman ME, Morrow M,
Hellman S, eds. Diseases of the Breast. Phila-
delphia: Lippincott- Raven; 1996.
89. Ramsay DT, Kent JC, Hartmann RA, Hart-
mann PE. Anatomy of the lactating human
breast redefined with ultrasound imaging. J
Anat. 2005;206:525–534.
90. Neville MC. Anatomy and physiology of lacta-
tion. Pediatr Clin North Am. 2001;48:13–34.
91. Hartmann PE, Cregan MD, Ramsay DT, Sim-
mer K, Kent JC. Physiology of lactation in
preterm mothers: initiation and maintenance.
Pediatr Ann. 2003;32:351–355.
92. Kuhn NJ. Lactogenesis: The search for trigger
mechanisms in different species. In: Peaker M,
ed. Comparative Aspects of Lactation. London:
Academic Press; 1977.
93. Chen DC, Nommsen- Rivers L, Dewey KG,
Lönnerdal B. Stress during labor and delivery
and early lactation performance. Am J Clin
Nutr. 1998;68:335–344.
94. Morton JA. The clinical usefulness of breast
milk sodium in the assessment of lactogenesis.
Pediatrics. 1994;93:802–806.
95. Neville MC, Keller R, Seacat J, et al. Studies
in human lactation: milk volumes in lactating
women during the onset of lactation and full
lactation. Am J Clin Nutr. 1988;48:1375–1386.
96. Neville MC, Allen JC, Archer PC, et al. Studies
in human lactation: milk volume and nutrient
composition during weaning and lactogenesis.
Am J Clin Nutr. 1991;54:81–92.
97. Kulski JK, Hartmann PE, Martin JD, Smith
M. Effects of bromocriptine mesylate on the
composition of the mammary secretion in
non- breast- feeding women. Obstet Gynecol.
1978;52:38–42.
98. Aperia A, Broberger O, Herin P, Zetterström
R. Salt content in human breast milk during
the three first weeks after delivery. Acta Paedi-
atr Scand. 1979;68:441–442.
99. Neville MC. Lactogenesis in women: a cascade
of events revealed by milk composition. In:
Jensen RD, ed. e Composition of Milks. San
Diego: Academic Press; 1995.
100. Kuhn NJ. The biochemistry of lactogenesis.
In: Mepham TB, ed. Biochemistry of Lactation.
Amsterdam: Elsevier; 1983.
101. Neubauer SH, Ferris AM, Chase CG, et al.
Delayed lactogenesis in women with insulin-
dependent diabetes mellitus. Am J Clin Nutr.
1993;58:54–60.
102. Neifert MR, McDonough SL, Neville MC. Fail-
ure of lactogenesis associated with placental
retention. Am J Obstet Gynecol. 1981;140:477–
478.
103. Sözmen M. Effects of early suckling of
cesarean- born babies on lactation. Biol Neo-
nate. 1992;62:67–68.
104. Chapman DJ. Risk factors for delayed lacto-
genesis among women with gestational diabe-
tes mellitus. J Hum Lact. 2014;30:134–135.
105. De Bortoli J, Amir LH. Is onset of lactation de-
layed in women with diabetes in pregnancy? A
systematic review. Diabet Med. 2016;33:17–24.
106. Matias SL, Dewey KG, Quesenberry Jr CP,
Gunderson EP. Maternal prepregnancy obe-
sity and insulin treatment during pregnancy
are independently associated with delayed
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

References 185.e3
lactogenesis in women with recent gestational
diabetes mellitus. Am J Clin Nutr. 2014;99:115–
121.
107. Jackson EB. Pediatric and psychiatric aspects
of the Yale room- in project. Conn State Med J.
1950;14.
108. Dimitraki M, Tsikouras P, Manav B, et al.
Evaluation of the effect of natural and emo-
tional stress of labor on lactation and breast-
feeding. Arch Gynecol Obstet. 2016;293:317–
328.
109. Brownell E, Howard CR, Lawrence RA, Dozier
AM. Delayed onset lactogenesis II predicts the
cessation of any or exclusive breastfeeding. J
Pediatr. 2012;161:608–614.
110. Ohyama M, Watabe H, Hayasaka Y. Manual
expression and electric breast pumping in the
first 48 h after delivery. Pediatr Int. 2010;52:39–
43.
111. Peterson WE, Ludwick TM. Humoral nature
of the factor causing let down of milk. Federa-
tion Proc. 1942;1:66–67.
112. Gardner H, Kent JC, Hartmann PE, Ged-
des DT. Asynchronous milk ejection in hu-
man lactating breast: case series. J Hum Lact.
2015;31:254–259.
113. Gardner H, Kent JC, Lai CT, et al. Milk ejec-
tion patterns: an intra- individual comparison
of breastfeeding and pumping. BMC Pregnan-
cy Childbirth. 2015;15:156.
114. Prime DK, Geddes DT, Hepworth AR, Tren-
gove NJ, Hartmann PE. Comparison of the
patterns of milk ejection during repeated
breast expression sessions in women. Breast-
feed Med. 2011;6:183–190.
115. Pedersen CA, Caldwell JD, Walker C, Ayers G,
Mason GA. Oxytocin activates the postpartum
onset of rat maternal behavior in the ventral
tegmental and medial preoptic areas. Behav
Neurosci. 1994;108:1163–1171.
116. Jackson EB, Olmsted RW, et al. A hospital
rooming- in unit for four newborn infants and
their mothers; descriptive account of back-
ground, development, and procedures with
a few preliminary observations. Pediatrics.
1948;1:28–43.
117. Barnes Jr GR, Lethin Jr AN, Jackson EB, Shea
N. Management of breast feeding. J Am Med
Assoc. 1953;151:192–199.
118. Berens PD. Prenatal, intrapartum, and post-
partum support of the lactating mother. Pedi-
atr Clin North Am. 2001;48:365–375.
119. Righard L, Alade MO. Effect of delivery room
routines on success of first breast- feed. Lancet.
1990;336:1105–1107.
120. e Mother and Child Health and Education
Trust: Initiation of Breastfeeding by Breast
Crawl. Breast Crawl; 2016. Video at. http://
breastcrawl.org/.
121. Holmes AV, McLeod AY, Bunik M. ABM
Clinical Protocol #5: Peripartum breastfeed-
ing management for the healthy mother and
infant at term, revision 2013. Breastfeed Med.
2013;8:469–473.
122. Oce on Women’s Health in the U.S. Depart-
ment of Health and Human Services: Get-
ting a Good Latch. ; 2018. https://www.
womenshealth.gov/breastfeeding/learning-
breastfeed/getting- good- latch. [Accessed Jan-
uary 30, 2021].
123. Ramsay DT, Mitoulas LR, Kent JC, Larsson M,
Hartmann PE. The use of ultrasound to char-
acterize milk ejection in women using an elec-
tric breast pump. J Hum Lact. 2005;21:421–
428.
124. Fredeen RC. Cup feeding of newborn infants.
Pediatrics. 1948;2:544–548.
125. Malhotra N, Vishwambaran L, Sundaram KR,
Narayanan I. A controlled trial of alternative
methods of oral feeding in neonates. Early
Hum Dev. 1999;54:29–38.
126. Collins CT, Makrides M, Gillis J, McPhee AJ.
Avoidance of bottles during the establishment
of breast feeds in preterm infants. Cochrane
Database Syst Rev. 2008. Cd005252.
127. Berens P, Eglash A, Malloy M, Steube AM.
ABM Clinical Protocol #26: persistent
pain with breastfeeding. Breastfeed Med.
2016;11:46–53.
128. Geddes D, Sakalidis V. Breastfeeding: how
do they do it? Infant sucking, swallowing and
breathing. Infant. 2015;11:146–150.
129. Flaherman VJ, Schaefer EW, Kuzniewicz MW,
Li SX, Walsh EM, Paul IM. Early weight loss
nomograms for exclusively breastfed new-
borns. Pediatrics. 2015;135:e16–e23.
130. Penn State Health. NEWT Newborn Weight
Tool; 2020. https://www.newbornweight.org.
[Accessed January 5, 2021].
131. Phillips RM, Goldstein M, Hougland K, et al.
Multidisciplinary guidelines for the care of
late preterm infants. J Perinatol. 2013;33(suppl
2):S5–S22.
132. Hand I, Noble L. Premature infants and
breastfeeding. In: Lawrence RA, Lawrence
RM, eds. Breastfeeding a Guide for the Medical
Profession. St. Louis, MO: Elsevier; 2021.
133. Humenick SS, Hill PD, Anderson MA. Breast
engorgement: patterns and selected outcomes.
J Hum Lact. 1994;10:87–93.
134. Witt AM, Bolman M, Kredit S, Vanic A.
Therapeutic breast massage in lactation for the
management of engorgement, plugged ducts,
and mastitis. J Hum Lact. 2016;32:123–131.
135. Berens PD. Breast pain: engorgement, nip-
ple pain, and mastitis. Clin Obstet Gynecol.
2015;58:902–914.
136. Berens P, Brodribb W. ABM Clinical Protocol
#20: engorgement, revised 2016. Breastfeed
Med. 2016;11:159–163.
137. Mangesi L, Zakarija- Grkovic I. Treatments for
breast engorgement during lactation. Cochrane
Database Syst Rev. 2016. 2016:Cd006946.
138. Dennis CL, Jackson K, Watson J. Interventions
for treating painful nipples among breastfeed-
ing women. Cochrane Database Syst Rev. 2014.
Cd007366.
139. Ureño TL, Buchheit TL, Hopkinson SG, Berry-
Cabán CS. Dysphoric milk ejection reflex: a
case series. Breastfeed Med. 2018;13:85–88.
140. Ureño TL, Berry- Cabán CS, Adams A, Buch-
heit TL, Hopkinson SG. Dysphoric milk ejec-
tion reflex: a descriptive study. Breastfeed Med.
2019;14:666–673.
141. Galipeau R, Dumas L, Lepage M. Perception
of not having enough milk and actual milk
production of first-time breastfeeding moth-
ers: is there a difference? Breastfeed Med.
2017;12:210–217.
142. Schanler RJ, Dooley S, Gartner LM, et al., eds.
Breastfeeding Handbook for Physicians. Wash-
ington DC: American College of Obstetricians
and Gynecologists, American Academy of Pe-
diatrics; 2006.
143. Kellams A, Harrel C, Omage S, Gregory C,
Rosen- Carole C. ABM Clinical Protocol #3:
supplementary feedings in the healthy term
breastfed neonate, revised 2017. Breastfeed
Med. 2017;12:188–198.
144. Brodribb W. ABM Clinical Protocol #9: Use of
galactogogues in initiating or augmenting ma-
ternal milk production, second revision 2018.
Breastfeed Med. 2018;13:307–314.
145. Grzeskowiak LE, Wlodek ME, Geddes DT.
What evidence do we have for pharmaceuti-
cal galactagogues in the treatment of lactation
insufficiency?—a narrative review. Nutrients.
2019;11.
146. Anderson PO, Valdés V. A critical review of
pharmaceutical galactagogues. Breastfeed Med.
2007;2:229–242.
147. Mortel M, Mehta SD. Systematic review of the
efficacy of herbal galactogogues. J Hum Lact.
2013;29:154–162.
148. Zapantis A, Steinberg JG, Schilit L. Use of
herbals as galactagogues. J Pharm Pract.
2012;25:222–231.
149. Committee on Nutrition; Section on Breast-
feeding; Committee on Fetus and Newborn:
Donor human milk for the high-risk infant:
preparation, safety, and usage options in the
United States. Pediatrics. 2017;139(1). https://
doi.org/10.1542/peds.2016- 3440. e20163440.
150. Meier P, Patel A, Esquerra- Zwiers A. Donor
human milk update: evidence, mechanisms,
and priorities for research and practice. J Pedi-
atr. 2017;180:15–21.
151. Schanler RJ. The use of human milk for
premature infants. Pediatr Clin North Am.
2001;48:207–219.
152. Maastrup R, Hansen BM, Kronborg H, et al.
Factors associated with exclusive breastfeeding
of preterm infants. Results from a prospective
national cohort study. PLoS One. 2014;9(2).
e89077.
153. Maastrup R, Hansen BM, Kronborg H, et al.
Breastfeeding progression in preterm infants is
influenced by factors in infants, mothers and
clinical practice: the results of a national co-
hort study with high breastfeeding initi ation
rates. PLoS One. 2014;9(9). e108208.
154. Meier PP, Patel AL, Bigger HR, Rossman B,
Engstrom JL. Supporting breastfeeding in the
neonatal intensive care unit: Rush Mother’s
Milk Club as a case study of evidence- based
care. Pediatr Clin North Am. 2013;60:209–226.
155. Wilson E, Christensson K, Brandt L, Altman
M, Bonamy AK. Early provision of mother’s
own milk and other predictors of successful
breast milk feeding after very preterm birth:
a regional observational study. J Hum Lact.
2015;31:393–400.
156. Chan GM, Lee ML, Rechtman DJ. Effects of
a human milk- derived human milk fortifier
on the antibacterial actions of human milk.
Breastfeed Med. 2007;2:205–208.
157. Gromada KK, Spangler AK. Breastfeeding
twins and higher- order multiples. J Obstet Gy-
necol Neonatal Nurs. 1998;27:441–449.
158. Berlin CM. “Exclusive” breastfeeding of qua-
druplets. Breastfeed Med. 2007;2:125–126.
159. Ahrens KA, Hutcheon JA, Ananth CV, et al.
Report of the Office of Population Affairs’
expert work group meeting on short birth
spacing and adverse pregnancy outcomes:
Methodological quality of existing studies and
future directions for research. Paediatr Perinat
Epidemiol. 2019;33(1):O5–O14. https://doi.
org/10.1111/ppe.12504. [Epub 2018 Oct 9].
160. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S.
medical eligibility criteria for contraceptive use.
MMWR Recomm Rep. 2016;65(No. RR- 3):1–104.
https://doi.org/10.15585/mmwr.rr6503a1. 2016.
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

References
185.e4
161. Department of Reproductive Health and Re-
search, World Health Organization. Medical
Eligibility Criteria for Contraceptive Use. 5th edi-
tion. Geneva, Switzerland: WHO Press, World
Health Organization; 2015. https://www.who.
int/publications/i/item/9789241549158. 2015.
162. Phillips SJ, Tepper NK, Kapp N, Nanda K,
Temmerman M, Curtis KM. Progestogen-
only contraceptive use among breastfeeding
women: a systematic review. Contraception.
2016;94:226–252.
163. Berens P, Labbok M. ABM Clinical Protocol
#13: contraception during breastfeeding, re-
vised 2015. Breastfeed Med. 2015;10:3–12.
164. American College of Obstetricians and Gy-
necologists: ACOG Practice Bulletin No. 206.
Use of hormonal contraception in women
with coexisting medical conditions. Obstet Gy-
necol. 2019;133:e128–e150.
165. American College of Obstetricians and Gynecol-
ogists: Postpartum Birth Control. https://www.
acog.org/womens- health/faqs/postpartum-
birth- control. [Accessed January 30, 2021].
166. American Academy of Pediatrics. Diphtheria.
In: Kimberlin DW, Brady MT, Jackson MA,
Long SS, eds. Red Book 2018: Report of the
Committee on Infectious Diseases. Elk Grove
Village, IL: American Academy of Pediatrics;
2018:319–323.
167. Snider Jr DE, Powell KE. Should women tak-
ing antituberculosis drugs breast- feed? Arch
Intern Med. 1984;144:589–590.
168. Centers for Disease Control and Prevention.
Brucellosis Reference Guide: Exposures, Testing,
and Prevention; 2017. https://www.cdc.gov/
brucellosis/pdf/brucellosi- reference- guide.
pdf. [Accessed January 30, 2021].
169. Centers for Disease Control and Prevention.
Recommendations for breastfeeding/infant feed-
ing in the context of Ebola; 2017. http://files.en-
nonline.net/attachments/2177/CDC_Ebola-
and- Breastfeeding- Guidance_Final_Cleared.
pdf. [Accessed January 31, 2017].
170. World Health Organization. Guidelines for
the management of pregnant and breastfeeding
women in the context of Ebola virus disease;
2020. https://apps.who.int/iris/bitstream/ha
ndle/10665/330851/9789240001381- eng.pdf.
[Accessed January 30, 2021].
171. Israel- Ballard K, Chantry C, Dewey K, et al.
Viral, nutritional, and bacterial safety of flash-
heated and pretoria- pasteurized breast milk to
prevent mother- to- child transmission of HIV
in resource- poor countries: a pilot study. J Ac-
quir Immune Dec Syndr. 2005;40:175–181.
172. Barthel A, Gourinat AC, Cazorla C, Joubert C,
Dupont- Rouzeyrol M, Descloux E. Breast milk
as a possible route of vertical transmission of
dengue virus? Clin Infect Dis. 2013;57:415–417.
173. Arragain L, Dupont- Rouzeyrol M, O’Connor
O, et al. Vertical transmission of Dengue virus
in the peripartum period and viral kinetics in
newborns and breast milk: new data. J Pediat-
ric Infect Dis Soc. 2017;6:324–331.
174. Centers for Disease Control and Prevention. Pos-
sible West Nile virus transmission to an infant
through breast- feeding—Michigan. MMWR
Morb Mortal Wkly Rep. 2002;51:877–878. 2002.
175. Hinckley AF, O’Leary DR, Hayes EB. Trans-
mission of West Nile virus through hu-
man breast milk seems to be rare. Pediatrics.
2007;119:e666–e671.
176. Amir LH, the Academy of Breastfeeding Medi-
cine. ABM clinical protocol #4: Mastitis, revised
March 2014. Breastfeed Med. 2014;9:239–243.
177. Schleiss MR. Breast milk-acquired cytomega-
lovirus in premature infants: uncertain con-
sequences and unsolved biological questions.
JAMA Pediatr. 2020;174:121–123.
178. Weimer KED, Kelly MS, Permar SR, Clark RH,
Greenberg RG. Association of adverse hear-
ing, growth, and discharge age outcomes with
postnatal cytomegalovirus infection in infants
with very low birth weight. JAMA Pediatr.
2020;174:133–140.
179. Bardanzellu F, Fanos V, Reali A. Human breast
milk-acquired cytomegalovirus infection: cer-
tainties, doubts and perspectives. Curr Pediatr
Rev. 2019;15:30–41.
180. Kurath S, Halwachs- Baumann G, Müller W,
Resch B. Transmission of cytomegalovirus
via breast milk to the prematurely born in-
fant: a systematic review. Clin Microbiol Infect.
2010;16:1172–1178.
181. American Academy of Pediatrics. Human
milk. In: Kimberlin DW, Brady MT, Jackson
MA, Long SS, eds. Red Book: 2018 Report of the
Committee on Infectious Diseases. Itasca, IL:
American Academy of Pediatrics; 2018:113–
121.
182. Bhola K, McGuire W. Does avoidance of
breast feeding reduce mother- to- infant trans-
mission of hepatitis C virus infection? Archives
of Disease in Childhood. 2007;92:365–366.
183. Centers for Disease Control and Prevention,
Division of Hepatitis. Hepatitis C FAQs for the
public; 2017. https://www.cdc.gov/hepatitis/
hcv/cfaq.htm. [Accessed January 31, 2017].
184. Ruiz- Extremera A, Salmerón J, Torres C, et al.
Follow- up of transmission of hepatitis C to
babies of human immunodeficiency virus-
negative women: the role of breast- feeding in
transmission. Pediatr Infect Dis J. 2000;19:511–
516.
185. Pfaender S, Heyden J, Friesland M, et al. Inacti-
vation of hepatitis C virus infectivity by human
breast milk. J Infect Dis. 2013;208:1943–1952.
186. Dunn DT, Newell ML, Ades AE, Peckham CS.
Risk of human immunodeficiency virus type
1 transmission through breastfeeding. Lancet.
1992;340:585–588.
187. Chadwick EG, Ezeanolue EE, Committee on
Pediatrics AIDS. Evaluation and management
of the infant exposed to HIV in the United
States. Pediatrics. 2020;146(5). e2020029058.
188. World Health Organization. Rapid advice: use
of antiretroviral drugs for treating pregnant wom-
en and preventing HIV infection in infants; 2010.
http://www.who.int/hiv/pub/mtct/advice/en/
index.html. [Accessed January 30, 2017].
189. White AB, Mirjahangir JF, Horvath H, An-
glemyer A, Read JS. Antiretroviral interven-
tions for preventing breast milk transmission
of HIV. Cochrane Database Syst Rev. 2014.
Cd011323.
190. Ekpini ER, Wiktor SZ, Satten GA, et al. Late
postnatal mother- to- child transmission of
HIV- 1 in Abidjan, Côte d’Ivoire. Lancet.
1997;349:1054–1059.
191. Hino S. Milk- borne transmission of HTLV-
I as a major route in the endemic cycle. Acta
Paediatr Jpn. 1989;31:428–435.
192. Hino S, Katamine S, Kawase K, et al. Inter-
vention of maternal transmission of HTLV- 1
in Nagasaki, Japan. Leukemia. 1994;8(suppl
1):S68–S70.
193. Takezaki T, Tajima K, Ito M, et al. Short- term
breast- feeding may reduce the risk of vertical
transmission of HTLV- I. The Tsushima ATL
Study Group. Leukemia. 1997;11(suppl 3):60–62.
194. Mitchell KB, Johnson HM, Eglash A. ABM
Clinical Protocol #30: breast masses, breast
complaints, and diagnostic breast imag-
ing in the lactating woman. Breastfeed Med.
2019;14:208–214.
195. Mitchell KB, Johnson HM. Breast conditions
in the breastfeeding mother. In: Lawrence RA,
Lawrence RM, eds. Breastfeeding: A Guide for
the Medical Profession. St. Louis, MO: Elsevier;
2021.
196. American Academy of Pediatrics, Commit-
tee on Drugs. Transfer of drugs and other
chemicals into human milk. Pediatrics.
2001;108:776–789.
197. Briggs GG, Freeman RK, Towers CV, Forinash
AB. Drugs in Pregnancy and Lactation. 12th
edition. Philadelphia, PA: Wolters Kluwer/
Lippincott, Williams & Wilkins; 2021.
198. Hale TW. Hale’s Medications & Mothers’ Milk
2021: A Manual of Lactational Pharmacology.
New York, NY: Springer Publishing Co; 2021.
199. American College of Radiology: Manual
on contrast media v10.22016: https://www.
acr.org/Quality- Safety/Resources/Contrast-
Manual. [Accessed January 31, 2017].
200. Reece- Stremtan S, Campos M, Kokajko L, The
Academy of Breastfeeding Medicine. ABM
Clinical Protocol #15: analgesia and anesthe-
sia for the breastfeeding mother, revised 2017.
Breastfeeding Med. 2017;12(9):1–7. https://doi.
org/10.1089/bfm.2017.29054.srt.
201. Grawey AE, Marinelli KA, Holmes AV. ABM
Clinical Protocol #14: Breastfeeding- friendly
physician’s office: optimizing care for infants
and children, revised 2013. Breastfeed Med.
2013;8:237–242.
202. Colson SD, Meek JH, Hawdon JM. Optimal
positions for the release of primitive neonatal
reflexes stimulating breastfeeding. Early Hum
Dev. 2008;84:441–449.
203. Newborn Nursery at Lucille Packard Children’s
Hospital, Stanford Medicine: Hand expres-
sion of Breast Milk. . https://med.stanford.edu/
newborns/professional- education/breastfeed-
ing/abcs- of- breastfeeding/hand- expression- of-
breast- milk.html. [Accessed January 31, 2021].
204. Newborn Nursery at Lucille Packard Chil-
dren’s Hospital, Stanford Medicine: Hand
Expression of Breastmilk. [Video]. http://
med.stanford.edu/newborns/professional-
education/breastfeeding/hand- expressing-
milk.html. [Accessed January 31, 2021].
205. Eglash A, Simon L, the Academy of Breastfeed-
ing Medicine. ABM clinical protocol #8: hu-
man milk storage information for home use
for full-term infants, revised 2017. Breastfeed
Med. 2017;12:390–395.
206. Centers for Disease Control and Prevention.
Rates of Any and Exclusive Breastfeeding by
Socio- demographics among Children Born in
2016; 2019. [updated 08/01/2019; cited 2019
12/20/2019]. Available from: https://www.cdc.
gov/breastfeeding/data/nis_data/rates- any-
exclusive- bf- socio- dem- 2016.htm.
207. Klerman JA, Daley K, Pozniak A. Family and
Medical Leave in 2012: Technical Report; 2014.
Cambridge, MA https://www.dol.gov/sites/
dolgov/files/OASP/legacy/files/FMLA- 2012-
Technical- Report.pdf.
208. Committee on Health Care for Underserved
Women, American College of Obstetricians
and Gynecologists. Racial and ethnic dis-
parities in obstetrics and gynecology. Com-
mittee Opinion No. 649. Obstet Gynecol.
2015;126:e130–134.
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.

References 185.e5
209. Louis- Jacques AF, Stuebe AM. Enabling
breastfeeding to support lifelong health for
mother and child. Obstet Gynecol Clin N Am.
2020;46:363–381.
210. American College of Obstetricians and Gyne-
cologists Women’s Health Care Physicians.
Committee Opinion: No. 732. Healthcare
for Transgender Individuals. Obstet Gynecol.
2011;512:1454–1458. https://www.acog.org/-
/media/project/acog/acogorg/clinical/files/
committee- opinion/articles/2011/12/health-
care- for- transgender- individuals.pdf.
Downloaded for kate bresnahan ([email protected]) at Elsevier - Demonstration Account from ClinicalKey.com by Elsevier on
January 19, 2023. For personal use only. No other uses without permission. Copyright ©2023. Elsevier Inc. All rights reserved.
