
Former Principal
Maharashtra State Institute of Hotel Management
and Catering Technology, Pune
FOOD SCIENCE
NUTRITION
Third Edition
SUNETRA RODAY
© Oxford University Press. All rights reserved.
Oxford University Press
3
Oxford University Press is a department of the University of Oxford.
It furthers the University’s objective of excellence in research, scholarship,
and education by publishing worldwide. Oxford is a registered trade mark of
Oxford University Press in the UK and in certain other countries.
Published in India by
Oxford University Press
Ground Floor, 2/11, Ansari Road, Daryaganj, New Delhi 110002, India
© Oxford University Press 2007, 2012, 2016, 2018
The moral rights of the author/s have been asserted.
First Edition published in 2007
Second Edition published in 2012
Eight Impression 2016
Third Edition published in 2018
All rights reserved. No part of this publication may be reproduced, stored in
a retrieval system, or transmitted, in any form or by any means, without the
prior permission in writing of Oxford University Press, or as expressly permitted
by law, by licence, or under terms agreed with the appropriate reprographics
rights organization. Enquiries concerning reproduction outside the scope of the
above should be sent to the Rights Department, Oxford University Press, at the
address above.
You must not circulate this work in any other form
and you must impose this same condition on any acquirer.
ISBN-13: 978-0-19-948908-4
ISBN-10: 0-19-948908-4
Typeset in Garamond
by Archetype, New Delhi 110063
Printed in India by Magic International (P) Ltd., Greater Noida
Cover image: Lisovskaya Natalia / Shutterstock
Third-party website addresses mentioned in this book are provided
by Oxford University Press in good faith and for information only.
Oxford University Press disclaims any responsibility for the material contained therein.
© Oxford University Press. All rights reserved.
Oxford University Press
Preface to the
Third Edition
e second edition of Food Science and Nutrition received an overwhelming response and served the
purpose of arousing interest in this subject in students, sta, and the community at large. e simple
language and style of presentation has helped people with minimum science background understand
this important subject which has a direct impact on our health and wellbeing. Food safety is the key
to sound health and needs to be in place if citizens of our country are to be protected from food-
borne diseases and dietary deciencies. Just knowledge about nutrition and food science is incomplete
without adding the food safety aspect. Keeping this in mind, this new edition has been developed as a
comprehensive approach to preparing safe and nutritious food for all.
Like every decade, there have been major changes in the Indian consumer’s lifestyle and eating
habits. Likewise, the incidence of lifestyle related diseases and consumption of junk food is on the
increase, making it necessary for all food business operators and homemakers to understand and apply
the basic principles underlying preparation of products that are safe, nutritionally sound, and high in
quality. e important role played by regulatory agencies has become more signicant and now food
business operators are realizing the benets of adopting food standards and regulations in marketing
their products and services.
e aim of this text has been and remains the same—to provide students with a wide range of basic
principles and practices in the subject area, thus enabling them to apply their knowledge eciently—be
it at home, in the food industry, hospitality or health care sector, and provide safe, wholesome, and
quality food.
About the Book
Although the rst edition was initially designed both as an introduction to the subject and as a textbook
for students pursuing degree in Hotel Management and Catering Technology, it was well accepted
by students and faculty members from food technology, home science, and health sciences. Based on
feedback from faculty members, a need to expand the coverage of the book was felt. erefore, the
book now becomes a complete and exhaustive textbook for students from other related streams as well.
Two new chapters and many new topics have been added to ensure adequate coverage of latest trends
and make the book useful for students pursuing a course in food science, home science, and hospitality
studies.
© Oxford University Press. All rights reserved.
Oxford University Press
vi Preface to the Third Edition
In addition, 12 original chapters have been revised to include the latest examples and trends in the
food and beverage industry. Illustrations have been updated and augmented to add interest to the text.
As in the last edition, each chapter in the book is designed with a focus on the learning objectives.
Key terms are explained at the end of every chapter. Simple illustrations, formulae, and reactions are
added to portray concepts. Review questions are listed at the end of each chapter. A concise summary
to highlight the main points is given for every chapter.
Key Features
• Completely matches the National Council for Hotel Management & Catering Technology
(NCHMCT) syllabus for the subjects taught in the rst three semesters namely food science,
nutrition, and food safety
• Coverssubjectstaughtinhospitalityandhoteladministration,foodtechnology,appliedsciences,
home science, and nursing courses
• Providesampleexamples,reviewquestions,analyticalthinkingexercises,andupdatedreference
charts and tables
• InstructorsmanualwithMCQsforallchapters
New to the Third Edition
Based on changing trends in hospitality and food business operators as well as the invaluable feedback
received from reviewers, users, industry professionals, and academicians, this edition now includes the
following:
• Acompletesectioninthebookfocusesonallaspectsoffoodsafetycoveredinfourdedicated
chapters as well an appendix on First Aid.
• Chapter10onFoodMicrobiologyintroducesthereadertomicroorganismsthatareofsignicance
to the food industry. Both useful and harmful microbes that are of special importance to the
food industry, factors that aect and control the growth of microorganisms. An introduction
to hygienic handling measures of food items to prevent contamination, spoilage and spread of
foodborne disease, emerging pathogens.
• Chapter11onFoodProcessingandPreservationisbasedonshelf-life,spoilageandcontamination,
objectives of processing, methods of preservation, eect of processing on nutrients, and food
additives.
• Chapter 12 on Food Safety covers principles of safe food, GHP, GMP, hygiene of the food
establishment, FSMS guidelines, good habits, 7 C’s of food hygiene, common faults in food
preparation and service, and food allergies.
• Chapter13on FoodStandards,Regulations,andQualityManagementincludes thefollowing
topics—international and national regulations and standards, compulsory and voluntary laws and
standards—CODEX,ISO, BIS, Agmark;regulatory agencies—National —FSSAI, EIC, QCI,
CPA,REGULATORYAGENCIES—International—WHO,FAO,WTO,qualitymanagement
systems—TQM,FSMS,HACCP;foodfortication,geneticallymodiedfoods,foodlabelling.
© Oxford University Press. All rights reserved.
Oxford University Press
Preface to the Third Edition vii
Extended Chapter Material
e following additions have been made in the existing chapters:
• Chapter4onProteinshasanewsectiononproteinisolates,concentrates,andhydrolysates.
• Chapter6onFatsandOilsincludestransfattyacids,interesterication,andfatreplacers.
• Chapter10onFoodMicrobiologyincludesclassicationofhazardouscontaminants,modeof
spread of food borne pathogens, and emerging pathogens.
• Chapter11onFoodProcessingandPreservationincludesclassicationoffoodonthebasisofits
shelf-life.
• Chapter14onIntroductiontoNutritionhasthelatest‘recommendeddietaryallowances’table.
• Chapter16onProteinsinNutritionhasintroducedtheconceptoflimitingaminoacids.
• Chapter17onLipidshasanewtopiconnon-communicablediseases(NCDs).
• Chapter19onVitaminsincludesabsorptionofvitamins.
• Chapter20onMineralsincludesbioavailabilityofmineralsandSelenium.
• Chapter22onBalancedDietincludesmodicationsinthehealthyfoodplate.
• Chapter 24 on Modied Diets has two additional dietary guidelines for cancer patients and
Naturopathy.
• Chapter25onNewTrendsinFoodshasadditionalinformationonnewpackagingoptions,safety
concerns regarding plastics, and smart packaging.
Coverage and Structure
e text is divided into three sections: (1) food science, (2) food safety, and(3)nutrition.
Section 1—Food Science
e section on food science now comprises nine chapters that cover scientic principles and their
applications in the preparation of food and commercial food products. New commodities and processes
which are of relevance have been included. is section concentrates on the composition, structure, and
behaviour of food in relation to pre-preparation, cooking, packaging, and storage relevant to catering
operations.
Chapter 1 introduces students to the basic concepts of food science.
Chapter 2 is on Colloidal systems in foods and explains various colloidal systems encountered in
foods and their eect on food quality.
Chapter3introducesthestudenttoCarbohydratesandtypesofcerealsandcerealproductsthatare
available in the market.
Chapters4onProteinsexplainspulsestobeavegetariansourceofproteinandtheeectofsteeping,
sprouting, and cooking on pulses.
Chapter5onFruitsandVegetablesdiscussesthestructureandnaturalplantpigmentsaswellasthe
role of plants in human diet, various kinds of fruits and vegetables, as well as the structure and natural
plant pigments present in them.
Chapter6onFatsandOilsdiscussesconceptssuchasrancidity,reversion,rening,winterization,
and nuts and oilseeds.
© Oxford University Press. All rights reserved.
Oxford University Press
viii Preface to the Third Edition
Chapter 7 on Flavour explains the various aspects as well as the use of avours in food preparation
and dierent spices and herbs.
Chapter 8 on Browning Reactions discusses types of browning reactions and the role of browning
in food preparation.
Chapter9coversdierentmethodsoffoodevaluation.
Section 2—Food Safety
is section comprises four chapters covering all aspects of Food Safety.
Chapter10introducesthereadertomicroorganismsofsignicancetothefoodindustry.
Chapter11onFoodProcessingandPreservationdiscussestheobjectivesoffoodprocessing,methods
of food preservation, and the eect of processing on food constituents.
Chapter 12 is on Food Safety.
Chapter13dealswithFoodStandards,Regulations,andQualityManagement.
Section 3—Nutrition
e section on nutrition comprises 12 chapters related to nutrients and planning of diets for sustaining
a healthy lifestyle. Crucial issues such as weight control, eating disorders, and lifestyle-related diseases
are included in this section. Dietary guidelines for prevention of deciency and problems related to
excessive consumption have been covered.
Chapter14introducesreaderstotheconceptofnutrition.
Chapter 15 on Carbohydrates in Nutritioncoverstheclassication as well as dietary sourcesof
carbohydrates.
Chapter16onProteinsinNutritionintroducesstudentstoclassicationandfunctionsofproteins.
Chapter 17 on Lipids covers fatty acids, antioxidants, saturated fatty acids, cholesterol, and more.
Chapter 18 explains the various functions of water as well as the concept of water balance.
Chapter19discussesthevarioustypesofvitamins.
Chapter20discussestheclassicationandgeneralfunctionsofminerals.
Chapter 21 on Energy Metabolism covers forms of energy, energy requirements, and energy balance.
Chapter 22 discusses the various aspects of a balanced diet, planning balanced diets, various food
pyramids, and the latest concept of the food plate.
Chapter23introducesstudentstomenuplanningandmassfoodproduction.
Chapter24onModiedDietsdiscussesdiettherapyandvarioustypesofmodieddiets.
Chapter25discussesnewtrendsinfoodsandnutritionincludingthenutritionalevaluationofnew
products and highlights dierent nutraceuticals, such as prebiotics and probiotics, and their role in
maintaining health and the signicance of nutritional labelling.
Sunetra Roday
© Oxford University Press. All rights reserved.
Oxford University Press
Acknowledgements
Many people from the food industry and academia have helped me in successfully completing this
book and I am grateful for their contributions. It is practically impossible to name them all but I would
specially like to mention a few.
IamgratefultoDrEramS.Rao,AssociateProfessorofAppliedSciences,BhaskaracharyaCollegeof
AppliedSciences,UniversityofDelhiandPresident,AssociationofFoodScientistsandTechnologists
(AFST) Delhi Chapter, for her invaluable inputs, inspiration, and encouragement in completing this
project.
I would like to thank Ms Sumedha Jalgaonkar, Food Safety Consultant and Trainer, Foodiesys
Consulting,PuneandMsSurbhiDatta,Counsellor FoodSafetyandQuality,CII-FACE,fortheir
contributionsinChapter13onFoodStandards,Regulations,andQualityManagement.
I owe an immense debt of gratitude to my son, Mr Vikrant Roday, for providing all technical
assistanceandmydaughter,DrNehaVivekSaraf,forherinvaluablecontributionsespeciallytowards
the chapters on Nutrition.
I am also thankful to Dr Shashi A Chiplonkar, Hon. Senior Scientist, H C Jehangir Medical Research
Institute,Puneforhersuggestionsandhelp.
IwouldliketoappreciatethetimelysupportprovidedbytheLibrarianfromMSIHMCT,Pune,Ms
PratibhaWankhede,andAssistantLibrarian,MsDeepaliMarne.
I am immensely indebted to my husband and entire family for putting up with impossible hours
and schedules.
I would also like to thank all the reviewers—Sunita Badhwar, Assistant Professor, BCIHMCT;
SanjibKumarDey,SeniorLecturer,IHMRohtak;GeetikaJoshi,LPU,Jalandhar;andNavjotSahasi,
InstituteofHotelManagement,Catering&Tourism,Udaipur.
I would also like to sincerely thank my friend Dilip Summanwar, Ex-Instructor St John’s Ambulance
(Australia) and Trainer for First Aid classes for e Indian Red Cross Society for his invaluable
contribution to the section on First Aid.
IextendmythankstotheeditorialandproductionteamofOxfordUniversityPress,India.
Feedback on this book can be shared with me at [email protected].
© Oxford University Press. All rights reserved.
Oxford University Press
Preface to the
First Edition
e provision of food and beverages is one of the oldest services associated with the hospitality industry.
e food services industry has evolved and has come full circle. e need for providing nutritious meals
for balanced overall development has acquired greater signicance in the past few decades.
Eating out is no longer the occasional special event to be celebrated where people indulge. It has
becomeawayoflife.Weareforcedtoeatoutorordertake-awaymealsduetoworkpatterns,education,
or social commitments. e number of people depending on food service providers for meeting their
daily nutritional requirement is increasing at a rapid pace, making it necessary for professionals in the
hospitality sector to be able to oer healthy choices on the menu to the customers.
Food science and nutrition has gained added signicance with an increase in the number of lifestyle-
related diseases, such as high blood pressure, atherosclerosis, heart diseases, diabetes mellitus, and
obesity. Food manufacturers are introducing new products keeping these diseases in mind. A need was
felt for a book designed specially for students in hospitality-related courses that addresses all the basic
issues of food science and nutrition. Keeping this aspect in mind, I decided to write this book.
Food Science and Nutrition is designed both as an introduction to the subject and as a textbook
for ‘Principles of Food Science and Nutrition’ for students of the ‘B.Sc. in Hospitality and Hotel
Administration’ course oered by the National Council for Hotel Management and Catering
Technology, New Delhi.
e students who join catering courses have little or no background in food science. ey need a
textbook that relates to what they practice in practical sessions. ey nd science dicult to comprehend
and time-consuming to study.
From my experience of teaching the subject to students of catering, I have developed a text that
concentrates on those aspects of food science and nutrition of particular relevance to the catering
industry.
e text is divided into two sections: (1) food science and (2) nutrition.
e section on food science comprises 11 chapters that cover scientic principles and their applications
in the preparation of food and commercial food products. New commodities and processes which are
of current relevance have been included. is section concentrates on the composition, structure, and
behaviour of food in relation to pre-preparation, cooking, packaging, and storage relevant to catering
operations.
© Oxford University Press. All rights reserved.
Oxford University Press
Preface to the First Edition xii
e section on nutrition comprises 12 chapters pertaining to nutrients and planning of diets for
maintaining good health throughout the life cycle.Weight control, eating disorders, and lifestyle-
related diseases are included. Dietary guidelines for prevention of deciency and problems related to
excessive consumption have been covered.
Each chapter in the book is designed with a focus on the objectives. Key terms are explained at the
end of the chapter. Simple illustrations, formulae, and reactions are added to portray concepts. Review
questions are listed at the end of each chapter. A concise summary to highlight the main points is given
for every chapter.
Today’s consumers ask questions about the nutritional value and health benets of food. ey are
aware of the role the diet may play in maintaining and promoting good health. is makes it imperative
for the food service provider to understand the fundamentals underlying food science and nutrition
and put theory into practice.
Reader’s views and comments are most welcome and will be appreciated.
Sunetra Roday
© Oxford University Press. All rights reserved.
Oxford University Press

Features of the Book
Learning Objectives
Each chapter begins with learning
objectives that focus on learning
and the knowledge you should
acquire by the end of the chapter.
Well-labeled illustrations
Each chapter is interspersed
with numerous illustrations
that supplement the
explanation in the text.
Tables
All chapters contain tables that
provide an outline of the topics
discussed in the chapter.
1
1
IntroductIon
The food industry, be it the processing industry or the catering industry, is one of the largest and most
needed industries in the world today fullling one of our three basic needs, i.e., food. Its growth rate
is phenomenal, growing by leaps and bounds to provide three square meals to our rapidly increasing
population and keeping pace with the ever-changing demands of the people.
e developments in the food industry can be traced back to surplus food which needed to be
preserved for a rainy day. Food preservation is not a new phenomenon. Our forefathers understood
the basic principles underlying food preservation and practised them using natural ingredients and the
forces of nature, such as sunlight and ultraviolet (UV) rays, till newer and more scientic methods were
developed.
Improvement in equipment and machinery has made it possible to increase the capacity of food
processing plants greatly. e shelf life of perishable foods has increased dramatically with the invention
of the refrigerator and the use of dry ice.
With the advent of the wheel, surplus food was transported several hundred miles. As early as in
1850, milk was transported by special milk trains and tank trucks over a distance of several hundred
miles with negligible loss in quality. Food, which was perishable, was moved thousands of miles before
it was processed, stored, and consumed.
LearnIng objectIves
After reading this chapter, you should be able to
• appreciate the importance of food science to a caterer in the context of the processed
food revolution
• understand the relationship of food science to food chemistry, food micro biology,
and food processing
• appreciate the role of convenience foods in our day-to-day life
• appreciate the importance of understanding the basic concepts in physics, chemistry, and biology
• understand the applications of these concepts in the food industry
• interpret the weights and measures in recipes
• weigh and measure ingredients accurately
Introduction to
Food Science
Part
I
Food Science
FoodProcessingandPreservation 167
338
276
250
240
212
170
165
160
145
D
A
N
G
E
R
Z
O
N
E
120
60
41
39
32
0
No growth, some bacteria
survive freezing
Scant multiplication and survival
Some bacterial growth occurs
Bacteria multiply rapidly
Pathogenic bacteria killed above 60
°
C
survival and scant growth
Tr ichinella cysts killed, cooking
temperatures destroy most
bacteria (74−100
°
C)
No bacterial growth, some
survive, pathogens are killed
Vegetative cells die, spores survive
Bacterial load reduced to safe levels
Commercial sterilization
Bacterial spores killed
Complete sterilization
170
°
C
135
°
C
121
°
C
115
°
C
100
°
C
82
°
C
77
°
C
72
°
C
70
°
C
63
°
C
62
°
C
49
°
C
37
°
C
15
°
C
10
°
C
5
°
C
4
°
C
0
°
C
–18
°
C
–35
°
C
Sterilization by
dry heat
UHTS
Sterilization by
moist heat
Canning low
acid food
Canning high acid
food/blanching, boiling
Dish sanitization
Safe temperature
for pork
Pasteurization HTST
Internal temperature
of reheated food
Danger zone ends
Pasteurization LTH
Body temperature
Cool for refrigeration,
thawing cabinet
temperature
Dry food store ideal
temperature
Danger zone begins
Refrigerated storage
Freezing point of water
Freezer storage
Blast freezing
°
F
Fig. 11.1 Temperaturesusedinthefoodindustryandtheireffectonmicroorganisms
ProteinsinNutrition 269
should be includedin the diet. Protein requirement increases during illness or disease as protein is needed
for rebuilding new tissue. In traumatic injury, surgery, burns, and fever,there is breakdown of tissues,
which need to be repaired. Extra physical activity does not require any additional intake of protein.
Adequacy of calorie intake e diet should contain adequate carbohydrates and fats to have a protein
sparing eect.
Quality of protein and eciency of digestion e quantity of protein required will be more if the
protein quality is poor. Plant proteins have a lower digestibility. e method of cooking also aects the
availability of protein. Overcooking and toughening of animal proteins aects digestibility.
Previous state of nutrition Malnourished and underweight individuals require more protein as
compared to healthy individuals.
ReCOMMeNDeD DIeTaRY aLLOwaNCes
e amount of protein required depends on the quality of protein consumed, i.e., on the amino acid
composition of the protein rather than the quantity of protein present in a food. On a mixed vegetarian
diet, adults require 1g/kg body weight (ideal weight). Requirements are higher in terms of body weight
for infants and children because proteins are needed for growth as well as maintenance.
Pregnant women require an additional +23g/day, while lactating mothers need +19g to +13g/day for
0 to 6 months and 6 to 12 months, respectively.
Table 16.5 gives the recommended protein allowances for Indians.
Table 16.5 RecommendedproteinallowancesforIndians
Group Particulars Body weight (kg) Protein (g/day)
Man Adult 60 60
Women Adult 55 55
Pregnancy +23
Lactation (0–6 months) +19
(6–12 months) +13
Infants 0–6 months 5.4 1.16 g/kg
6–12 months 8.4 1.69 g/kg
Children 1–3 years 12.9 16.7
4–6 years 18 20.1
7–9 years 25.1 29.5
Boys 10–12 years 34.3 39.9
13–15 years 47.6 54.3
16–18 years 55.4 61.5
Girls 10–12 years 35 40.4
13–15 years 46.6 51.9
16–18 years 52 55.5
DIeTaRY sOURCes
Proteins are present in both plant and animal foods.
© Oxford University Press. All rights reserved.
Oxford University Press

Summary
The summary at the
end of each chapter
draws together the main
concepts discussed within
the chapter. This will help
you to reflect and evaluate
important concepts.
Exercises
Each chapter contains
a series of exercises
that enhance learning
and can be used for
review and classroom
discussion.
Appendix
Appendix A at the end of the book
provides information on First Aid
techniques used in different situations
such as heart attack, accident, etc.
Vitamins 305
9. Do not add alkali (soda bicarbonate) to enhance green colour or hasten the cooking of pulses such
as kabuli channa as B-complex and vitamin C are readily destroyed in an alkaline medium.
10. Store food in a refrigerator, covered with a lid, aluminium foil, or cling lm to retain nutrients.
11. Reheat only what is required.
12. Pressure cooking helps in retaining vitamins as food is cooked in a covered container for a shorter
time.
13. Fat-soluble vitamins are lost during deep fat frying, if the food to be fried is not coated prior to
frying.
14. Vitamin A and carotene are lost due to oxidation and dehydration, so keep food covered to prevent
oxidative and moisture loss.
sUMMaRy
Vitaminsarevitalorganiccompoundsrequiredbythe
bodytoperformspecificfunctionssuchasthereleaseof
energyfromfoodandothergrowthrelated,protective
andregulatoryfunctions.Theyarerequiredinminute
amountsandhencearecategorizedasmicronutrients.
Theyarebroadlyclassifiedasfatsoluble(vitaminA,D,
E, and K) and water soluble (B-complex and vitamin
C)vitamins.Eachvitaminhasaspecificroletoperform
andcannotbereplacedbyanothervitamin.Fat-soluble
vitaminsrequirefatfortheirabsorptionandcanbestored
inthebody.Water-solublevitaminsarereadilyabsorbed
butarenotstoredinthebody.Excessiveintakeoffat-
solublevitaminsleadstotoxicityorhypervitaminosis.
VitaminAispresentinanimalfoodsonly.Carotene,a
precursorofvitaminA,ispresentinyellow,orange,and
redfruitsandvegetables,andingreenleafyvegetables.
WegetourrequirementofvitaminDfromsunlight.The
precursorintheskin‘7-dehydrocholesterol’isactivated
byUVraysfromsunlight.AdeficiencyofvitaminsEandK
israrelyseeninadultsasbothvitaminsarewide-spread
innature.
The B-complex vitamins are water soluble and
includeeightvitamins,namelythiamineorB
1
,riboflavin
orB
2
,niacin,pyridoxineorB
6
,folicacid,cyanocobalamine
or B
12
, pantothenic acid, and biotin. They mainly
functionasco-enzymesinthereleaseofenergyfrom
carbohydrates, fats, and proteins. Three B-complex
vitaminsaredesignated‘anaemiapreventingvitamins’
astheyareneededforsynthesisofhaemeandforthe
maturation of red blood cells. Apart from the food
sources,thebacterialfloraintheintestinearecapableof
synthesizingvitamins,namelyvitaminKandB-complex
vitamins.
VitaminCisthemostsusceptibleofallvitamins.Itis
presentin fresh fruits and vegetablesandinsprouted
grain.Itisdestroyedbyoxidation,heat,andanalkaline
medium.Propercookingpracticesneedtobefollowed
ifvitamincontentoffoodhastoberetained.
Key TeRMs
Anaemia AconditioninwhichnumberofRBCsor
haemoglobincontentofbloodisreduced.
Antagonist Asubstancethatinterfereswiththe
actionofanothersubstance.
Antioxidant Asubstancenaturallypresentoradded
toaproducttopreventitsbreakdownbyoxygen.
b-carotene Afat-solublecarotenoidpigmentwhich
ispresentinplantsandisaprecursorofvitaminA.
Carotene Reddishorangecolourpigmentinyellow/
orange/redfruitsandvegetablesandgreenleafy
vegetableswhichincludea-,b-,andg-carotenes
andcryptoxanthin.
Cheilosis Swollen,cracked,andredlips.
Co-enzyme Asubstancethatmustbepresentalong
withanenzymeforaspecificreactiontooccur.
Collagen Intercellularcementingsubstanceswhichis
proteinmatrixofcartilage,connectivetissue,and
bone.
Glossitis Inflammationofthetongue.
158 f oodscienceandn utrition
andincludesbothnaturalandprocessedfoods
suchasmilkandmilkproducts,proteinrichmoist
foodssuchasmeat,fish,poultry,cookedriceand
pulses,etc.
Prions Theseareinfectious,proteinaceousparticles
whicharecapableofcausingTSEsinhumans
andanimals.Theyaresmallerthanvirusesand
donotcarryanygeneticmaterials,resisthigh
temperaturesandchemicalsandareextremely
difficulttodestroy.
Psychrophiles Thesearecold-loving
microorganismsthatcansurviveattemperatures
aslowas-28 °C(–18.4 °F)andcanmultiplyat
temperaturesashighas20 °C(68 °F).
Putrefaction Itistheanaerobicdecompositionof
proteinsbybacteriawiththedevelopmentof
foul-smellingcompounds.
Sanitation Thewordsanitationcomesfrom
theLatinwordsanuswhichmeans‘soundand
healthy’or‘cleanandwhole’.Inthefoodindustry,
sanitationmeanscreatingandmaintaininghygienic
andhealthfulconditions.
Sanitize Itmeanstoreducethenumberofdisease-
causingbacteriatosafelevels.
Saprophyte Anyorganismthatlivesondeador
decayingorganicmatterforitssurvivaliscalled
saprophyte.
Sewage Itisthewastematterfromthetoilets,
bathrooms,kitchen,andotherdrainscarriedoff
bysewers.Liquidwastefromabovesourcesis
alsocalledgreywater.
Spore Abacterialsporeisaresistantstructure
formedinsomerod-shapedbacterialcellsthat
canwithstandunfavourableconditions.Itremains
dormanttillconditionsbecomefavourableand
germinateintoavegetativecellthatcanmultiply
andgrow.
Survival Continuetoliveinspiteofunfavourable
conditionsandcanmultiplyorgrowwhen
conditionsforgrowtharesuitableorfavourable
Thermophiles Microbesthatgrowbetterat
temperaturesfrom45 °Cto60 °Candsometimes
higher
Ubiquitous Presenteverywhereinour
environment,inandonhumans,plants,animals,
water,air,soil,andinanimateobjects
Vaccination Itistheintroductionofanykindof
deadorweakenedmicrobesintothebodyofa
livingbeingtodevelopresistanceorimmunity
againstthatspecificdisease.
Water activity (a
w
)
Itistheamountofwaterin
foodthatisavailabletomicroorganismsfortheir
growth.Somewaterisboundtoothersubstances
andhencedoesnotsupportmicrobialgrowth.
Wholesomefood Itisfood thatishealthful,atthe
rightstageofmaturity,freefrompollutants,and
contaminantsandisfitforconsumption.
revIew exercIses
1. Whydoesafoodhandlerneedtohaveknowledge
aboutfoodmicrobiology?
2. Classify the different microorganisms which are
presentinourfood.
3. Discussthebeneficialeffectsofmicroorganisms.
4. Explainthetermscross-contamination,sanitation,
foodborneillnesses,anddangerzone.
5. Differentiate between food poisoning and food
infection.
6. List the various factors which affect microbial
growthandexplainanytwofactors.
7. Definethefollowingterms:
(a) Anaerobes (c) Thermophiles
(b) Infestation (d) Saprophytes
(e) Contamination (g) High-riskfoods
(f) Endospores
8. With the help of a line diagram, explain how
diseasesaretransmitteddirectlyandindirectly.
9. Discusstheharmfuleffectsofmicroorganismswith
respecttofoodspoilageandfoodborneillnesses.
assIgnment
Visitthekitchenofyourcollegecanteenandlistmeasuresyouwouldsuggesttothecaterertoensureserviceof
safeandwholesomefood.
260 food s cience and nutrition
Fill in the blanks
1. Dietaryfibreprovides__________kcal/gofenergy.
2. Thehumanbodystorescarbohydrateintheform
of__________inthemusclesandliver.
3. The water-soluble fibre __________ is used for
settingjamsandjellies.
4. Thesugar__________ispresentintheblood
stream.
5. Thehormone__________secretedbythe
__________regulatesbloodsugarlevels.
1. Diseasecausedduetoinsufficientinsulin
2. Elevatedbloodglucoselevels
3. Disaccharidemadeupofglucoseandgalactose
4. Dietaryfibrethatisnotacarbohydrate
5. Bloodlevelofasubstanceatwhichitcannotbe
reabsorbedbythekidneys
Give one word for the following
260 food s cience and nutrition
Fill in the blanks
1. Dietaryfibreprovides__________kcal/gofenergy.
2. Thehumanbodystorescarbohydrateintheform
of__________inthemusclesandliver.
3. The water-soluble fibre __________ is used for
settingjamsandjellies.
4. Thesugar__________ispresentintheblood
stream.
5. Thehormone__________secretedbythe
__________regulatesbloodsugarlevels.
1. Diseasecausedduetoinsufficientinsulin
2. Elevatedbloodglucoselevels
3. Disaccharidemadeupofglucoseandgalactose
4. Dietaryfibrethatisnotacarbohydrate
5. Bloodlevelofasubstanceatwhichitcannotbe
reabsorbedbythekidneys
Give one word for the following
318 food scienceandn utrition
sUmmaRy
Mineralelementsareinorganiccompoundspresentin
bodytissuesandfluidsinsmallamountsandarereferred
toasmicronutrients.Theyconstitute4percentofbody
weightanddonotprovideanyenergy.Theyareclassified
intothreegroupsbasedonthequantityrequiredbythe
body.Majormineralsormacromineralsarerequiredin
largeamounts exceeding 100mg/day. Minor minerals
are required in amounts less than 100 mg / day and
traceelementsarethosewhoserequirementisafew
milligramsorinmicrogramsperday.
Themineralsofimportancetothebodyarecalcium,
phosphorus, sodium, chloride,magnesium potassium,
sulphur,allofwhicharemajorminerals.Iron,manganese,
fluorine,zinc,molybdenum,copper,cobalt,andiodine
arerequiredinmuchsmallerquantities.
Mineralsoccurinthebodyascomponentsoforganic
compounds,ascomponentsofinorganiccompounds,and
asfreeionsinallcells.Theyperformvariousfunctions
related to growth and maintenance and regulation of
bodyfunctions.
Theyarewidelydistributedinnatureandabalanced
dietwithvarietyinchoiceoffoodsensuresanadequate
intake and prevention of deficiency. However, many
factorsaffecttheabsorptionandutilizationofminerals
andtheseneedtobeknowntoenhanceavailabilityof
minerals.
Key TeRms
Betel leaf LeafofthecreeperPiperbetle,whichis
consumedalongwithslakedlimeandbetelnut
foritsdigestiveproperties.
Bioavailability Bioavailabilityistheproportionor
theamountofanutrient/mineralthatisabsorbed
andentersthecirculation,andcanbeutilizedby
thebody.
Co-factor Amineralelementwhichactivatesan
enzyme.
Electrolyte Anelementorcompoundwhich
dissociates,wheninsolution,intoions.
Garden cress seeds Maroonredseeds,which
whensoakedinwaterdevelopamucilaginous
covering.
Haeme iron Ironassociatedwiththehaemoglobin
moleculeandisbetterabsorbedthannon-haemeiron.
Niger seeds Alsocalledblackgingellyseedsor
karal.
Non-haeme iron Ironpresentinplantfoodsand
partlyinmeat,fish,andpoultry,whichisnot
associatedwithhaemoglobin.
Oxalic acid Anorganicacidpresentingreenleafy
vegetablesandcocoa.
Phytic acid Anorganicacidpresentinouterlayers
ofcerealswhichcombineswithcalciumforming
insolublecalciumphytate.
RevIew exeRCIses
1. List the seven major minerals. Describe the func-
tionsofmineralsingeneral.
2. Classifymineralelements,givingtwoexamplesfor
each.
3. State the factors which affect absorption of iron
andcalciuminthebody.
4. Definethefollowingterms:
(a)Mineralelements
(b)Co-factor
(c)Traceelements
(d)Goitrogens
5. Listthevarioussourcesofsodiuminourdiet.
6. Theiodinecontentoffooddependsontheiodine
contentofthesoilonwhichithasgrown.Explain
thisstatement.
7. Match the following minerals in column I with a
deficiencysymptomincolumnII.
I II
(i)Calcium (a) Toothdecay
(ii)Iron (b) Cretinism
(iii)Sodium (c) Musclecramps
(iv)Iodine (d) Alkalosis
(v)Fluorine (e) Spoon-shapednails
(vi)Chloride (f) Macrocyticanaemia
(vii)Phosphorus (g) Osteoporosis
(h) Glossitis
(i) Tetany
(j) Nightblindness
© Oxford University Press. All rights reserved.
Oxford University Press

Brief Contents
Preface to the ird Edition v
Preface to the First Edition xi
Features of the Book xiv
PART I FOOD SCIENCE
1. Introduction to Food Science 2
2. ColloidalSystemsinFoods 19
3. Carbohydrates 31
4. Proteins 54
5. FruitsandVegetables 78
6. FatsandOils 91
7. Flavour 109
8. BrowningReactions 119
9. EvaluationofFood 126
PART II FOOD SAFETY
10. FoodMicrobiology 142
11. FoodProcessingandPreservation 162
12. FoodSafety 185
13. FoodStandards,Regulations,andQualityManagement 208
PART III NUTRITION
14. IntroductiontoNutrition 240
15. CarbohydratesinNutrition 253
16. ProteinsinNutrition 265
17. Lipids 278
18. Water 291
19. Vitamins 297
20. Minerals 311
21. EnergyMetabolism 323
22. BalancedDiet 338
23. MenuPlanningandMassFoodProduction 350
24. ModiedDiets 372
25. NewTrendsinFoods 389
Appendix: First Aid 415
Index 437
About the Author 443
© Oxford University Press. All rights reserved.
Oxford University Press

Detailed Contents
Preface to the ird Edition v
Preface to the First Edition xi
Features of the Book xiv
PART I FOOD SCIENCE
1. Introduction to Food Science 2
Introduction 2
Inter-relationship between Food Chemistry,
Food Microbiology, and
FoodProcessing 3
NeedforConvenienceFoods 4
FoodScienceConcepts 5
BasicSIUnitsofLength,Area,Volume,and
Weight 5
MeasurementofLength 5
MeasurementofVolume 6
MeasurementofWeightorMass 6
Density 7
Relative Density 7
Temperature 8
Conversion of Farenheit Scale to
Celsius Scale 8
Conversion of Celsius Scale to
Farenheit Scale 8
Typesofermometers 9
PotentialHydrogenorpH 9
Applications of pH 11
Important Terminologies, their Denition,
and Relevance 12
BoilingPoint 12
ApplicationsofBoilingPoint 12
Applications of Boiling under
Pressure 12
Evaporation 12
Applications of Evaporation 12
MeltingPoint 13
Applications of Melting
Point 13
SurfaceTension 14
Applications of Surface
Tension 14
Osmosis 15
ApplicationsofOsmosis 15
Humidity 15
ApplicationsofHumidity 15
FoodRheology 15
ApplicationsofElasticity 16
2. Colloidal Systems in Foods 19
Introduction 19
ConstituentsofFood 19
TrueSolution 20
Suspension 20
ColloidalSystems 20
Stability of Colloidal Systems 21
Types of Colloidal Systems in Food 22
Sol 22
© Oxford University Press. All rights reserved.
Oxford University Press
xviii Detailed Contents
Gel 23
Emulsion 24
eoryofEmulsication 24
StabilityofanEmulsion 25
Some Common Food Emulsions 27
3. Carbohydrates 31
Introduction 31
ClassicationofCarbohydrates 33
Monosaccharides 33
Disaccharides 34
Oligosaccharides 34
Polysaccharides 35
StructureofCarbohydrates 35
ReducingSugars 36
Non-reducingSugar 36
Polysaccharides 36
Starch 36
EectofCookingonStarch 40
FactorswhichAecttheProperty
of Starch as a ickening
Agent 41
EectofAddedIngredients 41
Gelation 41
TypeofStarch 42
ConcentrationofStarch 42
DurationofHeating 42
Stirring 42
OtherIngredients 42
AgingofaGel 43
Retrogradation 43
Dextrinization 43
TypesofFoodStarches 43
UnmodiedStarches 43
ModiedStarches 44
CerealsandCerealProducts 44
CerealsusedintheCateringIndustry 45
MaltingofCereals 47
Sugar 48
SugarCookery 48
MakingPreserves 49
Honey 49
ArticialSweeteners 49
SolubleFibres—Pectins,Gums,and
Mucilages 50
Pectin 50
Agar 50
Algin 50
Gums 50
UsesofCarbohydratesinFood
Preparation 51
4. Proteins 54
Introduction 54
BasicStructureandProperties 54
PeptideLinkage 54
ClassicationofProtein 55
ClassicationofProteinsBased
onStructure 55
Classication Based on
Composition 56
Classication Based on
Function 56
NativeProteins 57
DenaturedProteins 58
StagesinHeatDenaturation 58
EectsofDenaturation 58
FactorsAectingDenaturation 58
FunctionalPropertiesofSpecicProtein-
RichFoods 59
Gelatin 59
Milk 60
MilkCookery 60
Eect of Heat on Milk
Proteins 60
EectofAcidonCasein 60
Eggs 61
EggWhite 61
EggYolk 61
EectofHeatonEggProteins 61
EggCookery 62
EggFoams 62
StagesofFreshEggWhiteFoam
Formation 62
FactorsAectingEggWhite
FoamFormation 63
SoueandFondue 65
Cake 65
© Oxford University Press. All rights reserved.
Oxford University Press
Detailed Contents xix
FunctionsofCakeIngredients 65
Eggs 65
Sugar 65
Acid 65
Flour 65
RoleofProteininBreadmaking 66
Meat 66
Post-mortemChanges 67
RipeningorAging 67
ChangesinMeatduringCooking 67
TendernessofMeat 68
CuredMeat 69
Pulses 69
EectofSteeping 70
Eect of Cooking 71
SproutingofPulses 72
CommercialusesofProteins 72
ProteinConcentrates,Isolates
and Hydrolysates, and their
Applications 73
ProteinConcentrates 73
ProteinIsolates 73
ProteinHydrolysates 74
TexturedVegetableProtein 74
OtherCommercialUsesof
Protein 75
5. Fruits and Vegetables 78
Introduction 78
ClassicationofFruitsandVegetables 78
PlantTissueSystems 80
DermalTissueSystem 80
VascularTissueSystem 80
GroundTissueSystem 80
ParenchymaCells 80
Collenchyma Tissue 81
Sclerenchyma Cells 81
Enzymes 82
Organic Acids 82
Maturation and Ripening 82
Post-HarvestChanges 83
ClimactericFruits 83
Non-climactericFruits 83
NaturalColouringPigments 84
Chlorophylls 84
Eect of Cooking on
Chlorophyll 85
Carotenoids 86
Classication of Carotenoid
Pigments 86
CarotenoidPigmentsand
VitaminAActivity 86
Eect of Cooking on Carotenoid
Pigments 87
Flavonoids 87
Betalains 88
Eect of Cooking on Flavonoid
Pigments 88
6. Fats and Oils 91
Introduction 91
Structure 92
Properties 93
Rancidity 93
TypesofRancidity 94
Reversion 95
Factors Leading to Rancidity and
Reversion 96
Temperature 96
Moisture 96
Air 96
Light 96
Metals 96
DegreeofUnsaturation 96
AbsenceofAntioxidants 96
PreventionofRancidity 96
EectofHeatonFatsandOils 97
Polymerization 97
CareofFatsandOils 97
ExtractionofFatsandOils 98
Rendering 98
Pressing 98
SolventExtraction 98
Rening 98
Winterization 99
HydrogenationofOils 99
Cis-TransConguration 100
Interesterication 100
© Oxford University Press. All rights reserved.
Oxford University Press

xx Detailed Contents
Shortenings 100
ShorteningPowerofFatsandOils 100
DietMargarines 101
WhippedButterandMargarine 102
Soft(Tub)Margarines 102
PeanutButter 102
StickMargarine 102
PopularFatsandOilsAvailable 102
Oils 102
Butter 102
Spreads 102
Vanaspati 102
Margarine 102
CompoundFats 103
Suet 103
Dripping 103
OliveOil 103
CanolaOil 103
FreshCream 103
NutsandOilseeds 103
Role of Nuts and Oilseeds in
Cookery 104
Uses 105
CommercialUsesofFatsandOils 105
ShorteningPower 106
FatReplacers 106
TypesofFatReplacers 106
7. Flavour 109
Introduction 109
Denitions 110
NaturalFlavours 110
SomeFlavoursomePlantProducts 110
ProcessedFlavours 111
Added Flavours 111
SpicesandHerbs 113
UseofFlavoursinFoodPreparation 115
8. Browning Reactions 119
Introduction 119
TypesofBrowningReactions 119
EnzymaticBrowning 120
PreventionofEnzymatic
Browning 120
Non-enzymatic Browning 121
Maillard Reaction 121
Conditions which Favour
Maillard Reaction 122
Caramelization 123
AscorbicAcidBrowning 123
LipidBrowning 124
RoleofBrowninginFoodPreparation 124
DetrimentalEectsofBrowning 124
9. Evaluation of Food 126
Introduction 126
Importance of Evaluation to the
FoodIndustry 126
Methods of Evaluation 127
Subjective Evaluation 127
PreferenceTests 127
DierenceTesting 129
ObjectiveEvaluation 133
PhysicalMethods 134
TestforVolume 134
TestforMoisture 135
ChemicalMethods 137
ProximateCompositionofFood
Constituents 138
ProximateAnalysisofFood
Constituents 138
PART II FOOD SAFETY
10. Food Microbiology 142
Introduction 142
Important Microorganisms in Food
Microbiology 143
Viruses 143
Bacteria 143
Fungi 145
Yeasts 145
Molds 146
Algae 147
Parasites 147
© Oxford University Press. All rights reserved.
Oxford University Press
Detailed Contents xxi
FactorsAectingtheGrowthofMicrobes 147
Food 147
Warmth 148
Moisture 148
Time 148
pHLevel 149
OsmoticPressure 149
Oxygen 149
DangerZone 149
UsefulandHarmfulMicrobes 149
FoodFermentations 150
AlcoholicFermentations 150
FermentationofMilk 151
ManufactureofVinegar 151
ContaminationofFood 153
Contaminant 155
CrossContamination 155
Food-BorneIllnesses 155
HowPathogensSpreadtoFood 156
Food, Fingers, Faeces, Fomites, and
Flies 156
TypesofFoodBorneIllnesses 156
FoodPoisoning 156
FoodInfection 156
DierencebetweenFoodPoisoning
andFoodInfection 157
ereeGoldenRulestoControl
ofFoodBorneDiseases 157
EmergingPathogens 158
Bovine Spongiform Encephalopathy
(BSE) 159
11. Food Processing and Preservation 162
Introduction 162
CausesofFoodSpoilage 163
ObjectivesofFoodProcessing 164
MethodsofFoodPreservation 165
UseofLowTemperatures 165
Refrigeration/Chilling 165
Cook–chill 165
Freezing 166
FreezeFlow 167
Cook–freeze 167
CreatingaVacuum 167
VacuumPacking 167
UseofHighTemperatures 167
WetHeat 168
DryHeat 169
RemovalofMoisturefromFood 169
UseofMechanicalDryers 169
FreezeDrying 169
UseofPreservatives 171
ClassIPreservatives 171
ClassIIPreservatives 171
Controlled Atmosphere Storage 172
PreservationbyFermentation 173
PreservationbyRadiation 173
UltravioletIrradiation 173
Milling 175
EectofProcessing(Heat,Acid,andAlkali)
onFoodConstituents 176
Food Additives 177
Numbering of Additives 177
Types of Additives 178
Preservatives 178
Antioxidants 180
EmulsifyingAgents 180
Stabilizers and ickening
Agents 180
Humectants 180
GasesandPropellants 181
Food Colours 181
Non-nutritive Sweeteners 181
Anti-caking Agents 181
Flavouring Agents 181
Nutrient Supplements 182
Bleaching Agents 182
Sequestrants or Chelating
Agents 182
Clarifying Agents 182
Acids and Acidity Regulators 182
12. Food Safety 185
Introduction 185
ImportantTerms 185
GHPandGMP 186
Hygiene of the Food Establishment 187
Food Safety Management Systems
© Oxford University Press. All rights reserved.
Oxford University Press
xxii Detailed Contents
Guidelines 187
Location, Layout, and Facilities 187
Location and Surroundings 187
Layout and Design of Food
EstablishmentPremises 188
Equipment,WorkSurfaces,and
Containers 188
Facilities 189
MaterialHandling 189
PurchasingandReceiving 189
FoodStorage 190
FoodPreparation 190
Cleaning 190
Washing 190
Pre-preparation 189
awing 191
Cross-contamination 191
Cooking 191
ReheatingofFood 191
Chilling 192
Handling High Risk Foods and Deep Fat
Frying 192
Holding,Packaging,Distribution,Serving,
andTransportation 193
Holding—HotandCold 193
Packaging 193
Food Distribution/Service and
Transportation 193
PersonalHygiene 193
HealthStatus 193
BehaviouralandPersonalCleanliness 194
HandWashing 195
HandWashingProcedure 195
Attention 196
BasicRequirementsForPersonal
Hygiene 196
SupportServices 198
PestControlandManagement 198
PestControl 198
Pests’Management 198
CleaningandMaintenance 199
Precautions 199
DishClothandDisposables 200
DrainageandWasteDisposal 200
TypesofWasteandGarbage
Bins 200
Training 201
e7C’sofFoodHygiene 201
Check 201
Clean 201
Cover 201
CrossContaminationAvoid 202
Cook 202
Cool/Chill 202
Consume 202
e10CommonFaultsinFood
PreparationandServices 203
FoodAllergies 203
Foods which Commonly Contain
Allergens 204
SymptomsofAllergy 204
ControlofFoodAllergies 205
13. Food Standards, Regulations, and Quality
Management 208
Introduction 208
FoodStandards 208
InternationalStandards 209
VoluntaryStandards 209
ISOStandards 209
IndianStandards 210
BureauofIndianStandards 210
CerticationSystem 210
TypesofLicensing 210
AgriculturalProduce(Grading
andMarketing)Act,1937
(AGMARK) 210
Regulatory Agencies 211
PreventionofFoodAdulteration
(PFA)Act,1954 211
e Food Safety and Standards
Act(FSSA2006) 211
e Export Inspection
Council 213
QualityCouncilofIndia 213
InternationalOrganizations 214
WorldHealthOrganization 214
Food and Agricultural
© Oxford University Press. All rights reserved.
Oxford University Press

Detailed Contents xxiii
Organization 215
WorldTradeOrganization 215
QualityManagement 216
QualityManagement
Systems 217
QualityControl 217
QualityAssurance 217
QualityImprovement 218
QualityPlanning 218
QualityAssessment 218
TotalQualityManagement 218
Food Safety Management
System 218
Hazard Analysis and Critical
ControlPoints 219
Hazards 220
DevelopingaHACCP
Plan 220
UseofHACCPinCatering 228
BenetsofHACCP 228
Food Fortication 228
GeneticallyModiedFoods 230
BenetsofGMFoods 230
SafetyConcerns 231
IdenticationofGMFoods 231
FoodLabelling 231
HealthClaims 232
Nutrition Information
Panel 234
NutrientContentClaims 234
TracLightSignalsonPackages 235
Adulteration 235
Mislabelling/Misbranding/
Misdescription 236
PART III NUTRITION
14. Introduction to Nutrition 240
Introduction 240
SomeImportantDenitions 240
RelationofFoodandHealth 241
Malnutrition 241
Undernutrition 241
Overnutrition 241
Diet 241
Kilocalorie(kcal) 241
Health 242
NutritionalStatus 242
FoodanditsFunctions 242
PhysiologicalFunctions 243
PsychologicalFunction 243
SocialFunction 243
Factors Aecting Food Intake and Food
Habits 244
GeographicReasons 244
EconomicReasons 244
ReligiousReasons 244
SocialReasons 245
Health 245
Otherfactors 245
ClassicationofNutrients 245
Classication on the Basis of Amounts
RequiredEveryday 245
Classication on the Basis of
Function 246
Classication on the Basis of Chemical
Properties 246
Classication on the Basis of
Essentiality 246
RecommendedDietaryAllowances 246
Digestion, Absorption, and Metabolism of
Food 248
DigestionofFood 249
FactorsthatAectDigestion 249
Absorption 250
Metabolism 251
15. Carbohydrates in Nutrition 253
Introduction 253
ClassicationofCarbohydrates 254
Digestion, Absorption, and
Metabolism 255
Sources 257
© Oxford University Press. All rights reserved.
Oxford University Press
xxiv Detailed Contents
Functions 257
Deciency 258
ExcessCarbohydrates 258
RoleofDietaryFibreinPreventionand
TreatmentofDisease 258
Recommended Dietary Intake for
Adults 261
ArticialSweeteners 261
Alcohol 261
16. Proteins in Nutrition 265
Introduction 265
EssentialAminoAcids 266
Non-essentialAminoAcids 266
ProteinQuality 267
LimitingAminoAcid 267
BiologicalValue 267
ClassicationofProteins 267
ClassicationbyComposition 268
ClassicationbyQuality 268
FunctionsintheHumanBody 268
StructuralFunctions 268
RegulatoryFunctions 269
Energy 269
Digestion, Absorption, and
Metabolism 269
MethodsofImprovingProtein
Quality 271
FactorsInuencingProtein
Requirements 272
Recommended Dietary
Allowances 273
DietarySources 273
AnimalFoodSources 274
PlantFoodSources 274
SpecialProteinSupplements 274
EectofDeciency 275
EectofExcess 275
17. Lipids 278
Introduction 278
Classication of Lipids 278
Classication based on Structure 278
FattyAcids 279
UnsaturatedFattyAcids 280
Monounsaturated Fatty Acids 281
PolyunsaturatedFattyAcids 281
Essential Fatty Acids 281
Antioxidants 282
SaturatedFattyAcids 283
Phospholipids 283
Lipoproteins 283
Glycolipids 283
Cholesterol 284
Sources 284
FunctionsofFats 284
Digestion, Absorption, and Metabolism
ofFats 285
Digestion 285
Absorption 285
Metabolism 286
Food Sources 287
PlantSources 287
Animal Sources 287
Invisible Sources 287
Deciency of Fats 287
Symptoms of Excessive Intake 288
RecommendedDietaryAllowances 289
18. Water 291
Introduction 291
Functions 291
DailyIntakeofWater 292
DailyLossofBodyWater 292
WaterBalance 293
DeciencyofWater 293
RetentionofWater 294
DailyRequirement 294
Beverages 294
NutritiveValueofBeverages 295
19. Vitamins 297
Introduction 297
Classication 297
Fat-SolubleVitamins 298
VitaminA 298
VitaminD 300
VitaminE 301
© Oxford University Press. All rights reserved.
Oxford University Press
Detailed Contents xxv
VitaminK 301
Water-SolubleVitamins 302
B-ComplexVitamins 302
iamine (vitamin B
1
) 303
Riboavin (vitamin B
2
) 304
Niacin 304
Anaemia-preventingVitamins 305
Folic Acid or Dietary
Folate 305
Cyanocobalamin(Vitamin
B
12
) 306
Pyridoxine(VitaminB
6
) 306
VitaminC 307
EectofCookingonVitamins 308
20. Minerals 311
Introduction 311
Classication 312
GeneralFunctionsofMinerals 312
BioavailabilityofMinerals 315
Calcium 315
Phosphorus 316
Iron 317
Iodine 318
Fluorine 319
Sodium 319
Potassium 320
Magnesium 320
21. Energy Metabolism 323
Introduction 323
FormsofEnergy 324
UnitsofMeasurement 324
EnergyValueofFood 325
Calorimetry 325
ProximateComposition 325
EnergyNeedsoftheBody 326
TotalEnergyRequirement 327
BasalMetabolicRate 327
TestforBasalMetabolism 327
FactorsAectingtheBMR 328
SpecicDynamicAction 328
PhysicalActivity 329
EnergyBalance 329
Overweight 330
CausesofObesity 330
Underweight 331
CalculatingtheEnergyValueBasedon
ProximatePrinciples 331
DietarySources 332
EstimationofEnergyRequirements 332
CalculatingEnergyRequirements 334
ModifyingEnergyContentofMeals 335
Underweight 335
ToGainWeight 335
Overweight/Obesity 335
ToReduceWeight 335
22. Balanced Diet 338
Introduction 338
RecommendedDietaryAllowance 338
RDAsforSpecicNutrients 339
Denition 340
BasicFoodGroups 340
CerealandMilletsGroup 342
ProteinorBody-buildingFood
Group 343
ProtectiveFoodGroup 344
SecondaryProtectiveGrouporOther
FruitsandVegetables 344
FatsandOils,Sugar,andJaggery 345
GuidelinesforusingtheBasicFood
Group 345
eFoodPyramid 345
23. Menu Planning and Mass Food
Production 350
Introduction 350
FactorsInuencingMealPlanning 351
NutritionalAdequacy 351
EconomicConsiderations 352
TypeofFoodService 352
EquipmentandWorkSpace 352
LeftoverFood 352
FoodHabits 352
Availability 352
MealFrequencyandPattern 352
Variety 352
© Oxford University Press. All rights reserved.
Oxford University Press
xxvi Detailed Contents
PlanningBalancedMeals 353
Protective/RegulatoryFoods 353
Body-buildingFoods 353
Energy-givingFoods 355
StepsinPlanningBalancedMeals 355
SampleBalancedDiet 356
CalculatingtheNutritiveValueofa
Recipe 358
NutritiveValueofShrewsbury
Biscuits 360
SpecialNutritionalRequirements 361
Pregnancy 361
Lactation 362
Infancy 362
Childhood 363
Adolescence 364
OldAge 365
EectofQuantityCookingandProcessing
onNutrients 367
BenetsofCookingFood 367
CommonFoodProcessing
Techniques 367
24. Modied Diets 372
Introduction 372
PurposeofDieterapy 372
ClassicationofModiedDiets 373
ModicationsinConsistency 373
Modications in Nutrient
Content 375
ModicationsinFibre 375
ModicationsinQuantity 376
Modications in Method of
Feeding 376
DietsforCommonDisorders 377
DiabetesMellitus 377
SymptomsofDiabetes 377
TreatmentofDiabetes 377
FeversandInfection 378
CardiovascularDiseases 378
DisordersoftheGastrointestinal
Tract 381
LiverDisorders 382
KidneyDisorders 383
Cancer 384
Naturopathy 385
GuidelinesforaNaturopathy-based
BalancedDiet 385
Disadvantages of Cooking
Food 385
Advantages of Consuming
UncookedFood 386
Precautionstobetakenwhile
PreparingNaturalFoods 386
Suggested Sample Menu for a
NaturopathyDiet 386
25. New Trends in Foods 389
Introduction 389
SoyaFoods 390
FoodFads 390
OrganicFoods 391
HealthFoods 391
NaturalFoods 392
LiveFoods 392
NewTrendsinFoodPackaging 392
WhyPackagingisNecessary 393
TypesofPackages 394
Multi-layeredPackaging 394
PackagingTypes 394
Intentionally Added Substances and Non-
IntentionallyAddedSubstances 396
PlasticsforFoodPackaging 396
SafetyConcernsforPlastics 397
GeneralPrecautionswhileusing
Plastics 397
PackagingWaste 397
SmartPackaging/Intelligent
Packaging 398
BiodegradablePackaging 399
EdibleFilmsandCoatings 399
SafetyConcernsRegardingUseofPlasticsas
FoodContactMaterial 400
Need for Serving Nutritional and
Health-specicMeals 401
NutritiveValueofFastFoodandJunk
Food 402
Nutritional Evaluation of Newly Launched
© Oxford University Press. All rights reserved.
Oxford University Press
Detailed Contents xxvii
Products 403
TransFattyAcids 404
NutritionalandProductEvaluationof
NewlyLaunchedProducts 405
Nutraceuticals 406
PrebioticsandProbiotics 409
Prebiotics 409
SourcesofPrebiotics 409
BenecialEectsofPrebiotic
Fibre 409
Probiotics 410
SourcesofProbiotics 410
Antioxidants 411
RoleofPhytochemicals 411
Appendix: First Aid 415
Index 437
About the Author 443
© Oxford University Press. All rights reserved.
Oxford University Press

INTRODUCTION
The food industry, be it the processing industry or the catering industry, is one of the largest and most
needed industries in the world today fullling one of our three basic needs, i.e., food. Its growth rate
is phenomenal, growing by leaps and bounds to provide three square meals to our rapidly increasing
population and keeping pace with the ever-changing demands of the people.
e developments in the food industry can be traced back to surplus food which needed to be
preserved for a rainy day. Food preservation is not a new phenomenon. Our forefathers understood
the basic principles underlying food preservation and practised them using natural ingredients and the
forces of nature, such as sunlight and ultraviolet (UV) rays, till newer and more scientic methods were
developed.
Improvement in equipment and machinery has made it possible to increase the capacity of food
processing plants greatly. e shelf life of perishable foods has increased dramatically with the invention
of the refrigerator and the use of dry ice.
With the advent of the wheel, surplus food was transported several hundred miles. As early as in
1850, milk was transported by special milk trains and tank trucks over a distance of several hundred
miles with negligible loss in quality. Food, which was perishable, was moved thousands of miles before
it was processed, stored, and consumed.
LEARNING OBJECTIVES
After reading this chapter, you should be able to
• appreciate the importance of food science to a caterer in the context of the processed
food revolution
• understand the relationship of food science to food chemistry, food micro biology,
and food processing
• appreciate the role of convenience foods in our day-to-day life
• appreciate the importance of understanding the basic concepts in physics, chemistry, and biology
• understand the applications of these concepts in the food industry
• interpret the weights and measures in recipes
• weigh and measure ingredients accurately
Introduction to
Food Science
1
© Oxford University Press. All rights reserved.
Oxford University Press

Introduction to Food Science 3
Over the past few decades, the food industry has witnessed a signicant change. e market has
witnessed a ood of food commodities, superior in quality and available all year round. Ice cream lled
cones and nuts in ice cream retaining their crunch, fresh milk stored on the shelf for months, and crisp
croutons in a ready to serve cream soup are a few marvels of food science and technology. With these
advances in science and technology, the consumer has an unlimited choice of meals to choose from all
year round.
e aesthetic value of food is important. To be able to oer the consumer quality cuisine, basic
knowledge of food science and its applications is necessary. Every food handler should know the
composition, structure, and behaviour of food and the changes that take place during cooking, holding,
and storage as well as what happens to the food once it is consumed, i.e., its digestion, absorption, and
metabolism in the human body.
e study of food is today accepted as a separate discipline called
food science.
Denition Food science is a systematic study of the nature of food materials and the scientic
principles underlying their modication, preservation, and spoilage.
To understand food science, it is necessary to understand the basic concepts of physics, chemistry,
mathe matics, and biology and their applications, i.e., biochemistry, microbiology, and food technology,
in order to prepare, package, store, and serve wholesome, high quality products.
All foods are chemical compounds which undergo various chemical reactions at all stages from
production to consumption. ese reactions are based on the laws of chemistry. Many processes used
while preparing food involve physical changes apart from chemical changes.
Matter exists in three states—solid, liquid, and gas. In general, as the temperature is increased, a pure
substance will change from solid to liquid and then to gas, without change in chemical composition.
However, many organic compounds will decompose, undergoing various chemical reactions, rather
than a change of state when temperature is raised.
Many foods are complex mixtures of chemical substances. In processed foods, additives are added
to improve colour, texture, avour, etc., and these additives are also chemical compounds. It undergoes
further chemical changes during storage, cooking, processing, as well as in the human body during
digestion of food by action of chemical substances.
Physical aspects of food such as the various food systems are of colloidal dimensions. Food is
subjected to various physical conditions during preparation and storage which aect its quality such as
temperature and pressure changes.
INTER-RELATIONSHIP BETWEEN FOOD CHEMISTRY, FOOD MICROBIOLOGY, AND FOOD PROCESSING
Food chemistry is the science that deals with the composition, structure, and properties of food, and
with chemical changes that take place in food. It forms a major part of food science and is closely related
to food microbiology. e chemical composition of food dictates which microorganisms can grow on
it and the changes which take place in the food because of their growth. e changes may be planned
and desirable or may result because of contamination, causing disease, i.e., causing food poisoning
and food infection or just spoiling the food rendering it unt for consumption. Microorganisms have
basic growth requirements, namely food, moisture, temperature, time, osmotic pressure, pH, and the
presence or absence of oxygen.
© Oxford University Press. All rights reserved.
Oxford University Press
4 F ood Science and Nutrition
Food chemistry and food microbiology are intimately related to food processing because the
processes to which food needs to be subjected to improve its taste, texture, avour, and aroma depend
on its composition and ingredients. e time and temperature for food processing depend not only
on the chemical compo sition of food but on its microbial load and the type of packaging to be used.
e growing public demand for meals away from home has made the problem of serving safe
wholesome food more critical and challenging. is makes it imperative for food handlers to understand
and implement the basic principles of food science to enable them to prepare and serve high quality
products over extended meal hours.
NEED FOR CONVENIENCE FOODS
Rapid urbanization and changes in social and cultural practices have modied the food habits of the
community. Industrial development in Indian cities has compelled labour from villages to migrate to
cities in search of employment. It is estimated that within the next ve years, half the world’s population
will be living and working in urban areas. Increase in buying power and long hours spent away from
home commuting to work places, make convenience foods a necessity in every home.
e ever-increasing market for convenience foods, be it tinned, canned, chilled, frozen, or preserved,
presents a whole array of complex operations in food processing. is weaning away from the traditional
fare of yesteryears provides a tremendous and urgent challenge to the food industry—serving safe,
attractive, and nutritious food that is wholesome and bacteriologically safe, at the same time conforming
with quality standards.
e urban workforce does not have the time or inclination to follow the traditional recipes and
prefers picking up packed, clean, and rea sonably priced meals rather than returning home from work
and doing domestic chores.
Most food consumed in developed countries is in the formof convenience foods. Convenience foods
are foods that require little labour and time to prepare. A packet of frozen green peas is a convenience
food since it requires no shelling. A packet of whole wheat our is also a convenience food as it has
already been milled.A packet of instant idli mix is more of a convenience food; with ‘ready toeat’ or
‘heat and eat’ foods, such as chicken keema matar or canned palak paneer, are most convenient since
they need no further cooking.
Many dierent types of convenience foods are available in the market today. e speed and eciency
of cooking and service increases dramatically with the use of convenience foods, giving the caterer,
homemaker, or working profess ional more time to devote to other activities. e convenience food
revolution is possible because of a wide variety of chemicals which are added to food not only to
preserve it but to enhance its overall quality. ese numerous chemicals, tested and permitted by law to
be added to food, are called food additives.
Today, convenience foods are being specially packed for caterers and are available in large catering
packs. Manufacturers of specialized food supplies pack food so that it ts into standard catering
equipment, e.g., catering packs that t into vending machines. e caterer can choose between smaller
packs or larger packs that are economical.
Convenience foods need to be handled with care because one source of infection can contaminate
thousands of pre-packed items. Take-away meals should not be kept for a long time, hygiene should be
practised in processing plants, and time and temperature control should be observed during storage.
Leftover contents in large catering packs should not be stored in the open.
© Oxford University Press. All rights reserved.
Oxford University Press

Introduction to Food Science 5
Convenience foods help by saving considerable time and eort. However, the cost of convenience
foods compared to home-prepared foods should be considered before purchase. Some foods may not
be costlier while others may work out to be expensive. For people who have to rush home from work
and prepare a meal, such foods purchased on the way home or stacked in the deep freezer are not only
time-saving but also convenient.
Convenience foods vary widely in their palatability, nutrient content, and cost. e consumer can
choose from a bewildering display of snacks, soups, sauces, fruit chunks and juices, desserts, meat, and
vegetable preparations and gravies in the ready-to-eat and ready-to-cook form. Some only need to be
warmed up in a microwave oven before they are served.
Canned foods, commercially prepared chapattis, snacks both sweet and savoury, main course,
vegetable preparations, soups, gravies, sauces, breakfast cereals, bakery items, deep frozen foods, dry
ready mixes, etc., are not only time saving but convenient to cook and store as well.
us, food science covers all aspects of food, from the properties of food materials and inuences
of all factors aecting food, beginning from growing the food to harvesting or slaughter, i.e., all stages
from the farm to the table, from raw food till it is consumed such as processing, nutritive value, shelf
life, novel sources of food, fabri cated food and food analogs, conservation and reuse of resources to
make more food.
A study of food science, food safety, and nutrition will be of benet to all food professionals.
Let us begin by understanding the basic concepts of food science.
FOOD SCIENCE CONCEPTS
Food science concepts are discussed in the following sections.
BASIC SI UNITS OF LENGTH, AREA, VOLUME, AND WEIGHT
Weights and measures are set standards which are used to nd the size of substances. To obtain a high-
quality product and carry out a protable business, accurate weighing and measuring of all ingredients
is essential.
e SI or International System of measurement is used universally for measurement of matter.
In this system, prexes such as ‘deci’, ‘centi’, and ‘milli’, and units such as ‘litre’, ‘gram’, ‘metre’, and
derived units such as ‘joule’ and ‘pascal’ are used.
Prexes represent numbers or numerical quantities symbolized by letters.
mega = M = 1,000,000 = one million
kilo = k = 1,000 = one thousand
deci = d = 1/10 = one tenth
centi = c = 1/100 = one hundredth
milli = m = 1/1,000 = one thousandth
micro = m = 1/1,000,000 = one millionth
Measurement of Length
e unit for measuring length is the metre (m).
Length is measured using a measuring tape or ruler.
One thousand metres (1,000 m) = one kilometre (km)
A metre is divided into hundred parts. Each part is called a centimetre (cm).
1 metre (m) = 100 centimetres (cm)
© Oxford University Press. All rights reserved.
Oxford University Press

6 F ood Science and Nutrition
Each centimetre is made up of ten smaller parts called millimetre (mm).
1 centimetre = 10 millimetres (mm)
e simplest instrument for measuring length is a scale/ruler measuring one metre, or a measuring
tape.
Measurement of Volume
Volume and capacity is measured in litres. A litre is made up of 10 decilitres (dl). Each decilitre is made
up of 10 centilitres (cl). A centilitre is made up of 10 millilitres (ml), which means that a litre is made
up of one thousand millilitres (1,000 ml).
Most measuring cups and jugs are marked in millilitres and litres. e capacity of cups and spoons
is listed below.
1 tablespoon = 15 ml
1 teaspoon = 5 ml
1 breakfast cup = 240 ml
1 coee cup = 100–120 ml
1 teacup = 150–180 ml
1 water glass = 280–300 ml
e volume of solids that is not greatly aected by water can be measured by the water displacement
method. Solids are immersed in the displacement can and the volume of water displaced, equal to the
volume of the solid, is noted.
e seed method is used to measure the volume of cake and bread. A large tin box is lled to the
brim with seeds and the volume of seeds required to ll the box is measured in a measuring cylinder.
e cake, whose volume is to be measured, is placed in the empty tin and covered with seeds. e
volume of seeds remaining after covering the cake is equal to the volume of the cake.
Measurement of Weight or Mass
Weight is the pull experienced on the body by the earth’s force of gravity. Mass is the amount of matter
contained in a known volume of substance. Mass always remains constant but weight may change in
dierent parts of the world because the force of gravity varies from place to place.
Weight is measured on a weighing scale (see Fig.
1.1). e kilogram is the unit for measuring weight
and is made up of one thousand smaller parts called
grams.
1 kilogram (kg) = 1,000 grams (g)
Each gram is further divided into one thousand
smaller parts called milligrams (mg).
1 g = 1,000 mg
Each milligram is further divided into 1,000 mi-
crograms (mg).
1 mg = 1,000 mg
From the above we conclude that
1 kg = 1,000,000 mg and a measure of 1 ppm
means 1 mg in 1 kg of a substance.
10 kg
Adjustment
knob
Pointer
Measuring pan
Fig. 1.1 Single pan weighing scale
© Oxford University Press. All rights reserved.
Oxford University Press

Introduction to Food Science 7
DENSITY
Density is the relationship between the weight and volume of a substance expressed as
Density =
weight in kg
volume in m
2
It is expressed in kilograms per cubic metre and is used to compare the heaviness or lightness of
dierent foods.
A fruit cake has a greater density as compared to a sponge cake. e density of liquids is measured
in g/cm
3
. Water has a density of 1 g/cm
3
.
Relative Density
Relative density (RD) is the ratio of the mass of a known volume of a substance to the mass of the same
volume of water. It tells us the number of times the volume of a substance is heavier or lighter than an
equal volume of water. If the RD of a volume of lead is 11, it means that it is eleven times as heavy as
an equal volume of water.
Relative density =
mass or weight of a substance
weight of equal volume of wateer
A hydrometer is used to measure the relative density of dierent liquids. It is made up of a weighted
bulb with a graduated stem calibrated to measure the relative density of the liquid directly. e liquid
is kept at room temperature and the hydrometer is allowed to oat in the liquid. e depth to which
it sinks is read on the grad uated stem. Hydrometers are specically calibrated to measure the RD of
dierent liquids used in the catering industry.
Saccharometers are used to determine the concentration of sugar solutions, denoted in degrees Brix.
A 75 per cent sugar solution is called 75 degrees Brix.
Salinometers are used to determine the RD of
brine or sodium chloride solutions used for can-
ning vegetables or pickling ham.
Lactometers are used for checking the purity
of milk. Addition of water or removal of cream
aects the RD and is depicted on the graduated
scale on the stem. e scale is marked 1.00 to
1.04. ‘W’ denotes RD of water, ‘M’ denotes pure
milk, and ‘S’ denotes skim milk.
Alcoholometers are used to test the RD of alco-
holic beverages. It is used to check the number of
degrees proof or ethanol content of wines, beers, and
spirits, and whether it has been diluted.
Refractometers (see Fig. 1.2) are used to meas-
ure the sugar or total solids in solution (TSS)
while preparing jam, syrups, etc. ey measure
the refractive index of light reected through the
solution.
Observe
Place sample
here
50
30
10
40
20
0
Refractometer reading 28° Brix
Fig. 1.2 A refractometer
© Oxford University Press. All rights reserved.
Oxford University Press

8 F ood Science and Nutrition
Besides checking the purity of milk, ethanol content of alcoholic beverages, strength of salt solution,
and concentration or stage of ‘doneness’ for sugar syrups and preserves such as jam, sauce, and candied
fruit, the other applications of RD are
• testingeggsforfreshnesswheneggsaredippedina10percentsaltsolution,fresheggssinkand
stale eggs oat because of a large air space caused by staling;
• determiningthelightnessofcakes;and
• choosingpotatoesforboilingandfrying.PotatoesthathavealowRDshouldbeboiled,while
those that have a high RD should be baked or fried.
TEMPERATURE
Heat is a form of energy needed to carry out work. Energy is the capacity for doing work. Energy is
present in two forms:
1. Potential energy or stored energy, such as the energy stored in a bar of chocolate
2. Kinetic energy or active energy in motion, such as when a person is walking
Energy is present in many forms. Heat is one form of energy. Solar energy, electrical energy, and
chemical energy are some of the others.
Heat energy is measured in units called joules and the energy present in food is measured in
kilocalories. One kilocalorie is made up of 1,000 calories.
1 kilocalorie (kcal) = 4.2 kilojoules (kj)
1 calorie = 4.2 joules
Temperature refers to the relative hotness or coldness of a substance compared with melting ice at
0 °C and boiling water at 100 °C. ermometers are used to measure temperature.
Temperature is measured either in the Celsius or centigrade scale (°C) or in the Farenheit scale (°F).
Each scale has two xed points:
1. Melting point of ice (0°C or 32 °F)
2. Boiling point of water (100 °C or 212 °F)
e Celsius scale is divided into 100 degrees and the Farenheit scale into 180 degrees. e Celsius
scale is the international scale.
Conversion of Farenheit Scale to Celsius Scale
To convert temperature in °F into °C, the following formula is used
(°F – 32) ¥
()∞- ¥=∞FC32
5
9
= °C
To convert 212 °F into °C
(212 °F – 32) ¥
5
9
=180 ×
5
9
20
1
= 20 ¥ 5 = 100 °C
so 212 °F = 100 °C
Conversion of Celsius Scale to Farenheit Scale
To convert temperatures in °C to °F, the following formula is used.
°C ¥
9
5
+ 32 = °F
© Oxford University Press. All rights reserved.
Oxford University Press

Introduction to Food Science 9
To convert 37 °C into °F
37 ¥
9
5
+ 32 = °F
37 °C = 98.6 °F
e conversion of imperival units to metric equivalents is given in Table 1.1.
Table 1.1 Conversion of Imperial units to metric equivalents
Non-metric units Metric units
Length 1 inch (in) 2.5 centimetres (cm)
1 foot (ft ) 30.5 centimetres (cm)
39.4 inches (in)
100 centimetres (cm) or 1 metre
1 mile 1.6 kilometres
Volume 1 pint 568 millilitres (ml)
1 gallon 4.5 litres (l)
1.8 pints 1 litre (l)
Weight 1 ounce (oz.) 28.4 grams (g)
1 pound (lb) 454 grams (g)
2.2 pounds (lb) 1 kilogram (kg)
Energy 1 kilocalorie (kcal) 4.2 kilojoules (kJ)
1 calorie (cal) 4.2 joules ( J)
Temperature 32°Fahrenheit (F) 0°Celsius (C)
212°Fahrenheit (F) 100°Celsius (C)
Area 1 square inch (sq. in) 6.45 square centimetres (sq. cm)
1 square foot (sq. ft) 929 sq. cm
1 square mile 2.59 sq. km
TYPES OF THERMOMETERS
Most thermometers (see Fig. 1.3) are mercury in glass thermometers with dierent temperature ranges
depending on their purpose. Some common thermometers are listed below:
1. Sugar or confectionery thermometers (40°C to 180 °C)
2. Dough testing thermometers (10°C to 43 °C)
3. Meat thermometers with a special spike which can be pierced into meat and a round dial to record
temperature, also called probe thermometers
4. Refrigeration thermometers lled with red coloured ethanol (–30°C to –100°C)
POTENTIAL HYDROGEN or pH
When an acid is diluted with water, it dissociates into hydrogen ions and acid radical ions.
HClHCl
Hydrochloric acid
Hydrogen ion
Chloride ion
acid radic
=+
+-
( al)
© Oxford University Press. All rights reserved.
Oxford University Press
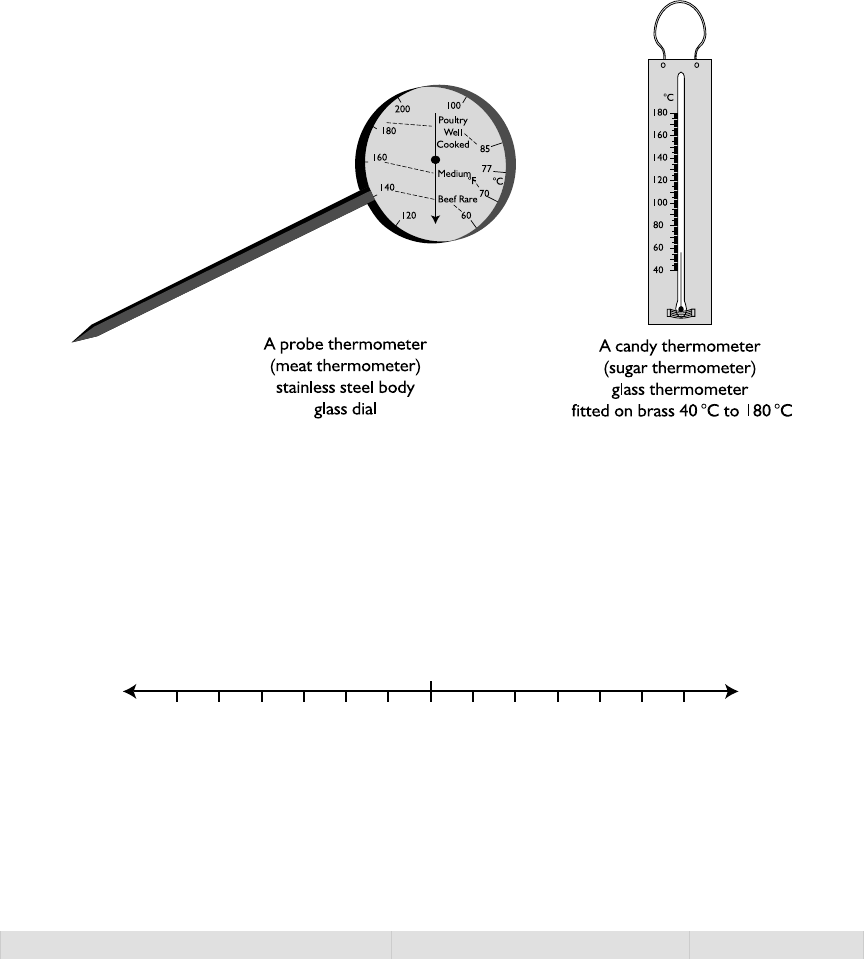
10 Food Science and N utrition
Fig. 1.3 Thermometers
e term pH (hydrogen ion concentration) is used to express the degree of acidity or alkalinity of a
food. It is dened as the negative logarithm to base 10 of the hydrogen ion concentration, i.e., higher
the hydrogen ion concentration, lower will be the pH and vice versa. Some foods, such as fruits, contain
organic acids and have an acid reaction while others such as milk are neutral. Bakery products leavened
with baking powder, have an alkaline reaction. Pure water is pH 7 or neutral.
e pH scale of pH 0 and pH 14 (see Fig. 1.4), i.e., from extremely strong acids to extremely strong
alkali is used to describe the acidity or alkalinity of food.
012345678910 11 12 13 14
NeutralAcid Alkali
Extremely
strong acid
Weak
acid
Weak
alkali
Extremely
strong alkali
Fig. 1.4 The pH scale
A reading between pH 1 and pH 6.5 indicates acidic food while between pH 7.5 and pH 14 indicates
alkaline food (see Table 1.2). e pH of a solution can be measured electrically using the pH meter or it
may be measured colorimetrically using pH papers which change colours according to the pH.
Table 1.2 pH values of some common foods
pH pH value Food
Strongly acidic 2.0
2.3
2.7
3.0
3.7
Vinegar
Lime juice
Pickles
Apples
Orange juice
Contd
© Oxford University Press. All rights reserved.
Oxford University Press

Introduction to Food Science 11
pH pH value Food
Mildly acidic
4.0
4.3
4.6
5.0
5.4
5.5
6.0
6.2
6.4
6.5
Fruit cake
Tomato
Banana
Bread
Spinach
Potatoes
Peas
Butter and Chicken
Salmon
Milk
Neutral 7.0 Chocolate
Mildly alkaline 8.0 Egg white
9.0 Soda bread
When hydrogen ions (H
+
) or hydroxide ions (OH
–
) are added, they can be absorbed by these systems
without altering the pH of the resulting solution.
Common buers are of two types:
1. Acetic acid and sodium acetate mixture
2. Citric acid and sodium citrate mixture
Buering action is very important in the human body and in food. e salts of calcium, phosphorus,
sodium, and potassium function as buers and maintain the pH of milk at a constant level of 6.5.
Applications of pH
1. Preparation of jam—e pectin in jam and marmalade does not form a gel until the pH is lowered
to 3.5. If fruit used for making these preserves does not contain sucient acid, small amounts of
citric acid should be added.
2. Retaining bright green colour in green vegetables—Green vegetables tend to get discoloured when
cooked. Green colour can be retained by adding a pinch of sodium bicarbonate to the cooking
liquor but B complex vitamins and vitamin C gets destroyed in an alkaline medium.
3. Food digestion—pH of the gastrointestinal juices aects our digestive process. e pH of gastric
juice is strongly acidic, between 1 and 2, and aids in digestion of food in the stomach while a
mildly alkaline pH, between pH 7 and pH 8, is needed to complete digestion in the intestine.
4. Texture of cakes—A signicant change in texture is observed with a change in pH while baking
cakes. Low pH gives a ne texture and high pH gives a coarse texture to the cake crumb.
5. pH of dough—In bread making, compressed yeast is used for fermentation. During fermentation,
yeasts convert simple sugars to ethyl alcohol and carbon dioxide.
(a) Ethyl alcohol takes up oxygen and forms acetic acid
(b) Carbon dioxide dissolves partially in water to form carbonic acid
(c) Chemical yeast food, i.e., ammonium sulfate and ammonium chloride if used produce
sulphuric acid and hydrochloric acid, respectively.
Table 1.2 Contd
© Oxford University Press. All rights reserved.
Oxford University Press

12 Food Science and N utrition
All these acids lower the pH of the dough from pH 6.0 to pH 4.5. is change in pH makes the
dough less sticky and more elastic.
IMPORTANT TERMINOLOGIES, THEIR DEFINITION, AND RELEVANCE
Boiling Point
Boiling is the use of heat to change a substance from a liquid to a gas. e change takes place throughout
the body of the liquid at a denite temperature.
Like the melting point, the boiling point of a pure substance is always constant. It changes if
impurities or dissolved substances are present or by changes in atmospheric pressure. Pure water boils
at 100 °C.
Applications of Boiling Point
1. Boiling vegetables in salted water increases the boiling point above 100°C.
2. In sugar cookery, the boiling points of sugar solutions is noted at various stages so that fondant,
fudge, toee, and caramel can be prepared.
Boiling under pressure When atmospheric pressure is low-
ered, water boils at a lower temperature of 70 °C. At hill sta-
tions, the atmospheric pressure is low so temperature is also
lower and food takes longer time to cook. When pressure is
increased, e.g., below sea level or boiling in a pressure cook-
er, water boils at higher temperatures and cooks food faster.
Applications of Boiling under Pressure
1. Food is cooked in pressure cookers to reduce cooking
time to one-fourth of ordinary cooking time as water
boils at a higher temperature under pressure.
2. Autoclaves are used for sterilization by moist heat
under pressure at 121 °C and 15 lb pressure for 20
minutes (see Fig. 1.5).
Evaporation
Evaporation is a change of state from liquid to gas which takes place continuo usly from the surface of
a liquid.
Volatile liquids vaporize easily, e.g., petrol and acetone.
Non-volatile liquids, such as oils, evaporate very gradually. Evaporation is faster when there is breeze
and low humidity in the air as well as a large surface area and high temperature.
Applications of Evaporation
1. Bread and cake if left uncovered, hardens and becomes stale because of loss of moisture. is can
be prevented by storing food in covered tins.
2. Cooking in shallow uncovered pans will cause greater evaporation and are used for preparing
mawa from milk.
Water
Perforated
stand
Gasket
Clamps
Pressure gauge
(15 lb pressure)
Handle
Te mp 121 °C
Operating valve
Electric coil
Fig. 1.5 An autoclave
© Oxford University Press. All rights reserved.
Oxford University Press

Introduction to Food Science 13
3. Milk powder is prepared by dehydration or spray drying in which water from milk is removed by
circulating hot air.
Melting Point
Melting or fusion is the change of state from a solid to a liquid.
e temperature at which a solid melts and turns into a liquid is called its melting point. e melting
point of fats depends on the percentage of saturated long chain fatty acids present in it.
e melting point for any chemical is xed and is used to measure the purity of a substance. It is
lowered by adding other substances.
Melting point of fats
Vanaspati 37–39 °C
Butter 36 °C
Lard 44 °C
Tallow 48 °C
Coconut oil 26 °C
Applications of Melting Point
1. Ice has a melting point of 0°C. If adequate sodium chloride is added to ice, the melting point falls
to –18 °C. is lowering of melting point is used in the setting of ice cream.
2. Fat is removed from adipose tissue of animals by a process called rendering, which is based on the
melting point. Boiling water or dry heat is used to liberate the oil from the fat cells.
Corn oil temperatures
Frying 180–195°C
Smoke point 232°C
Flash point 330 °C
Fire point 363°C
Smoke point When fats and oils are heated strongly above frying temperature, they decompose and
a stage is reached at which it emits a visible thin bluish smoke. is temperature is called the smoke
point (see Table 1.3).
e temperature varies with dierent fats and
ranges between 160 °C and 260 °C. e bluish vapour
is because of the formation of acrolein from overheated
glycerol. Acrolein has an acrid odour and is irritating
to the eyes. e eect of high temperature on fat is
shown in Fig. 1.6.
e smoking point is lowered by the following factors:
1. Presence of large quantities of free fatty acids
2. Exposure of large surface area while heating
3. Presence of suspended food particles
Table 1.3 Smoke point of some common fats
Oil Smoke point (°C)
Corn oil 232
Cotton seed 236
Soya bean 243
Ground nut 243
Butter 201
Lard 222
Beef dripping 163
© Oxford University Press. All rights reserved.
Oxford University Press

14 Food Science and N utrition
high temperature
3 R COOH +
Free fatty acids Glycerol
CH
2
OH
CHOH
CH
2
OH
heat to
smoke
point and
above
2H
2
O
CH
2
.CH.CHO
Water
Acrolein
CH
2
O. CO.R
Fat or oil
CH
2
O. CO.R
CHO. CO.R
+
Fig. 1.6 Effect of high temperature on fat
Flash point is is the temperature at which the decomposition products of fats and oils can be
ignited, but will not support combustion. e ash point varies with dierent fats and ranges between
290 °C and 330 °C.
Fire point is is the temperature at which the decomposition products of fats and oils support
combustion. It ranges between 340 °C and 360 °C for dierent fats. e oil or fat may catch re and burn.
e smoke point, ash point, and re point are lowered by the presence of free fatty acids.
Normal frying temperature for most oils is 180–195 °C. e smoke point is25–40 °C above normal
frying temperature. e application of smoke point is in frying foods. Fats and oils used for deep fat
frying should have a high smoke point. Moist foods should be coated well before frying as moisture
present in food tends to hydro lyse the fat and increase the free fatty acids present.
Surface Tension
Surface tension is a force experienced on the surface of a liquid. It is caused by cohesion, i.e., a force
that causes the molecules of a substance to be attracted to one another.
e molecules of a liquid that are below the surface are pulled by cohesive forces from all directions.
However, the molecules at the surface behave dierently because they are only pulled downwards or
sideways. is downward or sideways attraction causes a constant pull on the surface molecules which
makes the liquid behave as if it is covered by a thin elastic lm. For example, the surface of water can
support needles if they are placed carefully.
Due to surface tension, drops of liquid take a spherical shape, which has the smallest possible surface
area, e.g., dew drops.
Surface tension causes liquids to rise in a thin tube (capillary tube) when the tube is dipped in liquid.
is property of liquids is important in many food systems and in the action of detergents.
Surface tension is also dened as the force of attraction which exists between liquid and solid surfaces.
Applications of Surface Tension
1. Addition of detergent to liquids reduces the surface tension of water and the surface attraction
between the bre and greasy stain, and allows the soil to be removed from the fabric.
2. Release agents help prevent the paper lining the tin from sticking to the cake. ey contain silicone
compounds.
3. Silicones have a property of lowering the surface tension and is added to wood polishes to allow
the polish to spread easily.
4. Non-stick cookware is coated with polytetrauoroethane plastic or silicone to prevent attraction
between the food and pan.
© Oxford University Press. All rights reserved.
Oxford University Press
Introduction to Food Science 15
Osmosis
Osmosis is the passage of water from a weak solution to a stronger solution through a semi-permeable
membrane.
When raisins are soaked in a cup of water for sometime, the raisins swell because water from the cup
enters the raisins. Similarly, if raisins are placed in a concentrated sugar solution, they shrivel up after
some time because water from the raisins passes into the sugar solution because of osmosis.
Plant and animal cell membranes act as semi-permeable membranes and selectively permit water and
electrolytes to enter or leave the cell.
Applications of Osmosis
1. Osmosis plays an important role in food processing and preservation to retain the original shape
and size of canned fruits in syrup and of vegetables in pickles.
2. e freshness of fruits and vegetables depends on the osmotic pressure in the cells. Salads lose their
crisp crunchy texture and become limp if salt and sugar is sprinkled much in advance. Lettuce
leaves can be revived by immersing them in chilled water.
Humidity
Humidity refers to the presence of water vapour in the air. Water vapour is produced by respiration of
plants and animals, evaporation from food during cooking and from water bodies, from rain during
the monsoons, etc.
In catering establishments, moisture in the air is quite high because of large volumes of steam from
boilers, from cooking food, from dishwashers and laundry processes, and respiration and perspiration
of people in a conned area.
A humid atmosphere causes discomfort, headache, and tiredness.
e humidity of the air is measured with the help of a hygrometer. is instrument depicts the
percentage of water vapour in the air. It is a ratio between the amount of water vapour which air could
hold and what it actually holds at the same temperature. Humidity of 60–70 % is considered normal
and does not cause discomfort or undue spoilage of food.
Applications of Humidity
1. Spoilage organisms multiply and spores germinate at high moisture levels in the atmosphere.
2. Humidity needs to be controlled in air-conditioned rooms along with ventilation and heating
which is done by humidier water sprays which maintain 60–70 % humidity.
3. Processed foods are prevented from drying up by adding substances with hygroscopic properties
called humectants. Glycerine and sorbitol are used as humectants in jam.
FOOD RHEOLOGY
It is the science of measuring forces, which are needed to deform food materials or to study the ow
properties of liquid foods. It deals with the viscous behaviour of a system.
Solid food can be chopped up, ground, minced, sliced, torn apart, or broken while it is being
prepared or eaten. e texture is determined when we chew food and it is described as crisp, tough,
chewy, creamy, sticky, spongy, etc.
© Oxford University Press. All rights reserved.
Oxford University Press

16 Food Science and N utrition
Liquid foods are uid or viscous. Viscosity is dened as the resistance of a liquid to ow. It is
measured by an instrument called a viscometer. is property of a liquid is seen in batters, sauces,
syrups, etc.
Compression It is the pressure needed to squash foam or spongy foods to nd out their freshness or
tenderness. e compressimeter or tenderometer is used to measure the lightness of a product.
Adhesion Adhesive gum-like properties give stickiness to food which sticks to the teeth when chewed,
such as toee. Breaking strength of dry foods, such as spaghetti, biscuits, and potato wafers, are
measured by applying a load till the product breaks.
Shearing It is the force needed to cut or slice through meat, vegetables, fruits,etc., and indicates the
toughness of a food. Penetrometers measure the force needed to penetrate a food such as jelly, cooking
fat, canned and fresh fruits, and vegetables.
Rigidity It is the property of those substances which do not ow, e.g., bakedcustard and cake. Rigid
substances show either elastic property or plastic property.
Elastic substances ese substances do not ow, but ow when force is applied. However, when the
force is removed it regains its original shape, e.g., sponge cake.
Elasticity It is the property which permits a substance to change its shape when a force is applied to it
and to come back to its original shape once the force is removed, provided the force applied is within
elastic limits.
Applications of Elasticity
1. e stretching power of the dough can be tested before baking. e extensibility of our is due to
gluten formed in our. Over kneading of dough results in decreased elasticity.
2. Dough improvers are chemicals added to improve or strengthen the elasticity of bread dough.
3. Addition of malt our gives a softer-textured dough because of the enzymes present in malt.
Plastic substances ese substances resist ow to a certain point, but beyond that point they ow, i.e.,
they become plastic in nature.
Plasticity is an important property of margarine. A plastic fat is one which can be creamed as well as
forms a thin sheet or layer in dough when the dough is rolled out, e.g., aky pastry.
SUMMARY
The food industry is a fast-growing industry that applies
the principles of food science and technology to offer the
consumer a wide array of fresh and processed foods to
meet their nutritional needs, wants, and budget. These
foods are available under different brand names, all year
round in delectable flavours and assor ted preparations.
The aesthetic value of food is an important criterion in its
acceptability. Every food handler should be aware of the
composition, structure, and behaviour of food and what
happens to it during processing and after consumption.
The syste matic study of food is called food science. All
foods are chemical compounds and undergo physical as
well as chemical changes. The various food systems are
of colloidal dimensions and various physical conditions,
such as temperature and pressure, affect its quality.
Food science is intimately related to food chemistry,
food microbiology, and food processing. To understand
this, the basic concepts of physics, chemistry,
mathematics, and biology are necessary.
The growing demand for meals away from home has
made the problem of ser ving safe and wholesome food
critical and challenging. There is a shift in focus from
© Oxford University Press. All rights reserved.
Oxford University Press

Introduction to Food Science 17
REVIEW EXERCISES
1. What changes has the food industry witnessed in the
last century?
2. Why is knowledge of the principles of food science
necessary for a catering professional?
3. What do you understand by the term convenience
foods? What foods does it include? Give suitable
examples from your daily life.
4. Do you think convenience foods are necessary? Justify
your answer giving suitable examples.
5. Define the following terms:
(a) Viscosity
(b) Osmosis
(c) pH
(d) Smoke point
(e) Relative density
6. Give scientific reasons why:
(a) Food takes longer time to get cooked at high
altitudes
(b) Fat used for deep fat frying should have a high
smoke point
farm-grown fresh foods to partially or totally processed
convenience foods. These foods require little labour
and time to prepare and are useful to caterers and
homemakers. The shelf life and acceptability of these
foods are enhanced by the use of permitted additives.
A knowledge of basic physical, chemical, and biological
sciences is needed by all students studying catering.
Today, the SI or International System of measurement
is used universally for measuring matter. The unit for
measuring length is the metre and for volume, it is the
litre. Weight is measured in kilograms and may change
from place to place because of the force of gravity or
the pull of the earth. The hydrometer is used to measure
the relative density of different liquids and is specifically
calibrated to measure the relative density of different
substances. The lactometer is used to test the purity
of milk, the saccharometer is used to measure the
concentration of sugar solutions, alcoholometers are
used to check the degrees proof, and salinometers to
check the relative density of brine.
Temperature is measured in degrees Farenheit and
degrees Celsius, the potential hydrogen (pH) is used
to express the degree of acidity or alkalinity of a food.
Many terminologies are relevant and need to be
known and their applications understood by the caterers.
KEY TERMS
Acrolein A substance formed when glycerol from
fat is heated at high temperatures which is
irritating to the eyes and the respiratory tract.
Additives
All material added to food to improve
its shelf life, colour, flavour, texture, taste, and
quality such as flavouring agents, antioxidants, and
preservatives.
Convenience foods
Processed foods in which much
pre-preparation/preparation has already been
done by the manufacturer, e.g., frozen green
peas, breakfast cereals, and canned foods.
Dry ice
Solid carbon dioxide having temperature
of –79°C and used to refrigerate foodstuffs being
transported.
Food microbiology A study of bacteria, yeasts, and
moulds, and their harmful and useful effects on
food and its consumption.
Food science
A study of the physical and chemical
constituents of food and the scientific principles
underlying their modification, preservation, and
spoilage.
Food technology
Application of the principles of
food science to the preservation, processing,
pack aging, storage, and transportation of food
materials.
Hygroscopic
Readily absorbing water, such
substances are used as drying agents, e.g., silica
gel and calcium chloride.
Relative humidity
Method of measuring the
moisture present in air relative to saturation at
the same temperature.
Rendering
The process of removal of fat from the
fat cells of adipose tissue of animals by dry heat
method.
Silicone
Organic compounds of silicon used on non-
sticking wrapping paper.
© Oxford University Press. All rights reserved.
Oxford University Press
18 Food Science and N utrition
(c) The weight of a substance changes when weigh-
ed in different parts of the world
(d) Fresh eggs sink and stale eggs float in water
(e) Small amount of citric acid is added while
making jelly preserve.
7. List the main factors which affect the rate of evapo-
ration.
8. What does surface tension mean? Give two exam-
ples to explain this term.
9. How would you determine the density of a bread
roll?
10. Convert the following measurements:
(a) 2200 kcal into kJ
(b) 37 °C into °F
(c) 90°F into °C
(d) 5 ft 6 inches into cm
(e) 8 ozs into ml
© Oxford University Press. All rights reserved.
Oxford University Press
