gsk.com
Pipeline assets and clinical trials appendix
Q4 2023

Infectious disease
3
Innovation: Pipeline growth
Overview of potential new vaccines and medicines

Infectious disease
4
71 potential new vaccines and medicines in pipeline
Phase III / Registration – 18 assets
Arexvy
(RSV vaccine)
Recombinant protein, adjuvanted*
RSV older adults (50-
59 YoA)^
gepotidacin (2140944)
BTI inhibitor*
Uncomplicated UTI**
bepirovirsen (3228836)
Antisense oligonucleotide*
Chronic HBV infection
**
Bexsero
(MenB vaccine)
Recombinant protein, OMV
Meningitis B (infants US)
MenABCWY vaccine (3536819)
Recombinant protein, OMV, conjugated vaccine
MenABCWY, 1
st
Gen
tebipenem pivoxil (3778712)
Antibacterial carbapenem*
Complicated UTI
ibrexafungerp (5458448)
Antifungal glucan synthase inhibitor*
Invasive candidiasis
Nucala
(mepolizumab)
Anti
-IL5 antibody
COPD
depemokimab (3511294)
Long
-acting anti-IL5 antibody*
Asthma**
latozinemab (4527223)
Anti
-sortilin antibody* Frontotemporal dementia
1
**
camlipixant (5464714)
P2X3 receptor antagonist
Refractory chronic cough
Low carbon version of MDI
2
, Ventolin (salbutamol)
Beta 2 adrenergic receptor agonist
Asthma
3
Ojjaara/Omjjara
(momelotinib)
JAK1, JAK2 and ACVR1 inhibitor
*
Myelofibrosis^
4
Jemperli
(dostarlimab)
Anti
-PD-1 antibody*
Endometrial cancer^**
Zejula
(niraparib)
PARP inhibitor*
Ovarian cancer**
Blenrep
(belantamab mafodotin)
Anti
-BCMA ADC*
Multiple myeloma
cobolimab (4069889)
Anti
-TIM-3 antibody* Non-
small cell lung cancer
linerixibat (2330672)
IBAT inhibitor
Cholestatic pruritus in primary biliary cholangitis
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

Infectious disease
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
5
71 potential new vaccines and medicines in pipeline
3437949
Recombinant protein, adjuvanted*
Malaria fractional dose
4406371
Live, attenuated
MMRV new strain
3536852
GMMA*
Shigella
3528869
Viral vector with recombinant protein, adjuvanted*
Chronic HBV infection
5
**
4023393
Recombinant protein, OMV, conjugated vaccine
MenABCWY, 2
nd
Gen
5
4178116
Live, attenuated
Varicella new strain
5101956
MAPS*
Adult pneumococcal disease, 24-
valent
5101955
MAPS*
Paediatric pneumococcal disease, 24-
valent
4106647
Recombinant protein, adjuvanted*
Human papillomavirus
5
4348413
GMMA
Gonorrhoea
5
4382276
mRNA*
Seasonal flu
4396687
mRNA*
COVID-
19
3993129
Adjuvanted recombinant subunit
Cytomegalovirus
5
3943104
Recombinant protein, adjuvanted*
Therapeutic herpes simplex virus
5
5637608
Hepatitis B virus
-targeted siRNA*
Chronic HBV infection
4077164
Bivalent GMMA
Invasive non-
typhoidal salmonella**
3036656
Leucyl t
-RNA synthetase inhibitor*
Tuberculosis
sanfetrinem
cilexetil (GV118819)
Serine beta lactamase inhibitor*
Tuberculosis
BVL
-GSK098
Ethionamide booster*
Tuberculosis
3810109
Broadly neutralizing antibody*
HIV
3739937
Maturation inhibitor
HIV
4004280
Capsid protein inhibitor
HIV
4011499
Capsid protein inhibitor
HIV
4524184
Integrase inhibitor*
HIV
6
Benlysta
(belimumab)
Anti
-BLys antibody
Systemic sclerosis associated interstitial lung disease
3858279
Anti
-CCL17 antibody*
Osteoarthritis pain**
1070806
Anti
-IL18 antibody
Atopic dermatitis
4527226 (AL
-101)
Anti
-sortilin antibody*
Alzheimer’s disease
6
belrestotug
(4428859)
Anti
-TIGIT antibody* Non-
small cell lung cancer**
4532990
HSD17B13 siRNA*
Non-alcoholic
steatohepatitis
Phase II – 30 assets
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

Infectious disease
6
71 potential new vaccines and medicines in pipeline
Phase I – 23 assets
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
3536867
Bivalent conjugate*
Salmonella (
typhoid + paratyphoid A)
2556286
Mtb
cholesterol dependent inhibitor*
Tuberculosis
3186899
CRK
-12 inhibitor*
7
Visceral leishmaniasis
3494245
Proteasome inhibitor*
Visceral leishmaniasis
3772701
P. falciparum
whole cell inhibitor*
Malaria
4024484
P. falciparum
whole cell inhibitor*
Malaria
3882347
FimH antagonist*
Uncomplicated UTI
3923868
PI4K beta inhibitor
Viral COPD exacerbations
3965193
PAPD5/PAPD7 inhibitor
Chronic HBV infection
5
5251738
TLR8 agonist
*
Chronic HBV infection
cabotegravir (1265744)
Integrase inhibitor
HIV
3888130
Anti
-IL7 antibody*
Autoimmune disease
3915393
TG2 inhibitor*
Pulmonary fibrosis
3862995
Anti
-IL33 antibody
COPD
5462688
RNA
-editing oligonucleotide* Alpha-
1 antitrypsin deficiency
4347859
Interferon pathway modulator
Systemic lupus erythematosus
4381562
Anti
-PVRIG antibody*
Cancer
6097608
Anti
-CD96 antibody*
Cancer
XMT
-2056
9
(wholly owned by
Mersana Theraprutics)
STING agonist ADC*
Cancer
belantamab
(2857914)
Anti
-BCMA antibody
Multiple myeloma
4524101
DNA polymerase theta inhibitor*
Cancer
5
5733584 (HS
-20089)
ADC
-targeting B7-H4*
Gynecologic malignancies
4172239
DNMT1 inhibitor*
Sickle cell disease
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

Infectious disease
7
Infectious diseases pipeline
Phase III / Registration – 7 assets
Arexvy
(RSV vaccine)
Recombinant protein, adjuvanted*
RSV older adults (50-
59 YoA)^
gepotidacin (2140944)
BTI inhibitor*
Uncomplicated UTI**
bepirovirsen (3228836)
Antisense oligonucleotide*
Chronic HBV infection
**
Bexsero
(MenB vaccine)
Recombinant protein, OMV
Meningitis B (infants US)
MenABCWY vaccine (3536819)
Recombinant protein, OMV, conjugated vaccine
MenABCWY, 1
st
Gen
tebipenem pivoxil (3778712)
Antibacterial carbapenem*
Complicated UTI
ibrexafungerp (5458448)
Antifungal glucan synthase inhibitor*
Invasive candidiasis
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. Phase I study start imminent 9. GSK has an exclusive global license option to co-develop and commercialise the candidate
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
3437949
Recombinant protein, adjuvanted*
Malaria fractional dose
4406371
Live, attenuated
MMRV new strain
3536852
GMMA*
Shigella
3528869
Viral vector with recombinant protein, adjuvanted*
Chronic HBV infection
5
**
4023393
Recombinant protein, OMV, conjugated vaccine
MenABCWY, 2
nd
Gen
5
4178116
Live, attenuated
Varicella new strain
5101956
MAPS*
Adult pneumococcal disease, 24-
valent
5101955
MAPS*
Paediatric pneumococcal disease, 24-
valent
4106647
Recombinant protein, adjuvanted*
Human papillomavirus
5
4348413
GMMA
Gonorrhoea
5
4382276
mRNA*
Seasonal flu
4396687
mRNA*
COVID-
19
3993129
Adjuvanted recombinant subunit
Cytomegalovirus
5
3943104
Recombinant protein, adjuvanted*
Therapeutic herpes simplex virus
5
5637608
Hepatitis B virus
-targeted siRNA*
Chronic HBV infection
4077164
Bivalent GMMA
Invasive non-
typhoidal salmonella**
3036656
Leucyl t
-RNA synthetase inhibitor*
Tuberculosis
sanfetrinem
cilexetil
(GV118819)
Serine beta lactamase inhibitor*
Tuberculosis
BVL
-GSK098
Ethionamide booster*
Tuberculosis
Phase II – 19 assets
Phase I – 10 assets
3536867
Bivalent conjugate*
Salmonella (
typhoid + paratyphoid A)
2556286
Mtb
cholesterol dependent inhibitor*
Tuberculosis
3186899
CRK
-12 inhibitor*
7
Visceral leishmaniasis
3494245
Proteasome inhibitor*
Visceral leishmaniasis
3772701
P. falciparum
whole cell inhibitor*
Malaria
4024484
P. falciparum
whole cell inhibitor*
Malaria
3882347
FimH antagonist*
Uncomplicated UTI
3923868
PI4K beta inhibitor
Viral COPD exacerbations
3965193
PAPD5/PAPD7 inhibitor
Chronic HBV infection
5
5251738
TLR8 agonist
*
Chronic HBV infection

Infectious disease
3810109
Broadly neutralizing antibody*
HIV
3739937
Maturation inhibitor
HIV
4004280
Capsid protein inhibitor
HIV
4011499
Capsid protein inhibitor
HIV
4524184
Integrase inhibitor*
HIV
6
8
HIV pipeline
Phase II – 5 assets
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
Phase I – 1 asset
cabotegravir (1265744)
Integrase inhibitor
HIV

Infectious disease
9
Respiratory/Immunology pipeline
Phase III / Registration – 5 assets
Nucala
(mepolizumab)
Anti
-IL5 antibody
COPD
depemokimab (3511294)
Long
-acting anti-IL5 antibody*
Asthma**
latozinemab (4527223)
Anti
-sortilin antibody* Frontotemporal dementia
1
**
camlipixant (5464714)
P2X3 receptor antagonist
Refractory chronic cough
Low carbon version of MDI
2
, Ventolin (salbutamol)
Beta 2 adrenergic receptor agonist
Asthma
3
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
Benlysta
(belimumab)
Anti
-BLys antibody
Systemic sclerosis associated interstitial lung disease
3858279
Anti
-CCL17 antibody*
Osteoarthritis pain**
1070806
Anti
-IL18 antibody
Atopic dermatitis
4527226 (AL
-101)
Anti
-sortilin antibody*
Alzheimer’s disease
6
Phase II – 4 assets
Phase I – 5 assets
3888130
Anti
-IL7 antibody*
Autoimmune disease
3915393
TG2 inhibitor*
Pulmonary fibrosis
3862995
Anti
-IL33 antibody
COPD
5462688
RNA
-editing oligonucleotide* Alpha-
1 antitrypsin deficiency
4347859
Interferon pathway modulator
Systemic lupus erythematosus

Infectious disease
4381562
Anti
-PVRIG antibody*
Cancer
6097608
Anti
-CD96 antibody*
Cancer
XMT
-2056
8
(wholly owned by
Mersana Theraprutics)
STING agonist ADC*
Cancer
belantamab
(2857914)
Anti
-BCMA antibody
Multiple myeloma
4524101
DNA polymerase theta inhibitor*
Cancer
5
5733584 (HS
-20089)
ADC
-targeting B7-H4*
Gynecologic malignancies
Ojjaara/Omjjara
(momelotinib)
JAK1, JAK2 and ACVR1 inhibitor
*
Myelofibrosis^
4
Jemperli
(dostarlimab)
Anti
-PD-1 antibody*
Endometrial cancer^**
Zejula
(niraparib)
PARP inhibitor*
Ovarian cancer**
Blenrep
(belantamab mafodotin)
Anti
-BCMA ADC*
Multiple myeloma
cobolimab (4069889)
Anti
-TIM-3 antibody* Non-
small cell lung cancer
10
Oncology pipeline
Phase III / Registration – 5 assets
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
Phase II – 1 asset
Phase I – 6 assets
belrestotug
(4428859)
Anti
-TIGIT antibody* Non-
small cell lung cancer**

Infectious disease
4532990
HSD17B13 siRNA*
Non-alcoholic
steatohepatitis
11
Opportunity driven pipeline
Phase III / Registration – 1 asset
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
Phase II – 1 asset
Phase I – 1 asset
linerixibat
(2330672)
IBAT inhibitor
Cholestatic pruritus in primary biliary cholangitis
4172239
DNMT1 inhibitor*
Sickle cell disease

Infectious disease
12
Changes since Q3 2023
Changes on pipeline Achieved pipeline catalysts
Regulatory decisions
Nucala – severe asthma CN
Jemperli
1
– RUBY, dMMR/MSI-H 1L endometrial cancer EU
Omjjara: MOMENTUM, myelofibrosis EU
Regulatory submission acceptances
Arexvy – 50-59 YoA
EU, JP
Other events
Blenrep – DREAMM-7, 2L+ MM – Positive headline phase III data
Jemperli
1
– RUBY (Part 2), 1L EC – Positive phase III data readout
New to Phase I
4024484 – P. falciparum whole cell inhibitor, malaria
3862995 – Anti-IL33 antibody, COPD
5462688 – RNA-editing oligonucleotide, Alpha-1 antitrypsin deficiency
4347859 – Interferon pathway modulator, systemic lupus erythematosus
5733584 – ADC targeting B7-H4, gynecologic malignancies
New to Phase II
3943104 – Recombinant protein, adjuvanted, Therapeutic HSV
5637608 – HBV-targeted siRNA sequential combination, chronic HBV infection
4077164 – Bivalent GMMA, Invasive non-typhoidal salmonella**
4524184 – Integrase inhibitor, HIV
New to Phase III
Low carbon version of MDI, Ventolin – Beta 2 adrenergic receptor agonist
, asthma
Removed from Phase I
4429016 – Bioconjugated recombinant protein, adjuvanted, K. pneumoniae
4182137 (VIR7832) – Anti-spike protein antibody, COVID-19
Removed from Phase II
VIR2482 – Neutralising monoclonal antibody, influenza
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
** Additional indications or candidates also under investigation
1. Tesaro asset

Infectious disease
13
Upcoming pipeline catalysts: 2024 and 2025
H1 2024 H2 2024 2025
Regulatory
decision
Ojjaara
/Omjjara: MOMENTUM, myelofibrosis
JP
Arexvy
: 50-59 YoA
10
US, EU, JP
gepotidacin
: EAGLE-2/3, uUTI
11
US
Nucala
: CRSwNP
1
JP
gepotidacin
: EAGLE-1, GC
5
US
MenABCWY
vaccine 1st Gen
US, EU
depemokimab
: SWIFT-1/2, asthma
US
depemokimab
: ANCHOR-1/2, CRSwNP
1
US
Nucala
: CRSwNP
1
CN
Nucala
: MATINEE, COPD
12
US
Blenrep
: DREAMM-7/8, 2L+ MM
7
US, EU, CN, JP
Jemperli
2
: RUBY (Part 1)
3,
1L EC
4
US
linerixibat
: GLISTEN, cholestatic pruritus in PBC
14
US
Regulatory
submission and
acceptance
MenABCWY
vaccine 1st Gen
US
gepotidacin
: EAGLE-2/3, uUTI
11
US
gepotidacin
: EAGLE-1, GC
5
US
Nucala:
CRSwNP
1
CN
MenABCWY
vaccine 1st Gen
EU
tebipenem
pivoxil: PIVOT-PO, cUTI
15
US
Jemperli
2
: RUBY (Part 1)
3
, 1L EC
4
US
depemokimab
: SWIFT-1/2, asthma
US
camlipixant
: CALM-1/2, RCC
16
US, EU
depemokimab
: ANCHOR-1/2, CRSwNP
1
US
Nucala
: MATINEE, COPD
12
EU, CN
Nucala
: MATINEE, COPD
12
US
Blenrep
: DREAMM-7/8, 2L+ MM
7
US, EU, CN, JP
cobolimab
2
: COSTAR, 2L NSCLC
13
US, EU
linerixibat
: GLISTEN, cholestatic pruritus in PBC
14
US, EU, CN, JP
Late
-stage phase
III and phase II
readouts
gepotidacin
: EAGLE-1, GC
5
depemokimab
: ANCHOR-1/2, CRSwNP
1
tebipenem
pivoxil: PIVOT-PO, cUTI
15
depemokimab
: SWIFT-1/2, asthma
Nucala
: MATINEE, COPD
12
camlipixant
: CALM-1/2, RCC
16
Blenrep
: DREAMM-7
6
, 2L+ MM
7
Blenrep
: DREAMM-8, 2L+ MM
7
depemokimab
: OCEAN, EGPA
17
Zejula
2
: FIRST, 1L maintenance OC
8
cobolimab
2
: COSTAR, 2L NSCLC
13
Zejula
2
: ZEAL, 1L maintenance NSCLC
13
mRNA Seasonal flu
9
linerixibat
: GLISTEN, cholestatic pruritus in PBC
14
1. Chronic rhinosinusitis with nasal polyps 2. Tesaro asset 3. Overall population 4. Endometrial cancer 5. Urogenital gonorrhoea 6. Overall survival 7. Multiple myeloma 8. Ovarian cancer 9. Phase II 10. Years of age 11.
Uncomplicated urinary tract infection 12. Chronic obstructive pulmonary disorder 13. Non-small cell lung cancer 14. Treatment of cholestatic pruritus in primary biliary cholangitis 15. Complicated urinary tract infection 16.
Refractory chronic cough 17. Eosinophilic granulomatosis with polyangiitis
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

Infectious disease
14
Designations in our pipeline
Breakthrough Designation
1
5101956
MAPS*
Adult pneumococcal disease, 24-
valent
Qualified Infectious Disease Product Designation
2
gepotidacin
(2140944)
BTI inhibitor*
Uncomplicated UTI and urogenital
gonorrhoea
tebipenem
pivoxil (3778712)
Antibacterial carbapenem*
Complicated UTI
Orphan Drug Designation
5
ibrexafungerp
(5458448) US
Antifungal glucan synthase inhibitor*
Invasive candidiasis
Benlysta
(belimumab) US
Anti
-BLys antibody
Systemic sclerosis associated interstitial lung disease
latozinemab
(4527223) US, EU
Anti
-sortilin antibody*
Frontotemporal dementia
2
depemokimab
(3511294) JP
Long
-acting anti-IL5 antibody* Hypereosinophilic
syndrome
linerixibat
(2330672) US, EU
IBAT inhibitor
Cholestatic pruritus in primary biliary cholangitis
Fast Track
10
4382276
mRNA*
Seasonal flu
BVL
-GSK098
Ethionamide booster*
Tuberculosis
4348413
GMMA
Gonorrhoea
gepotidacin
(2140944)
BTI inhibitor*
Urogenital
gonorrhoea
tebipenem
pivoxil (3778712)
Antibacterial carbapenem*
Complicated UTI
3858279
Anti
-CCL17 antibody*
Osteoarthritis pain
3858279
Anti
-CCL17 antibody*
Diabetic peripheral neuropathic pain
latozinemab
(4527223)
Anti
-sortilin antibody*
Frontotemporal dementia
2
Jemperli
1
(dostarlimab)
Anti
-PD-1 antibody* Neoadjuvant dMMR/MSI-H 1L r
ectal cancer
4172239
DNMT1 inhibitor*
Sickle cell disease
BREAKTHROUGH DESIGNATION (US) – a process designed to expedite the
development and review of medicines intended to treat serious conditions,
where preliminary clinical evidence indicates the drug may demonstrate
substantial improvement over available therapy
FAST TRACK (US) – a program designed to facilitate the expedited
development and review of medicines to treat serious conditions and fill an
unmet medical need
OPHAN DRUG DESIGNATION – intended for treatment, diagnosis or prevention
of rare disease/disorders that affect fewer than 200,000 patients in the US, or
not more than 5 in 10,000 in the EU or that affect more than this number of
patients but are not expected to recover the costs of developing and marketing
a treatment drug, or if intended for use in less than 50,000 patients in Japan and
for which there is a high medical need
QUALIFIED INFECTIOUS DISEASE PRODUCT DESIGNATION (US) – an
antibacterial or antifungal drug for human use intended to treat serious or life-
threatening infections
*In-licence or other alliance relationship with third party
1. Tesaro asset 2. Phase III trial in patients with progranulin gene mutation
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
17
Infectious diseases
Arexvy (RSV Older Adults)
NCT04732871
- RSV OA=ADJ-004
Phase
III
Patient
Adults ≥60 years of age
Subjects
1653
Treatment
arms
Arm A: RSVPreF3 OA Day 1, 12 months & 24 months
Arm B: RSVPreF3 OA Day 1 and 24 months
Arm C: RSVPreF3 OA Day 1 then follow up
Description
A
randomised, open-label, multi-country trial to evaluate the immunogenicity,
safety, reactogenicity and persistence of a single dose of the RSVPreF3 OA
investigational vaccine and different revaccination schedules in adults aged
60 years and above
Timeline
Trial start: Q1 2021
Primary data
reported: Q2 2022
Key end
points
Humoral immune response following a 1 dose primary schedule up to 12 months
post dose 1
Clinicaltrials.g
ov
Link
NCT04886596
- RSV OA=ADJ-006
Phase
III
Patient
Adults ≥60 years of age
Subjects
24,966
Treatment
arms
Arm A: RSVPreF3 OA Lot 1
Arm B: RSVPreF3 OA Lot 2
Arm C: RSVPreF3 OA Lot 3
Arm D: RSVPreF3 OA Lot 4
Arm E: Placebo
Description
A
randomised, placebo-controlled, observer-blind, multi-country trial to
demonstrate the efficacy of a single dose and annual revaccination doses of
GSK's RSVPreF3 OA investigational vaccine in adults aged 60 years and above
Timeline
Trial start: Q2 2021
Primary data reported: Q2 2022; season two data reported Q2 2023
Key end
points
Efficacy of a single dose and annual revaccination doses of RSVPreF3 OA
vaccine in the prevention of RSV
-LRTD in adults ≥ 60 yoa
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
NCT04841577
- RSV OA=ADJ-007
Phase
III
Patient
Adults ≥60 years of age
Subjects
885
Treatment
arms
Arm A: 1 dose of RSVPreF3 OA + 1 dose of FLU
-QIV on Day 1
Arm B: 1 dose of FLU
-QIV on Day 1, 1 dose of RSVPreF3 OA on Day 31
Description
An open
-label, randomised, controlled, multi-country trial to evaluate the
immune response, safety and reactogenicity of RSVPreF3 OA investigational
vaccine when co
-administered with FLU-QIV vaccine in adults aged 60 years
and above
Timeline
Trial start: Q2 2021
Primary data reported: Q4 2022
Key end
points
Humoral immune response 1 month post vaccination upon co
-administration
compared to the immune response when vaccine is administered alone
Clinicaltrials.g
ov
Link
18
Infectious diseases
Arexvy (RSV Older Adults)
NCT05559476
- RSV OA=ADJ-008
Phase
III
Patient
Adults aged 65 years and above
Subjects
1028
Treatment
arms
Arm A: 1 dose of RSVPreF3 OA + 1 dose of Flu
-HD on day 1
Arm B: 1 dose of Flu HD on Day 1 ,1 dose of RSVPreF3 OA on Day 31
Description
An open
-label, randomised, controlled, multi-country trial to evaluate the
immune response, safety and reactogenicity of RSVPreF3 OA investigational
vaccine when co
-administered with FLU HD vaccine in adults aged 65 years
and above
Timeline
Trial start: Q4 2022
Primary data reported: Q2 2023
Key end
points
Humoral immune response 1 month post vaccination upon co
-administration
compared to the immune response when vaccine is administered alone
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
19
Infectious diseases
Arexvy (RSV Older Adults)
NCT05059301
- RSV OA=ADJ-009
Phase
III
Patient
Adults aged 60 years and above
Subjects
770
Treatment
arms
Arm A: 1 dose of a combination of the RSVPreF3 antigen Lot 1 and AS01E
adjuvant Lot A at day 1
Arm B: 1 dose of a combination of the RSVPreF3 antigen Lot 2 and AS01E
adjuvant Lot B at day 1
Arm C: 1 dose of a combination of the RSVPreF3 antigen Lot 3 and AS01E
adjuvant Lot C at Day 1
Description
A
randomised, double-blind, multi-
country trial to evaluate consistency, safety
and reactogenicity of 3 lots of RSVPreF3 OA investigational vaccine
administrated as a single dose in adults aged 60 years and above
Timeline
Trial start: Q4 2021
Trial end: Q2 2022
Key end
points
RSVPreF3
-binding IgG concentrations at 1 month post vaccination for three
lots of RSVPreF3 OA investigational vaccine
Clinicaltrials.g
ov
Link
NCT05568797
- RSV OA=ADJ-017
Phase
III
Patient
Adults aged 65 years and above
Subjects
880
Treatment
arms
Arm A: 1 dose RSVPreF3 OA investigational vaccine and 1 dose of FLU
aQIV
vaccine on Day 1
Arm B: one dose of Flu
aQIV on day 1 and 1 dose of RSVPreF3 OA on day 31
Description
An open
-label, randomised, controlled, multi-country trial to evaluate the
immune response, safety and reactogenicity of an RSVPreF3 OA
investigational vaccine when co
-administered with FLU aQIV (inactivated
influenza vaccine
– adjuvanted) in adults aged 65 years and above
Timeline
Trial start: Q4 2022
Primary data reported: Q2 2023
Key end
points
Humoral immune response 1 month post vaccination upon co
-administration
compared to the immune response when vaccine is administered alone
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
20
Infectious diseases
Arexvy (RSV Older Adults)
NCT05590403
- RSV OA-018
Phase
III
Patient
Adults 50
-59 years of age, including adults at increased risk of respiratory
syncytial virus lower respiratory tract disease, and older adults ≥60 years of
age
Subjects
1520
Treatment
arms
Arm A: adults HA
-RSVPreF3 OA Group
Arm B: adults HA
-Placebo Group
Arm C: adults AIR
-RSVPReF3 OA Group
Arm D: adults AIR
-Placebo Group
Arm E: OA
-RSVPReF3 OA Group ≥60 years of age
Description
An observer
-blind, randomised, placebo-controlled trial to evaluate the non-
inferiority of the immune response and safety of the RSVPreF3 OA
investigational vaccine in adults 50 59 years of age, including adults at
increased risk of respiratory syncytial virus lower respiratory tract disease,
compared to older adults ≥60 years of age
Timeline
Trial start: Q4 2022
Primary data reported: Q4 2023
Key end
points
Humoral immune response in healthy participants 50
-59 years of age and in
participants 50
-59 years of age at increased risk of RSV-LRTD compared to
OA (≥ 60
yoa)
Clinicaltrials.g
ov
Link
NCT05879107
- RSV OA=ADJ-019
Phase
III
Patient
Adults ≥60 years of age
Subjects
1090
Treatment
arms
Arm A (co
-ad group): RSVPreF3 OA investigational vaccine co-administered
with PCV20 vaccine
Arm B (control group): PCV20 vaccine on Day 1 and the RSVPreF3 OA
investigational vaccine on Day 31.
Description
An open
-label, randomised, controlled, multi-country study to evaluate the
immune response, safety and reactogenicity of RSVPreF3 OA investigational
vaccine when co
-administered with PCV20 in adults aged 60 years and older
Timeline
Trial start: Q2 2023
Data anticipated: H2 2024
Key end
points
Opsonophagocytic antibody titers for each of the pneumococcal vaccine
serotypes and RSV
-A & RSV-B serum neutralizing titers
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
NCT05966090
- RSV OA=ADJ-020
Phase
III
Patient
Adults aged 50 years and older
Subjects
530
Treatment
arms
Arm A: Participants will be administered first dose of HZ/
su vaccine and the
RSVPreF3 OA investigational vaccine together on Day 1. A second dose of the
HZ/
su vaccine will be administered at Day 61.
Arm B: Participants will be administered first dose HZ/
su vaccine on Day 1,
followed by the RSVPreF3 OA investigational vaccine on Day 31, and then
second dose of HZ/
su vaccine on Day 61.
Description
An open
-label, randomised, controlled, multi-country study to evaluate the
immune response, safety and reactogenicity of RSVPreF3 OA investigational
vaccine when co
-administered with Herpes Zoster recombinant subunit
(HZ/
su) vaccine in adults aged 50 years and older
Timeline
Trial start: Q3 2023
Data anticipated: H2 2024
Key end
points
Anti
-gE antibody concentrations expressed as group geometric mean
concentration ratio
RSV
-A & -B serum neutralizing titers expressed as group geometric mean titer
Clinicaltrials.g
ov
Link
21
Infectious diseases
Arexvy (RSV Older Adults)
NCT05921903
- RSV OA=ADJ-023
Phase
IIb
Patient
Immunocompromised (IC) adults 50 years of age and above
Subjects
375
Treatment
arms
Arm A: RSV_IC_1 group, IC patients receiving 1 dose of RSVPreF3 OA
investigational vaccine at Visit 1 (Day 1).
Arm B: RSV_IC_2 group, IC patients receiving 2 doses of RSVPreF3 OA
investigational vaccine at Visit 1 (Day 1) and Visit 3 (Visit 1 + 30
-60 days)
Arm C: RSV_HA group, healthy participants receiving 1 dose of RSVPreF3 OA
investigational vaccine at Visit 1 (Day 1).
Description
A
randomised, controlled, open-label trial to evaluate the immune response
and safety of the RSVPreF3 OA investigational vaccine in adults (≥50 years of
age) when administered to lung and renal transplant recipients comparing one
versus two doses and compared to healthy controls (≥50 years of age)
receiving one dose
Timeline
Trial start: Q3 2023
Data anticipated: 2025
Key end
points
RSV
-A & -B serum neutralizing titers expressed as mean geometric increase
post Dose 2 over post Dose 1
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
22
Infectious diseases
gepotidacin
NCT04010539
- EAGLE 1
Phase
III
Patient
Uncomplicated urogenital
gonorrhoea caused by Neisseria gonorrhoeae
Subjects
1531
Treatment
arms
Arm A: 2 x 3000 mg
gepotidacin for one day
Arm B: ceftriaxone (500mg IM), 1 g azithromycin
Description
A
randomised, multicentre, open-label trial in adolescent and adult
participants comparing the efficacy and safety of
gepotidacin to ceftriaxone
plus azithromycin
in the treatment of uncomplicated urogenital gonorrhoea
caused by
Neisseria gonorrhoeae
Timeline
Trial start: Q4 2019
Data anticipated: H1 2024
Key end
points
Number of participants with culture
-confirmed bacterial eradication 4-8 days
post treatment
Clinicaltrials.g
ov
Link
NCT04020341
- EAGLE 2
Phase
III
Patient
Females with
uUTI / acute cystitis
Subjects
1531
Treatment
arms
Arm A: 1500 mg BID
gepotidiacin + placebo x 5 days
Arm B: 100 mg BID nitrofurantoin + placebo x 5 days
Description
A
randomised, multicentre, parallel-group, double-blind, double-
dummy trial in
adolescent and adult female participants comparing the efficacy and safety of
gepotidacin
to nitrofurantoin in the treatment of uncomplicated urinary tract
infection (acute cystitis)
Timeline
Trial start: Q4 2019
Data reported: Q2 2023
Key end
points
Number of participants with therapeutic response (combined per participant
clinical and microbiological response)
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
23
Infectious diseases
gepotidacin
NCT04187144
- EAGLE 3
Phase
III
Patient
Females with
uUTI / acute cystitis
Subjects
1606
Treatment
arms
Arm A: 1500 mg BID
gepotidiacin + placebo x 5 days
Arm B: 100 mg BID nitrofurantoin + placebo x 5 days
Description
A
randomised, multicentre, parallel-group, double-blind, double-
dummy trial in
adolescent and adult female participants comparing the efficacy and safety of
gepotidacin
to nitrofurantoin in the treatment of uncomplicated urinary tract
infection (acute cystitis)
Timeline
Trial start: Q2 2020
Data reported: Q2 2023
Key end
points
Number of participants with therapeutic response (combined per participant
clinical and microbiological response)
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
24
Infectious diseases
bepirovirsen
NCT05630807
- B-WELL 1
Phase
III
Patient
Non
-cirrhotic nucleos(t)ide analogue treated patients with chronic hepatitis B
virus
Subjects
900
Treatment
arms
Arm A:
bepirovirsen for 24 weeks
Arm B: placebo
Description
Phase III
multicentre, randomised
, double blind trial to confirm the efficacy and
safety of treatment with
bepirovirsen in participants with chronic hepatitis B
virus
Timeline
Trial start: Q1 2023
Data anticipated: 2026+
Key end
points
Number of participants achieving functional cure (FC) with baseline HBsAg≤
3000IU/mL
Clinicaltrials.g
ov
Link
NCT05630820
- B-WELL 2
Phase
III
Patient
Non
-cirrhotic nucleos(t)ide analogue treated patients with chronic hepatitis B
virus
Subjects
900
Treatment
arms
Arm A:
bepirovirsen for 24 weeks
Arm B: placebo
Description
Phase III
multicentre, randomised
, double blind trial to confirm the efficacy and
safety of treatment with
bepirovirsen in participants with chronic hepatitis B
virus
Timeline
Trial start: Q1 2023
Data anticipated: 2026+
Key end
points
Number of participants achieving functional cure (FC) with baseline HBsAg≤
3000IU/mL
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
25
Infectious diseases
bepirovirsen
NCT04676724
- B-TOGETHER
Phase
IIb
Patient
Non
-cirrhotic patients with chronic hepatitis B virus on stable nucleos(t)ide
analog therapy
Subjects
108
Treatment
arms
Arm A:
bepirovirsen for 12 wks + PegIFN for =< 24 wks
Arm B:
bepirovirsen for 24 weeks + PegIFN =< 24 wks
Description
A
multicentre, randomised
, open label trial to assess the efficacy and safety of
sequential treatment with
bepirovirsen
followed by Pegylated Interferon Alpha
2a in participants with chronic hepatitis B virus
Timeline
Trial start: Q1 2021
Data reported: Q4 2023
Key end
points
Sustained response for 24 weeks post treatment
Clinicaltrials.g
ov
Link
NCT05276297
Phase
II
Patient
HBV suppressed subjects under
nucleo(s)tide treatment
Subjects
184
Treatment
arms
ASO24
-targeted immunotherapy group (GSK3228836 (24-week treatment)
followed by GSK3528869A)
ASO24 group (GSK3228836 (24
-week treatment) followed by non-active
control)
ASO12
-targeted immunotherapy group (GSK3228836 (12-week treatment)
followed by GSK3528869A)
ASO12 group (GSK3228836 (12
-week treatment) followed by non-active
control)
Description
A single
-blinded, randomised, controlled multi-country trial to evaluate the
safety, reactogenicity, efficacy and immune response following sequential
treatment with an anti
-sense oligonucleotide against Chronic Hepatitis B
(CHB) followed by Chronic Hepatitis B Targeted Immunotherapy (CHB
-TI) in
CHB patients receiving nucleos(t)ide analogue (NA) therapy
Timeline
Trial start: Q2 2022
Data anticipated: 2025
Key end
points
Number of subjects reporting local and general AEs and percentage of
participants with sustained virologic response
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
26
Infectious diseases
MenABCWY
NCT04707391
- MenABCWY-019
Phase
IIIb
Patient
Healthy adolescents and adults aged 15
-25 years
Subjects
1250
Treatment
arms
Arm A: 2 doses of MenABCWY days 1, 181 + placebo day 211
Arm B: 1 dose MenABCWY day 1; 2 doses of
MenB on Day 181 and Day 211
Description
A
randomised, controlled, observer-blind trial to evaluate safety and
immunogenicity of GSK’s meningococcal ABCWY vaccine when administered
in healthy adolescents and adults previously primed with meningococcal
ACWY vaccine
Timeline
Trial start: Q1 2021
Data reported: Q4 2023
Key end
points
hSBA
titers
Clinicaltrials.g
ov
Link
NCT04502693
- MenABCWY V72 72
Phase
III
Patient
Healthy adolescents and adults ages 10
-25 years
Subjects
3657
Treatment
arms
Arm A:
rMenB+OMV NZ (2/3 dose schedule) plus MenACWY
Arm B:
rMenB+OMV NZ (2 dose schedule) plus MenACWY plus placebo
Arm C: placebo + MenABCWY lot 1
Arm D: placebo + MenABCWY lot 2
Arm E: placebo + MenABCWY lot 3
Arm F:
rMenB+OMV NZ + MenACWY + placebo
Description
Effectiveness of GSK Biologicals S.A.'s Meningococcal Group B and combined
ABCWY vaccines in healthy adolescents and young adults
Timeline
Trial start: Q3 2020
Data reported: Q1 2023
Key end
points
hSBA
titers
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
27
Infectious diseases
MenABCWY
NCT05087056
- MenABCWY-020
Phase
IIb
Patient
Healthy adolescents ≥11 to <15 years of age
Subjects
300
Treatment
arms
Arm A: ABCWY
-24 Group
Arm B: ABCWY
-48 Group
Description
A
randomised, observer-blind trial to describe the safety, tolerability and
immunogenicity of MenABCWY administered on different dosing schedules in
healthy adolescents
Timeline
Trial start: Q4 2021
Data anticipated: 2026+
Key end
points
hSBA
titers ≥ LLOQ of each N. meningitidis serogroup B indicator strain
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
28
Infectious diseases
GSK 3437949 (Malaria fractional dose)
NCT03276962
Phase
IIb
Patient
Children aged 5
-17 months
Subjects
1498
Treatment
arms
R012
-20 Group: a full dose of RTS,S/AS01E at Month 0, Month 1, Month 2 and Month 20
R012
-14-mD Group: a full dose of RTS,S/AS01E at Month 0, Month 1, Month 2 Month 14, Month 26, Month 38
Fx012
-14-mFxD Group: a full dose of RTS,S/AS01E at Month 0, Month 1 and RTS,S/AS01E 1/5th dose at Month 2, Month 14, Month 26, Month 38
Fx017
-mFxD Group: a full dose of RTS,S/AS01E at Month 0, Month 1 and RTS,S/AS01E 1/5th dose at Month 7, Month 20, Month 32
Control Group: Subjects will receive rabies vaccine at Month 0, Month 1, Month 2
Description
A randomized, open
-label, controlled, multi-centre trial of the efficacy, safety and immunogenicity of GSK Biologicals’ candidate malaria
vaccine RTS,S/AS01E evaluating schedules with or without fractional doses, early Dose 4 and yearly doses, in children 5
-17 months of age living
in sub
-Saharan Africa.
Timeline
Trial start: Q3 2017
Data anticipated: H2 2023
Key end
points
Incremental efficacy of a schedule with a fractional third dose at Month 2 over the standard schedule. To demonstrate the su
periority of a 3-
dose schedule of GSK Biologicals’ malaria vaccine RTS,S/AS01E with a fractional third dose at Month 2 compared to a standard
schedule of
RTS,S/AS01E with three full doses in terms of vaccine efficacy against clinical malaria (primary case definition) over 12 mon
ths post-Dose 3.
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
29
Infectious diseases
GSK4406371 (MMRV new strain vaccine)
NCT05630846
Phase
II
Patient
Healthy children 4
-6 years of age
Subjects
800
Treatment
arms
Investigational MMRV(H)NS vaccine
Investigational MM(H)RVNS vaccine
Investigational M(L)M(L)R(L)V(L)NS vaccine
Marketed
MMRV_Lot 1 and Lot 2 vaccine
Description
A single
-blind, randomized, controlled trial to evaluate the immunogenicity
and safety of a measles, mumps, rubella, varicella vaccine compared with
ProQuad
, administered in healthy children 4-6 years of age
Timeline
Trial start: Q4 2022
Data anticipated: H2 2024
Key end
points
Anti
-measles, anti-mumps, anti-rubella, and anti-glycoprotein H antibodies
geometric mean concentrations
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
30
Infectious diseases
GSK3536852 (Shigella)
NCT05073003
Phase
I/II
Patient
Adults in Europe (Stage 1) followed by age de
-escalation from adults to children and infants and dose finding in infants in Africa (Stage 2)
Subjects
550
Treatment
arms
Drug:
altSonflex Placebo (adults stage 1 in Europe)
Biological:
altSonflex1-2-3 High Dose C (adults stage 1 in Europe, adults, children and infants stage 2 in Africa)
Biological:
altSonflex1-2-3 Medium Dose B (children and infants stage 2 in Africa)
Biological:
altSonflex1-2-3 Low Dose A (infants stage 2 in Africa)
Comparators: Menveo and Boostrix (adults stage 2 in Africa)
Comparators: Menveo and
Typhim Vi (children stage 2 in Africa)
Comparators: Menveo and Infanrix (infants stage 2 in Africa)
Description
A staged observer
-blind, randomised, controlled, multi-country trial to evaluate the safety, reactogenicity, and immune responses to the GVGH
altSonflex1
-2-3 vaccine against S. sonnei and S. flexneri serotypes 1b, 2a, and 3a, in adults in Europe (Stage 1) followed by age de-escalation
from adults to children and infants, and dose
-finding in infants in Africa (Stage 2)
Timeline
Trial start: Q4 2021
Data anticipated: 2025
Key end
points
Immune response to identify the preferred dose of each component of the altSonflex1
-2-3 vaccine (low, medium, or high) for infan
ts 9 months of
age in Africa (Stage 2). To evaluate the safety and reactogenicity of the altSonflex1
-2-3 vaccine in all participants in Europe
and Africa (Stage 1
and Stage 2)
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
31
Infectious diseases
GSK3528869 (Chronic HBV infection)
NCT03866187
Phase
I/II
Patient
HBV suppressed subjects under
nucleo(s)tide treatment
Subjects
148
Treatment
arms
ChAd155
-hIi-HBV low dose formulation
ChAd155
-hIi-HBV high dose formulation
HBc
-HBs/AS01B-4 low dose formulation
HBc
-HBs/AS01B-4 high dose formulation
MVA
-HBV low dose formulation
MVA
-HBV high dose formulation
Placebo
Description
A first time in human trial on GSK's therapeutic vaccines to evaluate the reactogenicity, safety, immunogenicity and efficacy
on
reduction of serum HBV surface antigen in HBV suppressed subjects under
nucleo(s)tide treatment.
Timeline
Trial start: Q1 2019
Data anticipated: 2025
Key end
points
Safety and reactogenicity, as well as percentage of patients with >1 log decline of HBsAg
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
32
Infectious diseases
GSK4023393 (MenABCWY, 2
nd
Gen)
NCT04886154
Phase
I/II
Patient
Healthy adults (phase I) and healthy adolescents and adults (phase II)
Subjects
1258
Treatment
arms
Combination Product: MenABCWY
-2Gen low dose vaccine
Combination Product: MenABCWY
-2Gen high dose vaccine
Combination Product: Placebo
Combination Product:
MenB vaccine
Biological:
MenACWY vaccine
Description
A
randomised, controlled trial to assess the safety, effectiveness and immune
response of meningococcal combined ABCWY vaccine when administered to
healthy adults (phase I) and to healthy adolescents and adults (phase II)
Timeline
Trial start: Q2 2021
Data anticipated: H1 2024
Key end
points
AEs, including all SAEs, AEs leading to withdrawal and AEs of special interest
(AESIs)
Immunological vaccine effectiveness by enc
-hSBA and immunogenicity by
hSBA
on indicator strains
Clinicaltrials.g
ov
Link
NCT05082285
Phase
II
Patient
Healthy infants
Subjects
724
Treatment
arms
Combination Product: MenABCWY
-2Gen low dose vaccine
Combination Product: MenABCWY
-2Gen high dose vaccine
Combination Product: MenABCWY
Combination Product: MenB + MenACWY
-TT
Description
A
randomised, partially blinded trial to assess the safety, tolerability and
immunogenicity of meningococcal combined ABCWY vaccine when
administered to healthy infants
Timeline
Trial start: Q4 2021
Data anticipated: 2025
Key end
points
AEs, including all SAEs, AEs leading to withdrawal and AEs of special interest
(AESIs), medical attended events (MAE)
I
mmunogenicity by hSBA to indicator strains
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
33
Infectious diseases
GSK4178116 (Varicella new strain)
NCT05084508
Phase
II
Patient
Healthy children between 12
-15 months
Subjects
800
Treatment
arms
Arm A: low potency varicella NS vaccine, plus routine schedule
Arm B: medium potency varicella NS vaccine, plus routine schedule
Arm C: high potency varicella NS vaccine, plus routine schedule
Arm D: marketed varicella vaccine lot 1, plus routine schedule
Arm E: marketed varicella vaccine lot 2, plus routine schedule
Description
A observer
-blind, randomised, controlled trial to evaluate the immunogenicity
and safety of a varicella vaccine at various potencies compared with
Varivax
as a first dose, administered in healthy children in their second year of life
Timeline
Trial start: Q4 2021
Data anticipated: H1 2024
Key end
points
Anti
-glycoprotein-E antibodies at day 43
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
34
Infectious diseases
GSK5101955 (Paediatric Pneumococcal disease, 24-valent)
NCT05412030
Phase
II
Patient
Healthy infants
Subjects
760
Treatment
arms
Arm A: 1 mcg AFX3772 administered intramuscularly 4 times within 12 months
Arm B: 2 mcg AFX3772 administered intramuscularly 4 times within 12 months
Arm C: 5 mcg AFX3772 administered intramuscularly 4 times within 12 months
Arm D: PCV13 administered intramuscularly 4 times within 12 months
Description
A
randomised, double-blind, multi-dose, dose finding trial to evaluate the
safety, tolerability and immunogenicity of AFX3772 compared with PCV13 in
healthy infants
Timeline
Trial start: Q2 2022
Data anticipated: 2025
Key end
points
Safety, tolerability profiles of 3 different dose levels of AFX3772 compared with
PCV13 with respect to the proportion of participants with AEs
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
35
Infectious diseases
GSK4106647 (Human papillomavirus)
NCT05496231
Phase
II
Patient
Healthy females 16 to 26 years of age
Subjects
1080
Treatment
arms
Arm A: HPV9 High formulation
Arm B: HPV9 Medium formulation
Arm C: HPV9 Low formulation
Arm D: Gardasil 9
Description
A randomized, observer
-blinded, multi-country trial to evaluate safety and
immunogenicity of investigational adjuvanted Human Papillomavirus Vaccine
in females (16 to 26 years of age)
Timeline
Trial start: Q3 2022
Data anticipated: H1 2024
Key end
points
AEs, SAEs, anti
-HPV IgG concentrations
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
36
Infectious diseases
GSK4348413 (Gonorrhoea)
NCT05630859
Phase
I/II
Patient
Healthy adults 18 to 50 years of age
Subjects
774
Treatment
arms
Phase I
NgG
low dose investigational vaccine
NgG
medium dose investigational vaccine
NgG
high dose investigational vaccine
Placebo
Phase II
NgG
HTD investigational vaccine
NgG
below HTD investigational vaccine
Placebo
Description
An observer
-blind, randomized, placebo-controlled multi-country trial to assess safety and efficacy of GSK Neisseria
gonorrhoeae
GMMA (NgG) investigational vaccine when administered to healthy adults 18 to 50 years of age
Timeline
Trial start: Q4 2022
Data anticipated: 2025
Key end
points
AEs and SAEs
Incidence rates of gonorrhoeae in trial phase II
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
37
Infectious diseases
GSK4382276 (mRNA Seasonal Flu)
NCT05446740
Phase
I
Patient
Healthy younger and older adults
Subjects
324
Treatment
arms
GSK4382276A Dose level 1
GSK4382276A Dose level 2
GSK4382276A Dose level 3
GSK4382276A Dose level 4
GSK4382276A Dose level 6
GSK4382276A Dose level 7
GSK4382276A Dose level 8
GSK4382276A Dose level 9
Combination Product: FDQ21A
-NH
Combination Product: FDQ22A
-NH
Description
A randomized, observer
-blind, dose-escalation trial to evaluate the safety,
reactogenicity and immunogenicity of an mRNA
-based monovalent influenza
vaccine candidate in healthy younger and older adults
Timeline
Trial start: Q3 2022
Final data anticipated: H1 2024
Key end
points
Safety and reactogenicity, including number of participants reporting systemic
and solicited administration site events
Serum anti
-influenza seroconversion rates and geometric mean titers
Clinicaltrials.g
ov
Link
NCT05823974
Phase
I/II
Patient
Healthy younger and older adults
Subjects
1253
Treatment
arms
Biological: Flu mRNA
Combination Product: Control 1
Combination Product: Control 2
Description
A randomized, dose
-finding/dose-confirmation study to evaluate the
reactogenicity, safety and immunogenicity of mRNA
-based multivalent
seasonal influenza vaccine candidates administered in healthy younger and
older adults
Timeline
Trial start: Q2 2023
Final data anticipated: H2 2024
Key end
points
Safety and reactogenicity, including number of participants reporting systemic
and solicited administration site events
Serum anti
-influenza antigen seroconversion rates and geometric mean titers
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
NCT05960097
Phase
II
Patient
Adults at least 18 years old
Subjects
415
Treatment
arms
Arm A: CV0701 bivalent high dose
Arm B: CV0701 bivalent medium dose
Arm C: CV0701 bivalent low dose
Arm D: CV0601 monovalent high dose
Arm E: Control vaccine
Description
A randomized, active
-controlled, observer-blind study to assess the safety, reactogenicity, and
immunogenicity of a booster dose of investigational COVID
-19 mRNA vaccines in healthy adults who
previously received a complete primary vaccination series with or without booster dose(s)
Timeline
Trial start: Q3 2023
Data anticipated: H2 2024
Key end
points
Serum neutralizing titers against
pseudoviruses bearing SARS-CoV-2 spike proteins at Day 29
Percentage of participants with solicited local AE during 7 days after vaccination
Clinicaltrials.g
ov
Link
38
Infectious diseases
GSK4396687 (mRNA COVID-19)

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
39
Infectious diseases
GSK3993129 (CMV)
NCT05089630
Phase
I/II
Patient
Healthy adults 18 to 50 years of age
Subjects
329
Treatment
arms
Arm A: pentamer (low)/
gB(low)/adjuvant vaccine
Arm B: pentamer (med)/
gB(low)/adjuvant vaccine
Arm C: pentamer (med)/
gB(med)/adjuvant vaccine
Arm D: pentamer (high)/
gB(med)/adjuvant vaccine
Arm F: placebo (saline)
Description
A
randomised, observer-blind, placebo-controlled, dose escalation trial to
assess safety, reactogenicity and immunogenicity of a candidate CMV vaccine
comprising recombinant protein and adjuvant
Timeline
Trial start: Q4 2021
Data anticipated: 2026+
Key end
points
Safety, reactogenicity and immunogenicity
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
40
Infectious diseases
GSK3943104 (Therapeutic HSV)
NCT05298254
Phase
I/II
Patient
Healthy participants aged 18
-60 years negative for HSV-2
HSV
-2 and HSV-1 patients with ≥3 episodes of GH in the previous year
Subjects
Part 1: 245; Part 2: 240
Treatment
arms
Arm A: non
-adjuvanted HSV formulation 1 - part 1 group
Arm B: non
-adjuvanted HSV formulation 2 - part 1 group
Arm C: non
-adjuvanted HSV formulation 3 - part 1 group
Arm D: HSV formulation 1 with adjuvant 1
- part 1 group
Arm E: HSV formulation 2 with adjuvant 1
- part 1 group
Arm F: HSV formulation 3 with adjuvant 1
- part 1 group
Arm G: HSV formulation 1 with adjuvant 2
- part 1 group
Arm H: HSV formulation 2 with adjuvant 2
- part 1 group
Arm I: HSV formulation 3 with adjuvant 2
- part 1 group
Arm J: part 1 group (placebo)
Arm K: selected formulation
- part 2 group
Arm L: selected formulation
- part 2 group
Arm M: part 2 group (placebo)
Description
An observer
-blind, randomised, placebo-controlled, multi-country trial to evaluate reactogenicity, safety, immune response and efficacy of an
HSV vaccine
Timeline
Trial start: Q1 2022
Data anticipated: 2026+
Key end
points
Part 1: Percentage of participants reporting each solicited administration site event; dose selection
Part 2: Clinical efficacy (TTFE)
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
41
Infectious diseases
GSK4077164 (iNTS Typhimurium + Enteritidis)
NCT05480800
Phase
I/
IIa
Patient
Healthy European and African adults
Subjects
155
Treatment
arms
Arm A: iNTS
-TCV low dose group - Europe
Arm B: iNTS
-GMMA and TCV low doses group - Europe
Arm C: Step 1 group (placebo)
- Europe
Arm D: iNTS
-TCV full dose_1 group - Europe
Arm E: iNTS
-GMMA and TCV full doses_1 group - Europe
Arm F: Step 2 group (placebo)
- Europe
Arm G:
iNTS-TCV full dose_2 group - Africa
Arm H:
iNTS-GMMA and TCV full doses_2 group - Africa
Arm I: Stage 2 group (control)
- Africa
Description
An observer
-blind, randomised, controlled, two-stage, multi-country trial to evaluate the safety, reactogenicity and immune response of the
trivalent vaccine against iNTS and Typhoid fever
Timeline
Trial start: Q3 2022
Data anticipated: H2 2024
Key end
points
To evaluate the safety,
reactogenicity and immunogenicity profile of iNTS-TCV vaccine in healthy European/African adults
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
42
Infectious diseases
GSK4077164 (iNTS S. typhimurium + S. enteritidis + S. Typhi)
NCT06213506
Phase
IIa
Patient
Adults, children and infants, including dose
-finding in infants in Africa (Ghana)
Subjects
20 adults/40 children/60 infants 9 months/ 396 infants 6 weeks
Treatment
arms
Stage 1: Age
-de-escalation
Adults (dose C or control)
Children (dose B or C or control)
Infants, 9 months (dose A, B, C or control)
Infants, 6 months (dose A, B, C, or control)
Stage 2: Dose finding in infants 6 weeks of age
Description
An observer
-blind, randomized, controlled, age-de-escalation, single center interventional study to evaluate the safety, reactogenicity, and
immune response of the GVGH
iNTS vaccine against S. typhimurium and S. enteritidis, in adults, children and infants, including dose-finding in
infants, in Africa (Ghana)
Timeline
Trial start: Q1 2024
Data anticipated: 2026+
Key end
points
To evaluate the safety,
reactogenicity and immunogenicity profile of iNTS-GMMA vaccine in adults, children and infants (Ghana)
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
43
Infectious diseases
GSK3036656 (Tuberculosis)
NCT05382312
Phase
IIa
Patient
Males and females aged 18 to 65 years inclusive with drug
-sensitive
(rifampicin
-susceptible) pulmonary tuberculosis
Subjects
70
Treatment
arms
Arm A: Participants receiving GSK3036656+bedaquiline
Arm B: Participants receiving GSK3036656+delamanid
Arm C: Participants receiving
bedaquiline+delamanid
Arm D: Participants receiving RIFAFOUR e
-275
Description
A parallel group,
randomised, open-label, 4 treatment arm trial to assess the
early bactericidal activity, safety and tolerability of oral GSK3036656 in
combination with either oral
delamanid or oral bedaquiline, oral delamanid in
combination with oral
bedaquiline, or standard of care in males and females
aged 18 to 65 years inclusive with drug
-sensitive (rifampicin-susceptible)
pulmonary tuberculosis
Timeline
Trial start: Q3 2022
Data anticipated: H2 2024
Key end
points
Change from baseline in log10 CFU of
Mycobacterium tuberculosis
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
44
Infectious diseases
GSK3536867 (Salmonella typhoid + paratyphoid A)
NCT05613205
Phase
I
Patient
Healthy adults aged 18
-50 years in Europe
Subjects
96
Treatment
arms
Arm A: Step 1a low dose without adjuvant group
Arm B: Step 1a control group
Arm C: Step 1b low dose with adjuvant group
Arm D: Step 1b control group
Arm E: Step 2 full dose without adjuvant group
Arm F: Step 2 full dose with adjuvant group
Arm G: Step 2 control group
Description
An observer
-blind, randomised, controlled, single-centre trial to evaluate the safety, reactogenicity and immune responses to an adjuvanted
and non
-adjuvanted conjugate vaccine against Salmonella Typhi and Salmonella Paratyphi A
Timeline
Trial start: Q4 2022
Data anticipated: H1 2024
Key end
points
Percentage of participants with solicited administration
-site events, systemic events, unsolicited adverse event and any serious adverse events
after the first vaccination
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
45
Infectious diseases
GSK2556286 (Tuberculosis)
NCT04472897
Phase
I
Patient
Healthy adults
Subjects
120
Treatment
arms
Arm A: Part A
- GSK2556286 with up to 11 cohorts
Arm B: Part A
- placebo
Arm C: Part B
- GSK2556286 with up to 4 cohorts
Arm D: Part B
- placebo
Description
A
randomised, double blind (sponsor unblinded), placebo-
controlled, first time
in human trial to evaluate the safety, tolerability and pharmacokinetics of
single and repeat oral doses and the food effect of GSK2556286
Timeline
Trial start: Q4 2020
Data anticipated: H2 2024
Key end
points
SAEs and non
-SAEs
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
46
Infectious diseases
GSK3494245 (Visceral leishmaniasis)
NCT04504435
Phase
I
Patient
Healthy adult males
Subjects
54
Treatment
arms
Cohort 1: maximum of 3 ascending doses GSK3494245 starting at 20 mg and
placebo (fasted)
Cohort 2: maximum of 3 ascending doses GSK3494245 starting at dose level 5
and placebo (fasted)
Cohort 3: Participants receiving GSK3494245 (fasted then fed)
Cohort 3: Participants receiving GSK3494245 (fed then fasted)
Description
A randomized, double
-blind, placebo-controlled, first time in human trial to
evaluate the safety, tolerability and pharmacokinetics of single (in both fed
and fasted states) doses of GSK3494245 in healthy participants
Timeline
Trial start: Q3 2020
Data anticipated: H2 2024
Key end
points
Number of participants with AEs and SAEs
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
47
Infectious diseases
GSK4024484 (Malaria)
NCT06171113
Phase
I
Patient
Healthy adults aged 18
-60 years
Subjects
54
Treatment
arms
Group/Arm 1: 6mg SAD GSK’484 or placebo (fasted state)
Group/Arm 2: 12mg SAD GSK’484 or placebo (fasted state)
Group/Arm 3: 24mg SAD GSK’484 or placebo (fasted state)
Group/Arm 4: 40mg SAD GSK’484 or placebo (fasted state)
Group/Arm 5: 60mg SAD GSK’484 or placebo (fasted state)
Group/Arm 6: 80mg SAD GSK’484 or placebo (fasted state)
Group/Arm 7: Food Effect (GSK’484 or placebo in fed state)
Group/Arm 8: 100 mg SAD GSK’484 or matching placebo
Group/Arm 9: Optional Group (dose escalation or dose level
modification flexibility)
Group/Arm 10: 10mg MAD GSK’484 or matching placebo
Group/Arm 11: 20mg MAD GSK’484 or matching placebo
Group/Arm 12: 30mg MAD GSK’484 or matching placebo
Description
A
randomised, double-blind placebo-controlled, First Time in Human Study to evaluate the safety and pharmacokinetics of
single and multiple oral doses and food effect of GSK4024484
Timeline
Trial start: Q4 2023
Data anticipated: H2 2025
Key end
points
Number of participants with AEs and SAEs
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
48
Infectious diseases
GSK3923868 (Viral COPD exacerbations)
NCT05398198
Phase
Ib
Patient
Participants with mild asthma
Subjects
68
Treatment
arms
Arm A: GSK3923868
Arm B: placebo
Description
A
randomised, double-
blind, placebo controlled, repeat dose trial to assess the
efficacy, safety, tolerability, pharmacokinetics and pharmacodynamics of
inhaled GSK3923868 during experimental human rhinovirus infection
participants with mild asthma
Timeline
Trial start: Q2 2022
Data anticipated: H1 2024
Key end
points
AUC of
CfB in LRTS score from day of inoculation up to discharge
Clinicaltrials.g
ov
Link

Innovation: Pipeline growth HIV Respiratory/ImmunologyInfectious diseases Oncology Opportunity driven Glossary
49
Infectious diseases
GSK3965193 (Chronic HBV infection)
NCT05330455
Phase
I/II
Patient
Healthy participants and those living with chronic hepatitis B infection
Subjects
132
Treatment
arms
Part 1 cohort 1: GSK3965193 and placebo
Part 1 cohort 2: GSK3965193 and placebo
Part 2A cohort 3: GSK3965193 or placebo
Part 2A cohort 4: GSK3965193 or placebo
Part 2A cohort 5: GSK3965193 or placebo
Part 2B cohort 6: GSK3965193
Part 3 cohort 7: GSK3965193 or placebo
Part 4 cohort 8: GSK3965193 and
bepirovirsen or placebo and bepirovirsen
Description
Four
-part, randomised, double-blind (Parts 1, 2A, 3 and 4), multi-centre, placebo-controlled trial to assess the safety, tolerability,
pharmacokinetics and pharmacodynamics of GSK3965193 monotherapy in healthy participants and in participants living with chron
ic
hepatitis
B infection; and GSK3965193 in combination with
bepirovirsen
Timeline
Trial start: Q2 2022
Data anticipated: 2026+
Key end
points
Number of participants with AEs, SAEs, and withdrawals due to AEs
Part 3: Change from Baseline in HBsAg levels
Part 4 : Number of participants achieving sustained virologic response
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth HIV Respiratory/Immunology Oncology Opportunity driven Glossary
51
HIV
VH3810109
NCT04871113
- B-NAB
Phase
II
Patient
Anti
-retroviral naïve HIV-1 infected adults
Subjects
62
Treatment
arms
Part 1
Cohort 1: ’109A infusion (40mg/kg)
Cohort 2: ’109A infusion (280 mg/kg)
Part 2
Cohort 3: ‘109A IV or SC
– dosing determined from part 1
Cohort 4: ‘109A IV or SC
– dosing determined from part 1
Cohort 5: ‘109A IV or SC
– dosing determined from part 1
Description
A
multicentre, randomised, open-label, two part adaptive design trial to
evaluate the antiviral effect, safety and tolerability of GSK3810109A, an HIV
-1
specific broadly neutralizing human monoclonal antibody in antiretroviral
-
naïve HIV
-1-infected adults
Timeline
Trial start: Q2 2021
Data anticipated: H2 2023
Key end
points
Safety, plasma HIV
-1 levels
Clinicaltrials.g
ov
Link
NCT05996471
Phase
IIb
Patient
Antiretroviral therapy (ART)
-experienced adults living with HIV
Subjects
150
Treatment
arms
Group 1: VH3810109 + cabotegravir
Group 2 VH3810109 + rHuPH20 + cabotegravir
Group 3: Active comparator
- Participants receiving standard of care (SOC)
antiretroviral therapy (ART)
Description
A
multicentre, randomised, open-
label, trial comparing the efficacy, safety, PK,
and tolerability of VH3810109, administered either intravenously or as a
subcutaneous infusion with rHuPH20, in combination with cabotegravir given
intramuscularly, to standard of care in virologically suppressed, antiretroviral
therapy (ART)
-experienced adults living with HIV
Timeline
Trial start: Q3 2023
Data anticipated: H2 2024
Key end
points
Safety, plasma HIV
-1 levels
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth HIV Respiratory/Immunology Oncology Opportunity driven Glossary
52
HIV
VH3739937
NCT06061081
Phase
II
Patient
Treatment
-naïve adults living with HIV-1
Subjects
26
Treatment
arms
Arm A: VH3738837
Arm B: placebo
Description
A randomized, double
-blind (sponsor-unblinded), placebo-controlled,
adaptive study to investigate the antiviral effect, safety, tolerability and
pharmacokinetics of VH3739937 in treatment
-naïve adults living with HIV-1
Timeline
Trial start: Q1 2024
Data anticipated: H1 2024
Key end
points
AEs and SAEs, concentrations of VH3738837
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth HIV Respiratory/Immunology Oncology Opportunity driven Glossary
53
HIV
VH4004280 & VH4011499
NCT06012136
Phase
I
Patient
Healthy adults
Subjects
160
Treatment
arms
Arm A: VH4004280
Arm B: Placebo
Arm C: VH4011499
Description
A double
-blind (sponsor-unblinded), placebo-controlled, randomized, single
dose escalation study to evaluate the safety, tolerability, and
pharmacokinetics of a parenterally administered suspension of investigational
capsid inhibitors in healthy adults
Timeline
Trial start: Q3 2023
Data anticipated: 2025+
Key end
points
AEs, PK
Clinicaltrials.g
ov
Link
NCT06039579
Phase
II
Patient
HIV
-1 infected treatment-naïve adults
Subjects
42
Treatment
arms
Arm A: VH4004280
Arm B: VH4011499
Arm C: VH4004280
-matching placebo
Arm D: VH4011499
-matching placebo
Description
A randomized, double
-blind (sponsor-unblinded), placebo-controlled trial to
investigate the antiviral effect, safety, tolerability and pharmacokinetics of
orally administered investigational capsid inhibitor monotherapy in
HIV-1
infected treatment
-naïve adults
Timeline
Trial start anticipated: H2 2023
Data anticipated: H1 2024
Key end
points
Maximum change from baseline (Day 1) in plasma HIV
-1 RNA
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth HIV Respiratory/Immunology Oncology Opportunity driven Glossary
54
HIV
VH4524184
NCT06214052
Phase
IIa
Patient
HIV
-1 infected treatment naïve adults
Subjects
28
Treatment
arms
Arm A: VH4524184
Arm B: Placebo
Description
A randomized, double
-blind (sponsor unblinded), placebo-controlled study to
investigate the antiviral effect, safety, tolerability and pharmacokinetics of
VH4524184 in HIV
-1 infected treatment naïve adults
Timeline
Trial start anticipated: H1 2024
Data anticipated: H2 2024
Key end
points
Maximum change from baseline in log10 plasma HIV
-1 RNA
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth HIV Respiratory/Immunology Oncology Opportunity driven Glossary
55
HIV
cabotegravir
NCT05418868
Phase
I
Patient
Healthy adult volunteers
Subjects
60
Treatment
arms
Part A: Participants receiving CAB 200 mg/mL with rHuPH20
Part C: Participants receiving CAB 400 mg/mL
Part D: Participants receiving CAB 400 mg/mL with rHuPH20
Description
A multi
-centre, open-label, single dose escalation trial to evaluate the
pharmacokinetics, safety and tolerability of long
-acting cabotegravir co-
administered with recombinant human hyaluronidase PH20 (rHuPH20) in
healthy adult volunteers
Timeline
Trial start: Q2 2022
Data anticipated: H1 2024
Key end
points
Plasma concentrations of cabotegravir
Clinicaltrials.g
ov
Link
NCT06033547
Phase
I
Patient
Healthy adult volunteers
Subjects
48
Treatment
arms
Part A: Participants receiving cabotegravir Formulation F
Part B: Participants receiving cabotegravir Formulation G
Description
An open
-
label, single dose escalation study to evaluate the pharmacokinetics,
safety and tolerability of two different formulations of long
-acting
cabotegravir administered to healthy adult participants
Timeline
Trial start: Q3 2023
Data anticipated: 2025
Key end
points
Plasma concentrations of cabotegravir
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
57
Respiratory/Immunology
Nucala (mepolizumab)
NCT04133909
- MATINEE
Phase
III
Patient
Participants with chronic obstructive pulmonary disease (COPD) experiencing
frequent exacerbations and
characterised by eosinophil levels
Subjects
806
Treatment
arms
Arm A: placebo
Arm B: mepolizumab
Description
A
multicentre randomised, double-blind, parallel-group, placebo-controlled
trial of mepolizumab 100 mg subcutaneously as add
-on treatment in
participants with COPD experiencing frequent exacerbations and
characterised
by eosinophil levels
Timeline
Trial start: Q4 2019
Data anticipated: H2 2024
Key end
points
Annualised
rate of moderate or severe exacerbations
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
58
Respiratory/Immunology
depemokimab
NCT04719832
- SWIFT-1
Phase
III
Patient
Adult and adolescents with severe uncontrolled asthma with an eosinophilic
phenotype
Subjects
395
Treatment
arms
Arm A:
depemokimab plus SoC
Arm B: placebo plus SoC
Description
A 52
-week, randomised, double-blind, placebo-controlled, parallel-group,
multi
-centre trial of the efficacy and safety of depemokimab adjunctive
therapy in adult and adolescent participants with severe uncontrolled asthma
with an eosinophilic phenotype
Timeline
Trial start: Q1 2021
Data anticipated: H1 2024
Key end
points
Annualised
rate of clinically significant exacerbations over 52 weeks
Clinicaltrials.g
ov
Link
NCT04718103
- SWIFT-2
Phase
III
Patient
Adult and adolescents with severe uncontrolled asthma with an eosinophilic
phenotype
Subjects
397
Treatment
arms
Arm A:
depemokimab plus SoC
Arm B: placebo plus SoC
Description
A 52
-week, randomised, double-blind, placebo-controlled, parallel-group,
multi
-centre trial of the efficacy and safety of depemokimab adjunctive
therapy in adult and adolescent participants with severe uncontrolled asthma
with an eosinophilic phenotype
Timeline
Trial start: Q1 2021
Data anticipated: H1 2024
Key end
points
Annualised
rate of clinically significant exacerbations over 52 weeks
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
59
Respiratory/Immunology
depemokimab
NCT05243680
- AGILE
Phase
III
Patient
Adult and adolescents with severe asthma with an eosinophilic phenotype
from studies SWIFT
-1 and SWIFT-2
Subjects
637
Treatment
arms
Participants diagnosed with asthma receiving
depemokimab
Description
A 52
-week, open label extension phase of SWIFT-1 and SWIFT-2 to assess the
long
-term safety and efficacy of depemokimab adjunctive therapy in adult
and adolescent participants with severe uncontrolled asthma with an
eosinophilic phenotype
Timeline
Trial start: Q1 2022
Data anticipated: 2025
Key end
points
Number of participants with AEs and SAEs and incidence of immunogenicity
over 52 weeks
Clinicaltrials.g
ov
Link
NCT04718389
- NIMBLE
Phase
III
Patient
Adult and adolescent severe asthmatic participants with an eosinophilic
phenotype treated with
depemokimab compared with mepolizumab or
benralizumab
Subjects
1700
Treatment
arms
Arm A: participants receiving
depemokimab plus placebo matching prior anti-
IL
-5/5R treatment
Arm B: participants receiving prior anti
-IL-5/5R treatment plus placebo
matching
depemokimab
Description
A 52
-week, randomised, double-blind, double-dummy, parallel group, multi-
centre
, non-
inferiority trial assessing exacerbation rate, additional measures of
asthma control and safety in adult and adolescent severe asthmatic
participants with an eosinophilic phenotype treated with
depemokimab
compared with mepolizumab or
benralizumab
Timeline
Trial start: Q1 2021
Data anticipated: 2025
Key end
points
Annualised
rate of clinically significant exacerbations over 52 weeks
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
60
Respiratory/Immunology
depemokimab
NCT05274750
- ANCHOR-1
Phase
III
Patient
Adults with chronic rhinosinusitis with nasal polyps (
CRSwNP)
Subjects
276
Treatment
arms
Arm A:
depemokimab
Arm B: placebo
Description
A randomized, double
-blind, parallel group trial to assess the efficacy and
safety of 100 mg subcutaneous
depemokimab in patients with CRSwNP
Timeline
Trial start: Q2 2022
Data anticipated: H2 2024
Key end
points
Change from baseline in total endoscopic nasal polyps (NP) score at week 52
Change from baseline in mean nasal obstruction verbal response scale (VRS)
score from Week 49 through to Week 52
Clinicaltrials.g
ov
Link
NCT05281523
- ANCHOR-2
Phase
III
Patient
Adults with chronic rhinosinusitis with nasal polyps (
CRSwNP)
Subjects
264
Treatment
arms
Arm A:
depemokimab
Arm B: placebo
Description
A randomized, double
-blind, parallel group trial to assess the efficacy and
safety of 100 mg subcutaneous
depemokimab in patients with CRSwNP
Timeline
Trial start: Q2 2022
Data anticipated: H2 2024
Key end
points
Change from baseline in total endoscopic nasal polyps (NP) score at week 52
Change from baseline in mean nasal obstruction verbal response scale (VRS)
score from
Week 49 through to Week 52
Clinicaltrials.g
ov
Link
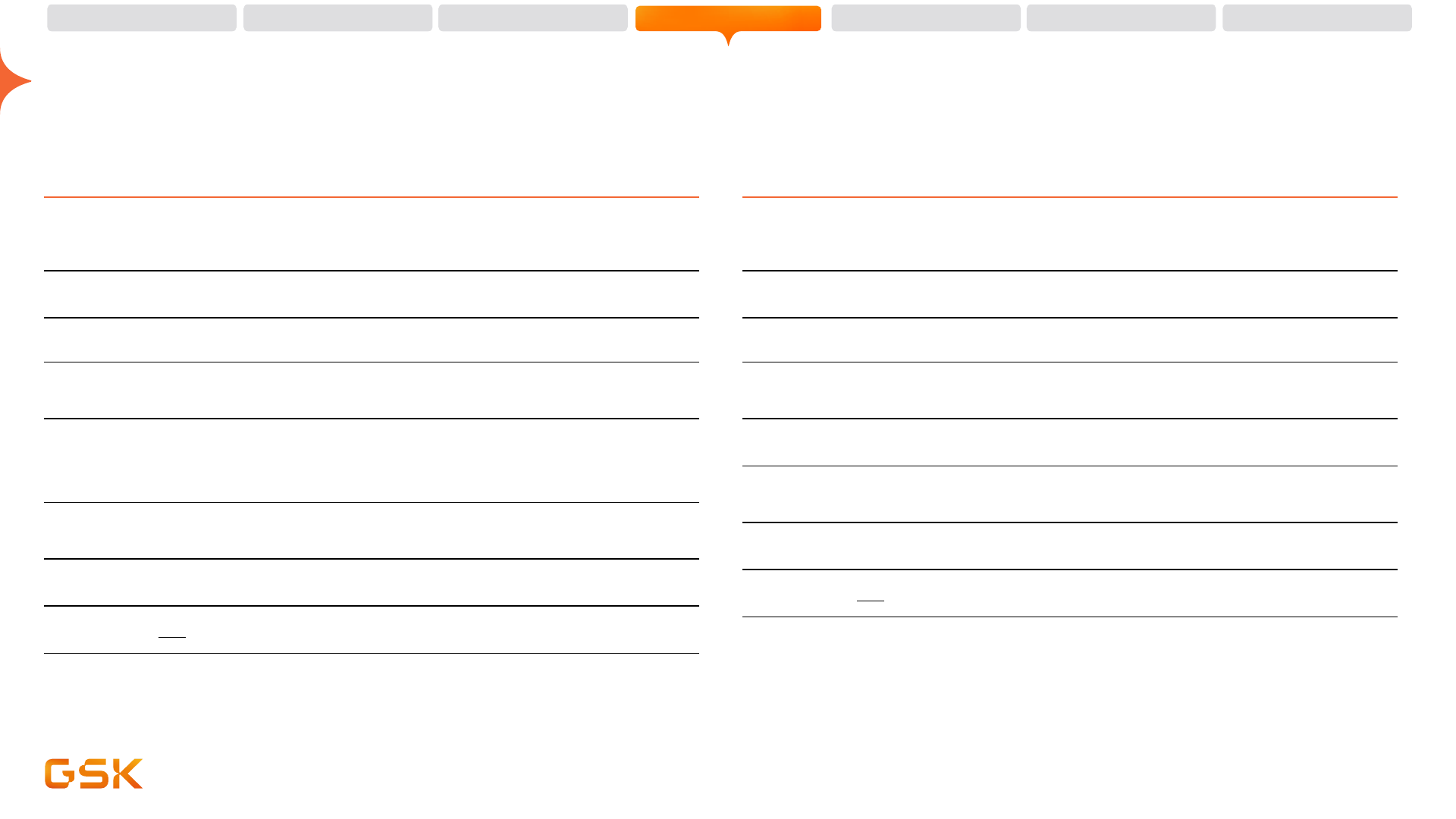
Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
61
Respiratory/Immunology
depemokimab
NCT05263934
- OCEAN
Phase
III
Patient
Adults with relapsing or refractory eosinophilic granulomatosis with
polyangiitis (EGPA) receiving standard of care therapy
Subjects
160
Treatment
arms
Arm A:
depemokimab + placebo matching mepolizumab
Arm B: mepolizumab + placebo matching
depemokimab
Description
A 52
-week randomised, double-blind, double-dummy, parallel-group,
multicentre
, non-inferiority trial to investigate the efficacy and safety of
depemokimab
compared with mepolizumab in adults with relapsing or
refractory EGPA receiving standard of care therapy
Timeline
Trial start: Q3 2022
Data anticipated: 2025
Key end
points
Number of participants with remission
Clinicaltrials.g
ov
Link
NCT05334368
- DESTINY
Phase
III
Patient
Adults with
hypereosinophilic syndrome (HES) receiving standard of care
therapy
Subjects
120
Treatment
arms
Arm A:
depemokimab
Arm B: placebo
Description
A
randomised, double-blind, placebo-controlled trial to investigate the
efficacy and safety of
depemokimab in adults with HES
Timeline
Trial start: Q3 2022
Data anticipated: 2026+
Key end
points
Frequency of HES flares
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
62
Respiratory/Immunology
camlipixant
NCT05599191
- CALM-1
Phase
III
Patient
Adult participants with refractory chronic cough, including unexplained
chronic cough
Subjects
825
Treatment
arms
Arm A:
camlipixant 25 mg twice a day
Arm B:
camlipixant 50 mg twice a day
Placebo twice a day
Description
A 52
-week, randomised, double-blind, placebo-controlled, parallel-arm
efficacy and safety study with open
-label extension of camlipixant in adult
participants with refractory chronic cough, including unexplained chronic
cough
Timeline
Trial start: Q4 2022
Data anticipated: 2025
Key end
points
24
-hour cough frequency
Clinicaltrials.g
ov
Link
NCT05600777
- CALM-2
Phase
III
Patient
Adult participants with refractory chronic cough, including unexplained
chronic cough
Subjects
825
Treatment
arms
Arm A:
camlipixant 25 mg twice a day
Arm B:
camlipixant 50 mg twice a day
Placebo twice a day
Description
A 24
-week, randomised, double-blind, placebo-controlled, parallel-arm
efficacy and safety study with open
-label extension of camlipixant in adult
participants with refractory chronic cough, including unexplained chronic
cough
Timeline
Trial start: Q1 2023
Data anticipated: 2025
Key end
points
24
-hour cough frequency
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
63
Respiratory/Immunology
Benlysta (belimumab)
NCT05878717
Phase
II/III
Patient
Adults with systemic sclerosis associated interstitial lung disease (
SSc-ILD)
Subjects
300
Treatment
arms
Arm A
: belimumab + standard therapy
Arm B
: placebo + standard therapy
Description
A randomized, double
-blind, placebo-controlled, parallel-group trial to
evaluate the efficacy and safety of belimumab administered subcutaneously in
adults with
SSc-ILD
Timeline
Trial start: Q4 2023
Data anticipated: 2026+
Key end
points
Absolute change from baseline in Forced Vital Capacity (FVC)
millilitre
(mL) at
week 52
Clinicaltrials.g
ov
Link
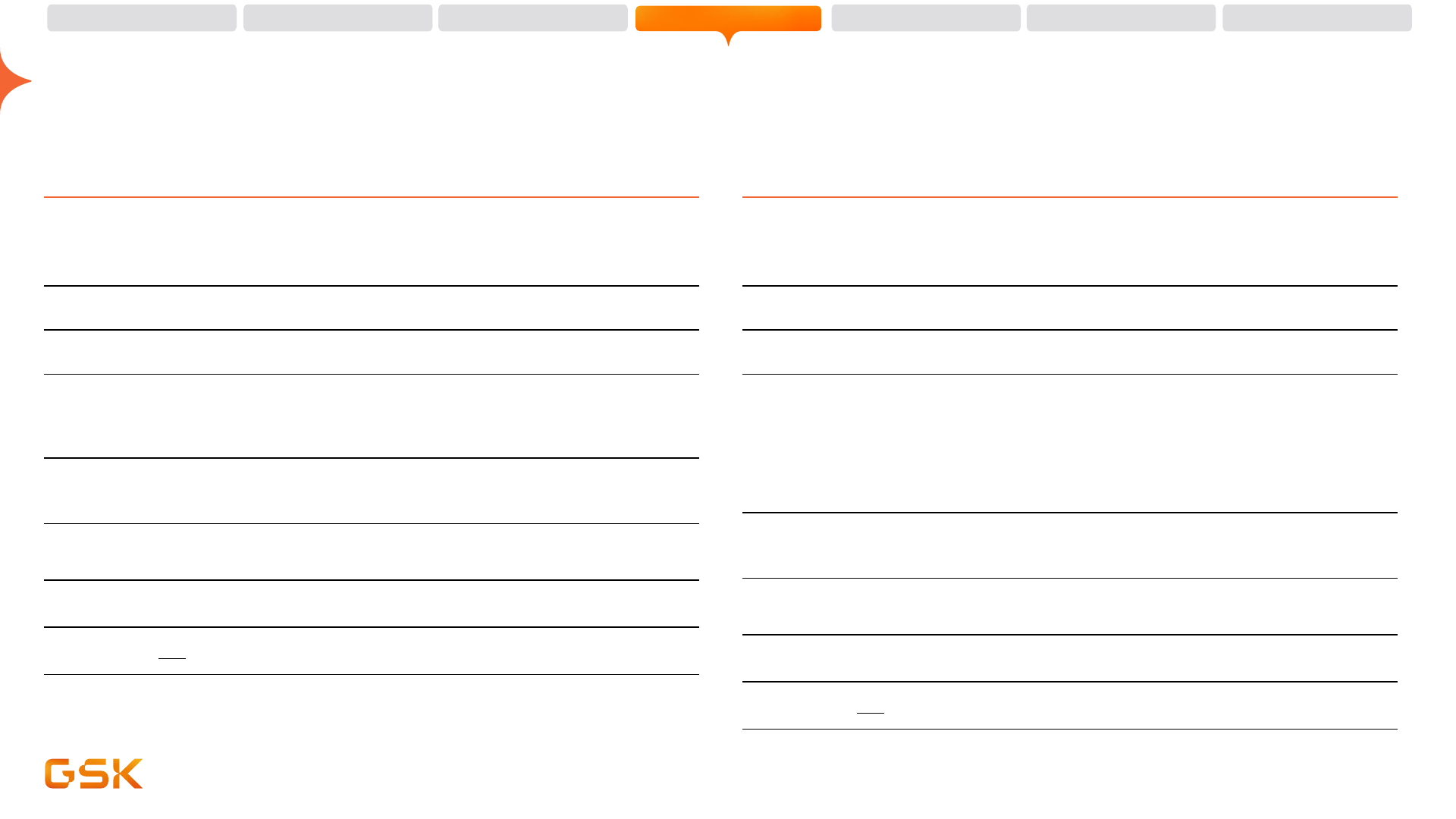
Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
64
Respiratory/Immunology
GSK3858279 (Osteoarthritis pain)
NCT05838755
- NEPTUNE-17
Phase
II
Patient
Adult participants with chronic diabetic peripheral neuropathic pain (DPNP)
Subjects
240
Treatment
arms
Arm A: GSK3858279 dose 1
Arm B: GSK3858279 dose 2
Arm C: placebo
Description
A
multicentre randomised, double-blind, placebo-controlled trial to evaluate
efficacy, safety, tolerability, pharmacokinetics and target engagement of
GSK3858279 in adult participants with chronic DPNP
Timeline
Trial start: Q4 2023
Data anticipated: 2025
Key end
points
Change from baseline in the weekly average of average daily pain intensity at
week 12, assessed on Numeric Rating Scale (NRS)
Clinicaltrials.g
ov
Link
NCT05838742
- MARS-17
Phase
II
Patient
Adult participants with moderate to severe pain due to knee osteoarthritis
Subjects
420
Treatment
arms
Arm A: GSK3858279 dose 1
Arm B: GSK3858279 dose 2
Arm C: GSK3858279 dose 3
Arm D: GSK3858279 dose 4
Arm E: placebo
Description
A
multicentre randomised, double-blind, placebo controlled, dose-finding trial
of GSK3858279 in adult participants with moderate to severe pain due to knee
osteoarthritis
Timeline
Trial start anticipated: H2 2023
Data anticipated: 2025
Key end
points
Change from baseline in the weekly average of average daily knee pain
intensity at week 12, assessed on Numeric Rating Scale (NRS)
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
65
Respiratory/Immunology
GSK1070806 (Atopic dermatitis)
NCT05999799
Phase
IIb
Patient
Patients with moderate to severe atopic dermatitis
Subjects
175
Treatment
arms
Arm A: GSK1070806 dose 1
Arm B: GSK1070806 dose 2
Arm C: GSK1070806 dose 3
Arm D: GSK1070806 dose 4
placebo
Description
A randomized, double
-blind, parallel group, placebo-controlled dose finding
study to evaluate the efficacy, safety, pharmacokinetics, and
pharmacodynamics of GSK1070806 SC injection
Timeline
Trial start: Q4 2023
Data anticipated: 2025
Key end
points
Percent change from baseline in eczema area and severity index (EASI) at
Week 16
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
66
Respiratory/Immunology
GSK3888130 (Autoimmune disease)
NCT05131971
Phase
I
Patient
Healthy participants aged 18
-55 inclusive
Subjects
54
Treatment
arms
Cohort 1: GSK3888130B at dose level 1 (placebo comparator)
Cohort 2: GSK3888130B at dose level 2 (placebo comparator)
Cohort 3: GSK3888130B at dose level 3 (placebo comparator)
Cohort 4: GSK3888130B at dose level 4 (placebo comparator)
Cohort 5: GSK3888130B at dose level 5 (placebo comparator)
Cohort 6: GSK3888130B at dose level 6 (placebo comparator)
Cohort 7: GSK3888130B at dose level 7 (placebo comparator)
Description
A
randomised, double-
blind, placebo controlled, single dose escalation trial to
evaluate safety, tolerability, pharmacokinetics and pharmacodynamics of
GSK3888130B
Timeline
Trial start: Q4 2021
Trial end: Q4 2023
Key end
points
Number of participants with AEs and SAEs
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
67
Respiratory/Immunology
GSK3862995 (COPD)
NCT06154837
Phase
I
Patient
Part A: Healthy participants
Part B: Participants with Chronic Obstructive Pulmonary Disorder
Subjects
130
Treatment
arms
Part A: Single ascending dose (SAD) of GSK3862995B
Part B, arm A: Repeat doses GSK3862995B
Part B, arm B: Placebo
Description
A two
-part randomized, double-blind, placebo-controlled study to investigate
safety, tolerability, immunogenicity, pharmacokinetics and pharmacodynamics
of GSK3862995B following single ascending doses in healthy participants and
repeat doses in participants with Chronic Obstructive Pulmonary Disease
(COPD)
Timeline
Trial start: Q4 2023
Data anticipated: 2026+
Key end
points
AEs and SAEs
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Respiratory/Immunology OncologyHIV Opportunity driven Glossary
68
Respiratory/Immunology
GSK4347859 (Systemic lupus erythematosus)
NCT06188507
Phase
I
Patient
Healthy participants
Subjects
44
Treatment
arms
Part 1, cohort 1: GSK4347859 or placebo
Part 1, cohort 2: GSK4347859 or placebo
Part 2, cohort 3: GSK4347859 (dose level A) or placebo
Part 2, cohort 4: GSK4347859 (dose level B) or placebo
Part 2, cohort 5: GSK4347859 (dose level C) or placebo
Description
A randomized, double
-blind, placebo-controlled study to evaluate the safety,
tolerability, pharmacokinetics and pharmacodynamics of GSK3996401
following single and multiple ascending doses of GSK4347859 in healthy
participants
Timeline
Trial start: Q1 2024
Data anticipated: 2025
Key end
points
AEs and SAEs
Maximum observed plasma concentration (
Cmax) of GSK3996401 following
administration of GSK4347859
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
70
Oncology
Ojjaara/Omjjara (momelotinib)
NCT03441113
Phase
II
Patient
Participants with primary myelofibrosis (PMF) or post
-polycythemia vera or
post
-essential thrombocythemia myelofibrosis (post-PV/ET MF)
Subjects
237
Treatment
arms
Arm A: Study GS
-US-352-0101
Arm B: Study GS
-US-352-1214
Arm C: Study GS
-US-352-1154
Arm D: Study SRA
-MMB-301
Description
Extended access and assess long
-term safety of momelotinib (MMB) in
participants with PMF or post
-PV/ET MF
Timeline
Trial start: Q3 2018
Anticipated trial end: 2026+
Key end
points
Number of patients who had access to and received the intervention
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
71
Oncology
Jemperli (dostarlimab)
NCT03981796
- RUBY ENGOT-EN6 GOG-3031
Phase
III
Patient
Patients with recurrent or primary advanced endometrial cancer
Subjects
785
Treatment
arms
Arm A:
dostarlimab + SoC followed by dostarlimab
Arm B: placebo + SoC followed by placebo
Arm C:
dostarlimab + SoC followed by dostarlimab+niraparib
Arm D: placebo (+chemo) followed by PBO
Description
A
randomised, double-blind, multi-centre trial of dostarlimab plus carboplatin
-
paclitaxel with and without niraparib maintenance versus placebo plus
carboplatin
-paclitaxel in patients with recurrent or primary advanced
endometrial cancer
Timeline
Trial start: Q3 2019
Part 1 data reported: Q4 2022; Part 2 data reported: Q4 2023
Key end
points
Part 1: PFS by IA (
dMMR/MSI-H and ITT) and OS (ITT)
Part 2: PFS (ITT)
Clinicaltrials.g
ov
Link
NCT04581824
- PERLA
Phase
II
Patient
Participants with metastatic non
-squamous non-small cell lung cancer
(NSCLC)
Subjects
244
Treatment
arms
Arm A:
dostarlimab + chemotherapy
Arm B: pembrolizumab + chemotherapy
Description
A
randomised, double-blind trial to evaluate the efficacy of dostarlimab plus
chemotherapy versus pembrolizumab plus chemotherapy in metastatic non
-
squamous NSCLC
Timeline
Trial start: Q4 2020
Primary data reported: Q4 2022
Key end
points
ORR, OS, PFS
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
72
Oncology
Jemperli (dostarlimab)
NCT02715284
- GARNET
Phase
I/II
Patient
Participants with advanced solid tumors
Subjects
740
Treatment
arms
Part 1:
dostarlimab at ascending weight doses
Part 2A:
dostarlimab
fixed dose of 500mg Q3W or 1000mg administered Q6W
dose
Part 2B: Cohort A1
dMMR/MSI-H endometrial
Part 2B: Cohort A2 MMR proficient/MSS endometrial
Part 2B: Cohort E: NSCLC
Part 2B: Cohort F non
-endometrial dMMR/MSI-H & POLE-mutation
Part 2B: Cohort G PROC without known BRCA
Description
A multi
-centre, open-label, first-in-human trial evaluating dostarlimab in
participants with advanced solid tumors who have limited available treatment
options
Timeline
Trial start: Q1 2016
Primary data reported: Q1 2019
Key end
points
ORR,
DoR, safety
Clinicaltrials.g
ov
Link
NCT05723562
- AZUR-1
Phase
II
Patient
Patients with untreated stage II/III mismatch repair deficient/high
microsatellite instability (
dMMR/MSI-H) locally advanced rectal cancer
Subjects
150
Treatment
arms
dostarlimab
monotherapy
Description
A single
-arm, open-label trial with dostarlimab monotherapy in participants
with untreated stage II/III
dMMR/MSI-H locally advanced rectal cancer
Timeline
Trial start: Q1 2023
Data anticipated: 2026+
Key end
points
Sustained
cCR for 12, 24 and 36 months, EFS at 3 years
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
73
Oncology
Jemperli (dostarlimab)
NCT05855200
- AZUR-2
Phase
III
Patient
Participants with untreated T4N0 or Stage III (
resectable), mismatch repair
deficient/high microsatellite instability (
dMMR/MSI-H) colon cancer
Subjects
711
Treatment
arms
Arm A:
dostarlimab
Arm B: Standard of care (FOLFOX/CAPEOX) or expectant observation post
surgery.
Description
An open
-label, randomized trial of perioperative dostarlimab monotherapy
versus standard of care in participants with untreated T4N0 or Stage III
dMMR
/MSI-H resectable colon cancer
Timeline
Trial start: Q3 2023
Data anticipated: 2026+
Key end
points
EFS assessed by Blinded Independent Central Review (BICR)
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
74
Oncology
Zejula (niraparib)
NCT03602859
- FIRST
Phase
III
Patient
Participants with Stage III or IV
nonmucinous epithelial ovarian cancer
Subjects
1402
Treatment
arms
Arm A: SOC (carboplatin + paclitaxel
± bevacizumab) +placebo
Arm B: SOC + niraparib
Arm C: SOC +
dostarlimab + niraparib
Description
A
randomised, double-blind comparison of platinum-based therapy with TSR-
042 and niraparib versus standard of care platinum
-based therapy as first-
line
treatment of Stage III or IV
nonmucinous epithelial ovarian cancer
Timeline
Study start: Q4 2018
Data anticipated: H1 2024
Key end
points
PFS for PD
-
L1 positive participants. Primary analysis is ARM B vs ARM C. This is
an adaptive study with ARM A closed post topline.
Clinicaltrials.g
ov
Link
NCT04475939
- ZEAL-1L
Phase
III
Patient
Participants whose disease has remained stable or responded to 1L platinum
based chemo with pembrolizumab for stage IIIB/IIIC or IV NSCLC
Subjects
666
Treatment
arms
Arm A: niraparib plus pembrolizumab
Arm B: placebo plus pembrolizumab
Description
A
randomised, double-blind, placebo-controlled, multicentre
study comparing
niraparib plus pembrolizumab versus placebo plus pembrolizumab as
maintenance therapy
Timeline
Study start: Q4 2020
Data anticipated: H2 2024
Key end
points
OS, PFS assessed by BICR using Response Evaluation Criteria in Solid Tumors
(RECIST)
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
75
Oncology
Blenrep (belantamab mafodotin)
NCT04126200
- DREAMM-5
Phase
I/II
Patient
Participants with relapsed/refractory multiple myeloma (RRMM)
Subjects
464
Treatment
arms
Substudy
1: belantamab mafodotin + OX40 (GSK3174998)
Substudy
2: belanatamab mafodotin + feladilimab
Substudy
3: belantamab mafodotin + nirogacestat (GSI)
Substudy
4: belantamab mafodotin + dostarlimab
Substudy
5: belantamab mafodotin + isatuximab
Substudy
6: belantamab mafodotin + nirogacestat + lenalidomide +
dexamethasone
Substudy
7: belantamab mafodotin + nirogacestat + pomalidomide +
dexamethasone
Description
A
randomised, open-label platform trial utilizing a master protocol to trial
belantamab
mafodotin as monotherapy and in combination with anti-cancer
treatments
Timeline
Trial start: Q4 2019
Data anticipated: 2026+
Key end
points
Dose escalation phase: DLT, safety, ORR
Cohort expansion phase: ORR, CBR, safety
Clinicaltrials.g
ov
Link
NCT03544281
- DREAMM-6
Phase
I/II
Patient
Participants with relapsed/refractory multiple myeloma (RRMM)
Subjects
152
Treatment
arms
Arm A:
belantamab mafodotin + lenalidomide + dexamethasone
Arm B:
belantamab mafodotin + bortezomib + dexamethasone
Description
An open
-label, dose escalation and expansion trial to evaluate safety,
tolerability and clinical activity of the antibody
-drug conjugate belantamab
mafodotin
administered in combination with lenalidomide plus
dexamethasone (Arm A), or bortezomib plus dexamethasone (Arm B)
Timeline
Trial start: Q3 2018
Data anticipated: H1 2024
Key end
points
DLT, safety, ORR, PK
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
76
Oncology
Blenrep (belantamab mafodotin)
NCT04246047
- DREAMM-7
Phase
III
Patient
Participants with relapsed/refractory multiple myeloma (RRMM)
Subjects
571
Treatment
arms
Arm A:
belantamab mafodotin + bortezomib + dexamethasone (B-Vd)
Arm B: daratumumab, bortezomib + dexamethasone (D
-Vd)
Description
A
multicentre, open-label, randomised
trial to evaluate the efficacy and safety
of the combination of
belantamab mafodotin, bortezomib and
dexamethasone (B
-Vd) compared with the combination of daratumumab,
bortezomib and dexamethasone (D
-Vd)
Timeline
Trial start: Q2 2020
Data readout: Q4 2023
Key end
points
PFS, CRR, ORR,
DoR, TTR, TTP, OS, PFS2, MRD negativity rate, safety
Clinicaltrials.g
ov
Link
NCT04246047
- DREAMM-8
Phase
III
Patient
Participants with relapsed/refractory multiple myeloma (RRMM)
Subjects
300
Treatment
arms
Arm A:
belantamab mafodotin+ pomalidomide + dexamethasone (B-Pd)
Arm B: Pomalidomide, bortezomib + dexamethasone (P
-Vd)
Description
A
multicentre, open-label, randomised
trial to evaluate the efficacy and safety
of
belantamab mafodotin in combination with pomalidomide and
dexamethasone (B
-Pd) versus pomalidomide plus bortezomib and
dexamethasone (
PVd)
Timeline
Trial start: Q4 2020
Data anticipated: H2 2024
Key end
points
PFS, MRD negativity rate, ORR, CRR, VGPR or better rate,
DoR, TTBR, TTR,
TTP, OS, PFS2, safety
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
77
Oncology
Blenrep (belantamab mafodotin)
NCT04091126
- DREAMM-9
Phase
I
Patient
Patients with newly diagnosed multiple myeloma (MM)
Subjects
144
Treatment
arms
Belantamab
mafodotin, selected doses
Bortezomib, administered subcutaneously or intravenously approximately 1
hour after the
belantamab mafodotin infusion until Cycle 8
Lenalidomide, administered as 25 or 10 mg orally, depending upon renal
function.
Dexamethasone, administered orally as 20 mg in cycles 1
-
8 and 40 mg in Cycle
9 onwards
Description
A
randomised, dose and schedule evaluation trial to investigate the safety,
pharmacokinetics, pharmacodynamics and clinical activity of
belantamab
mafodotin
administered in combination with standard of care
Timeline
Trial start: Q4 2019
Data anticipated: 2025
Key end
points
DLT, safety, RDI of lenalidomide and bortezomib, PK, PD, ORR, CRR, VGPR or
better
Clinicaltrials.g
ov
Link
NCT04398745
- DREAMM-12
Phase
I
Patient
Relapsed/refractory multiple myeloma (RRMM) who have normal and varying
degrees of impaired renal function
Subjects
36
Treatment
arms
belantamab
mafodotin monotherapy
Description
A trial to evaluate the pharmacokinetics and safety of
belantamab mafodotin
monotherapy
Timeline
Trial start: Q4 2020
Data anticipated: 2025
Key end
points
PK, change in vital signs, safety
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
78
Oncology
Blenrep (belantamab mafodotin)
NCT04398680
- DREAMM-13
Phase
I
Patient
Relapsed/refractory multiple myeloma (RRMM) who have normal and
impaired hepatic function
Subjects
28
Treatment
arms
belantamab
mafodotin monotherapy
Description
A trial to evaluate the pharmacokinetics and safety of
belantamab mafodotin
monotherapy in participants who have normal and impaired hepatic function
Timeline
Trial start: Q2 2021
Data anticipated: 2025
Key end
points
PK, change in vital signs, safety
Clinicaltrials.g
ov
Link
NCT05064358
- DREAMM-14
Phase
II
Patient
Participants with relapsed/refractory multiple myeloma (RRMM)
Subjects
180
Treatment
arms
Arm A:
belantamab mafodotin
Description
A
randomised, parallel, open-
label study to investigate the safety, efficacy and
pharmacokinetics of various dosing regimens of single
-agent belantamab
mafodotin
(GSK2857916)
Timeline
Study start: Q1 2022
Data anticipated: H2 2024
Key end
points
% of patients with >= Gr 2 ocular events, safety, ORR, TTR,
DoR, TTP, PFS, OS
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
79
Oncology
cobolimab
NCT04655976
- COSTAR LUNG
Phase
II/III
Patient
Patients with advanced non
-small cell lung cancer (NSCLC) who have
progressed on prior anti
-PD-(L)1 therapy and chemotherapy
Subjects
750
Treatment
arms
Arm A:
cobolimab + dostarlimab + docetaxel
Arm B:
dostarlimab + docetaxel
Arm C: docetaxel
Description
A
randomised, open label trial comparing cobolimab + dostarlimab +
docetaxel to
dostarlimab + docetaxel to docetaxel alone
Timeline
Trial start: Q4 2020
Data anticipated: H2 2024
Key end
points
OS, ORR, PFS, DoR, TTD
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
80
Oncology
belrestotug
NCT05565378
- GALAXIES LUNG-201
Phase
II
Patient
Participants with previously untreated, locally advanced/metastatic,
Programmed Death Ligand 1
-selected non small cell lung cancer (NSCLC)
Subjects
300
Treatment
arms
Comparator Arm: pembrolizumab monotherapy
Intervention Arm:
dostarlimab monotherapy
Substudy
1A: dostarlimab + belrestotug (Dose A)
Substudy
1B: dostarlimab + belrestotug (Dose B)
Substudy
1C: dostarlimab + belrestotug (Dose C)
Substudy
2: dostarlimab + belrestotug + GSK6097608
Description
A randomized, open
-
label, platform trial utilizing a master protocol to evaluate
novel immunotherapy combinations in participants with previously untreated,
locally advanced/metastatic, Programmed Death Ligand 1
-selected NSCLC
Timeline
Trial start: Q4 2022
Data anticipated: 2026+
Key end
points
ORR
Clinicaltrials.g
ov
Link
NCT06062420
- GALAXIES H&N-202
Phase
II
Patient
Participants with recurrent/metastatic PD
-L1 positive squamous cell
carcinoma of the head and neck
Subjects
360
Treatment
arms
Arm A:
dostarlimab monotherapy
Arm B:
dostarlimab and belrestotug
Arm C:
dostarlimab and GSK6097608
Arm D:
dosarlimab and belrestotug and GSK6097608
Description
A randomized, open
-label, platform study using a master protocol to evaluate
novel immunotherapy combinations as first
-line treatment in participants with
recurrent/metastatic PD
-
L1 positive squamous cell carcinoma of the head and
neck
Timeline
Trial start: Q4 2023
Data anticipated: 2026+
Key end
points
ORR
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
NCT03739710
– ENTRÉE
Phase
II
Patient
Participants with non
-small cell lung cancer (NSCLC)
Subjects
185
Treatment
arms
Part 1
Arm A:
feladilimab + ipilimumab
Arm B:
dostarlimab + GSK4428859A
Arm C:
dostarlimab
+ GSK4428859A +
GSK6097608
Part 2
SoC: docetaxel
feladilimab
and docetaxel
Description
A randomized, open
-label platform trial utilizing a master protocol to trial
novel regimens versus standard of care treatment in NSCLC participants
Timeline
Trial start: Q1 2019
Data anticipated: 2025+
Key end
points
Part 1: Number of participants with AEs, SAEs, DLT, clinically significant
changes in vital signs, physical examination and laboratory parameters.
Number of participants requiring dose modifications.
Part 2: Overall survival
Clinicaltrials.g
ov
Link
81
Oncology
belrestotug

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
82
Oncology
GSK4381562
NCT05277051
Phase
I
Patient
Participants with selected advanced solid tumors
Subjects
162
Treatment
arms
Arm A: GSK4381562 monotherapy
Arm B: GSK4381562 plus
dostarlimab
Arm C: GSK4381562 plus
dostarlimab plus belrestotug
Description
An open
-label study of GSK4381562 administered as monotherapy and in
combination with anticancer agents
Timeline
Study start: Q1 2022
Data anticipated: 2026+
Key end
points
Safety and PK
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
83
Oncology
GSK6097608
NCT04446351
Phase
I
Patient
Participants with advanced solid
tumours
Subjects
184
Treatment
arms
Arm A: GSK6097608
Arm B: GSK6097608 +
dostarlimab
Arm C:
dostarlimab
Arm D:
dostarlimab + belrestotug
Arm E:
dostarlimab + belrestotug + GSK6097608
Arm D:
dostarlimab + cobolimab
Description
A
first time in human, open-label trial of GSK6097608 administered as
monotherapy and in combination with anticancer agents
Timeline
Trial start: Q1 2020
Data anticipated: 2025
Key end
points
DLT, AEs and SAEs
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
84
Oncology
belantamab
NCT05714839
- DREAMM-20
Phase
I/II
Patient
Relapsed/refractory multiple myeloma (RRMM) [Parts 1 and 2]
Transplant
-ineligible newly diagnosed multiple myeloma (TI NDMM) [Part 3]
Subjects
124
Treatment
arms
Part 1:
belantamab (may switch to belantamab mafodotin in case of PD)
Part 2: Bela
-xRd and Belamaf-xRd. The combination treatment xRd includes lenalidomide (R) and dexamethasone (d). x will be either a
standard of care (SoC) or an emerging treatment.
Part 3: Participants with TI NDMM will receive Bela
-xRd and Belamaf-xRd. The combination treatment xRd includes lenalidomide (R) and
dexamethasone (d). x will be either a standard of care (SoC) or an emerging treatment
Description
An open
-lab multicentre, dose escalation and expansion trial to investigate the safety, tolerability and clinical activity of belantamab as
monotherapy and in combination with other treatments in participants with multiple myeloma
Timeline
Trial start: Q3 2023
Data anticipated: 2026+
Key end
points
Part 1: Safety and tolerability (including DLTs), PK and recommended Part 2 dose
Part 2: Safety and tolerability, PK and recommended phase II dose
Part 3: Safety and tolerability, PK and efficacy
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Oncology Opportunity drivenHIV Respiratory/Immunology Glossary
85
Oncology
GSK4524101
NCT06077877
Phase
I/II
Patient
Adult participants with solid tumors
Subjects
112
Treatment
arms
Arm A, Part 1: GSK4524101 monotherapy
Arm B, Part 1: GSK4524101 plus niraparib
Arm C, Part 1: GSK4524101 food effect cohort
Arm D, Part 2: GSK4524101 plus niraparib
Arm E, Part 2: Niraparib
Description
A first
-time-in-human, open-label, multicentre
, dose escalation and expansion
study of the oral DNA Polymerase Theta inhibitor (
POLQi
) GSK4524101 and the
PARP inhibitor (
PARPi) Niraparib in adult participants with solid tumors
Timeline
Trial start: Q4 2023
Data anticipated: 2025
Key end
points
DLTs, AEs, SAEs, ORR
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Opportunity driven GlossaryHIV Respiratory/Immunology Oncology
87
Opportunity driven
linerixibat
NCT04950127
- GLISTEN
Phase
III
Patient
Participants with primary biliary cholangitis (PBC)
Subjects
230
Treatment
arms
Arm A:
linerixibat
Arm B:
linerixibat followed by placebo
Arm C: placebo
Arm D: placebo followed by
linerixibat
Description
A two
-part randomised, placebo controlled, double blind, multicentre trial to
evaluate the efficacy and safety of
linerixibat for the treatment of cholestatic
pruritus in participants with primary biliary cholangitis
Timeline
Trial start: Q3 2021
Data anticipated: H2 2024
Key end
points
Change from baseline in monthly itch scores over 24 weeks using Numerical
Rating Scale (NRS)
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Opportunity driven GlossaryHIV Respiratory/Immunology Oncology
88
Opportunity driven
GSK4532990 (Non-alcoholic steathohepatitis)
NCT05583344
- HORIZON
Phase
IIb
Patient
Adults with non
-alcoholic steatohepatitis (NASH) and advanced fibrosis
Subjects
246
Treatment
arms
Arm 1: high dose GSK4532990
Arm 2: low dose GSK4532990
Arm 3: placebo
Description
A placebo
-controlled trial to evaluate the efficacy and safety of GSK4532990
in adults with pre
-cirrhotic non-alcoholic steatohepatitis (NASH)
Timeline
Trial start: Q1 2023
Data anticipated: 2025
Key end
points
Part 1: Percentage of participants achieving ≥ 1 stage improvement in
histological fibrosis with no worsening of
NASH (at week 52)
Part 2: Percentage of participants achieving NASH resolution with no
worsening of fibrosis (at week 52)
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth Opportunity driven GlossaryHIV Respiratory/Immunology Oncology
89
Opportunity driven
GSK4172239 (Sickle cell disease)
NCT05660265
Phase
I
Patient
Participants with sickle cell disease
Subjects
40
Treatment
arms
Cohort 1: GSK4172239D (Dose 1)
Cohort 2: GSK4172239D (Dose 2)
Cohort 3: GSK4172239D (Dose 3)
Cohort 4: GSK4172239D (Dose 4)
Cohort 5: GSK4172239D (Dose 5)
Food effect cohort
Description
A
randomised, placebo-controlled, double-blind (sponsor unblind), parallel
group, single dose, dose escalation to evaluate the safety, tolerability and
pharmacokinetics of GSK4172239D
Timeline
Trial start: Q3 2023
Data anticipated: 2025
Key end
points
Area under curve zero to time infinity (AUC 0
-
inf) for GSK4106401 after a single
oral dose of GSK4172239D
Clinicaltrials.g
ov
Link

Infectious diseasesInnovation: Pipeline growth GlossaryHIV Respiratory/Immunology Oncology Opportunity driven
91
ADC Antibody drug conjugate EGPA Eosinophilic granulomatosis with polyangiitis NSCLC Non-small cell lung cancer
AE Adverse event FVC Forced vital capacity OMV Outer membrane vesicle
AESI Adverse event of special interest GC Urogenital gonorrhea ORR Overall response rate
AUC Area under curve GMMA Generalised Modules for Membrane Antigens OS Overall surival
BCMA B-cell maturation antigen GSI Gamma secretase inhibitor PBC Primary biliry cholangitis
BICR Blinded Independent Central Review HA Healthy adults PFS Progression-free survival
BRCA Breast cancer HBV Hepatitis B virus PFS2 Time to second disease progression or death
CAE Corneal adverse events HES Hypereosinophilic syndrome PK Pharmacokinetic
CBR Clinical benefit rate Hgb Hemoglobin PMF
Primary myelofibrosis
cCR Complete clinical response hSBA Human serum bactericidal assay Post-PV/ET MF Post-essential thrombocythemia myelofibrosis
CKD Chronic kidney disease HZ Herpes zoster RCC Refractory chronic cough
CfB Change from baseline IC Immunocompromised RL Repeat dose level
CMV Cytomegalovirus ICR Independent central review RRMM Relapsed/refractory multiple myeloma
CN China iNTS Invasive non-typhoidal salmonella RSV Respiratory syncytial virus
COPD Chronic obstructive pulmonary disease ITT Intention-to-treat SAD Single ascending dose
CP Cholestatic pruritus JP Japan SAE Serious adverse event
CRR Complete response rate LLOQ Lower limit of quantitation siRNA Small interfering RNA
CRSwNP Chronic rhinosinusitis with nasal polyps LRTS Lower respiratory tract symptoms SoC Standard of care
cUTI Complicated urinary tract infection MAD Multiple ascending dose SSc-ILD
Systemic sclerosis associated interstitial lung disease
CV Cardiovascular MAE Medical attended events TOC Test of cure
DDI Drug-drug interaction MDI Metered dose inhaler TTBR Time to best response
DFS Disease-freee survival MAPS Mulitple Antigen Presenting System TTD Time to treatment discontinuation
DL Dose level MM Multiple myeloma TTP Time to tumour progression
DLT Dose-limiting toxicity MMR Measles, mumps and rubella TTR Time to treatment response
dMMR Deficient mismatch repair MMRV Measles, mumps, rubella and varicella UTI Urinary tract infection
DoR Duration of response MRD Multiple rising dose uUTI Uncomplicated urinary tract infection
DPNP Diabetic peripheral neuropathic pain MSI-H Microsatellite instability high VGPR Very good partial remission
EASI Eczema Area and Severity Index NASH Nonalcoholic steatohepatitis VSP Vital sign parameters
EGPA Eosinophilic granulomatosis with polyangiitis NRS Numeric Rating Scale YoA Years of age
Glossary