
Methyltert-butyl ether
1634-04-4
Hazard Summary
Methyltert-butyl ether is used as a gasoline additive. Exposure may occur by breathing air contaminated
with auto exhaust or gasoline fumes while refueling autos. Respiratory irritation, dizziness, and
disorientation have been reported by some motorists and occupationally exposed workers. Acute (short-
term) exposure of humans to methyltert-butyl ether also has occurred during its use as a medical
treatment to dissolve cholesterol gallstones. Chronic (long-term) inhalation exposure to methyltert-butyl
ether has resulted in central nervous system (CNS) effects, respiratory irritation, liver and kidney effects,
and decreased body weight gain in animals. Developmental effects have been reported in rats and mice
exposed via inhalation. EPA has not classified methyltert-butyl ether with respect to potential
carcinogenicity.
Please Note: The main sources of information for this fact sheet are EPA'sIntegrated Risk Information System(IRIS)
(2), which contains information on inhalation chronic toxicity of methyltert-butyl ether and theRfCand the Agency
for Toxic Substances and Disease Registry's (ATSDR's)Toxicological Profile for Methyl t-Butyl Ether. (4)
Uses
Nearly all methyltert-butyl ether produced in the United States is used as an additive in unleaded gasoline
to increase octane levels and reduce carbon monoxide emissions. (1,4,5)
It was used in the past to produce isobutene. (1,5)
Sources and Potential Exposure
The general population may be exposed to methyltert-butyl ether by breathing air contaminated with auto
exhaust or gasoline fumes while refueling cars. (1,4)
Workers may be occupationally exposed via inhalation or dermal contact. (1)
Assessing Personal Exposure
No information was located regarding the measurement of personal exposure to methyltert-butyl
ether.(4)
Health Hazard Information
Acute Effec ts:
Acute exposure of humans to methyltert-butyl ether has occurred via injection into the gallbladder during
its use as a medical treatment to dissolve cholesterol gallstones. Nausea, vomiting, and sleepiness have
been observed; in one case renal failure was reported. (1,2,4)
Acute inhalation exposure has resulted in CNS effects including ataxia and abnormal gait in rats. (2,4)
Acute animal tests in rats have demonstrated methyltert-butyl ether to havelowacute toxicity via
inhalation and moderateacute toxicity via ingestion. (3)
Chronic Effects (Noncancer):

Chronic Effects (Noncancer):
Motorists and gas station attendants have reported symptoms of coughing, burning sensations in the nose
and throat, headache, dizziness, and feelings of spaciness and disorientation that may have been
associated with methyltert-butyl ether exposure. (4)
CNS effects observed in animals following inhalation or oral exposure to methyltert-butyl ether include
ataxia, incoordination, loss of righting reflex, decreased startle and pain reflexes, prostration, drowsiness,
and hypoactivity. (4)
Other effects noted in rats and/or mice chronically exposed by inhalation include liver and kidney effects,
respiratory irritation, and decreased body weight gain. (4)
The Reference Concentration (RfC) for methyltert-butyl ether is 3.0 milligrams per cubic meter (mg/m
3
)
based on increased liver and kidney weights, increased prostration in females, and swollen periocular
tissues in male and female rats. TheRfCis an estimate (with uncertainty spanning perhaps an order of
magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups)
that is likely to be without appreciable risk of deleterious noncancer effects during a lifetime. It is not a
direct estimator of risk but rather a reference point to gauge the potential effects. At exposures
increasingly greater than theRfC, the potential for adverse health effects increases. Lifetime exposure
above theRfCdoes not imply that an adverse health effect would necessarily occur. (2)
EPA has medium confidence in the study on which theRfCwas based because it was well designed (e.g.,
with respect to exposure protocol, number of animals, and exposure duration), identified a consistent
lowest-observed-adverse-effect level (LOAEL) and no-observed-adverse-effect level (NOAEL) for a number
of organ systems, and involved extensive histopathology on both sexes. However, the results of the rat
study are confounded by the high mortality in the males, which is presumed to be the result of rat chronic
nephropathy. EPA has medium to high confidence in the database because of the existence of chronic and
subchronic bioassays in more than one species, developmental studies in several different species, and the
existence of single- and two-generation reproductive studies in the rat; and, consequently, medium to
high confidence in the RfC. (2)
EPA has not established a Reference Dose (RfD) for methyltert-butyl ether. (2)
Reproductive/Developmental Effects:
No information is available on the reproductive or developmental effects of methyltert-butyl ether in
humans.
In rats exposed via inhalation, reduced body weight and body weight gain in pups and decreased pup
viability have been reported. (2,4)
Developmental effects have been reported in mice. A decreased number of viable implantations, increased
maternal toxicity, late resorptions, and skeletal variations were observed in mice exposed via inhalation.
(2,4)
Cancer Risk:
No information is available on the carcinogenic effects of methyltert-butyl ether in humans.
In inhalation studies, an increased incidence of liver tumors was reported in mice. In male rats, increased
incidences of renal tubular adenoma and carcinoma and interstitial cell adenoma in the testes were
reported; however, the renal tumors may have resulted from accumulation of a protein unique to male rats
and testicular tumors are common in the strain of rats used. (4)
Lymphomas, leukemia, and testicular Leydig cell tumors were reported in orally exposed rats. (4)
EPA has not classified methyltert-butyl ether with respect to potential carcinogenicity. (2)
Physical Properties
Methyltert-butyl ether is also called MTBE andtert-butyl methyl ether. (1,3)
The chemical formula for methyltert-butyl ether is C
5
H
12
O, and its molecular weight is 88.15 g/mol.
(1,4,5)
Methyltert-butyl ether occurs as a colorless liquid, with a vapor pressure of 245 mm Hg at 25 °C. (1,4,5)
Methyltert-butyl ether occurs as a colorless liquid, with a vapor pressure of 245 mm Hg at 25 °C. (1,4,5)
It has a log octanol/water partition coefficient (log K
ow
) of 1.24. (5)
Conversion Factors:
To convert concentrations in air (at 25 °C) from ppm to mg/m
3
: mg/m
3
= (ppm) × (molecular weight of the
compound)/(24.45). For methyl tert-butyl ether: 1 ppm = 3.61 mg/m
3
.
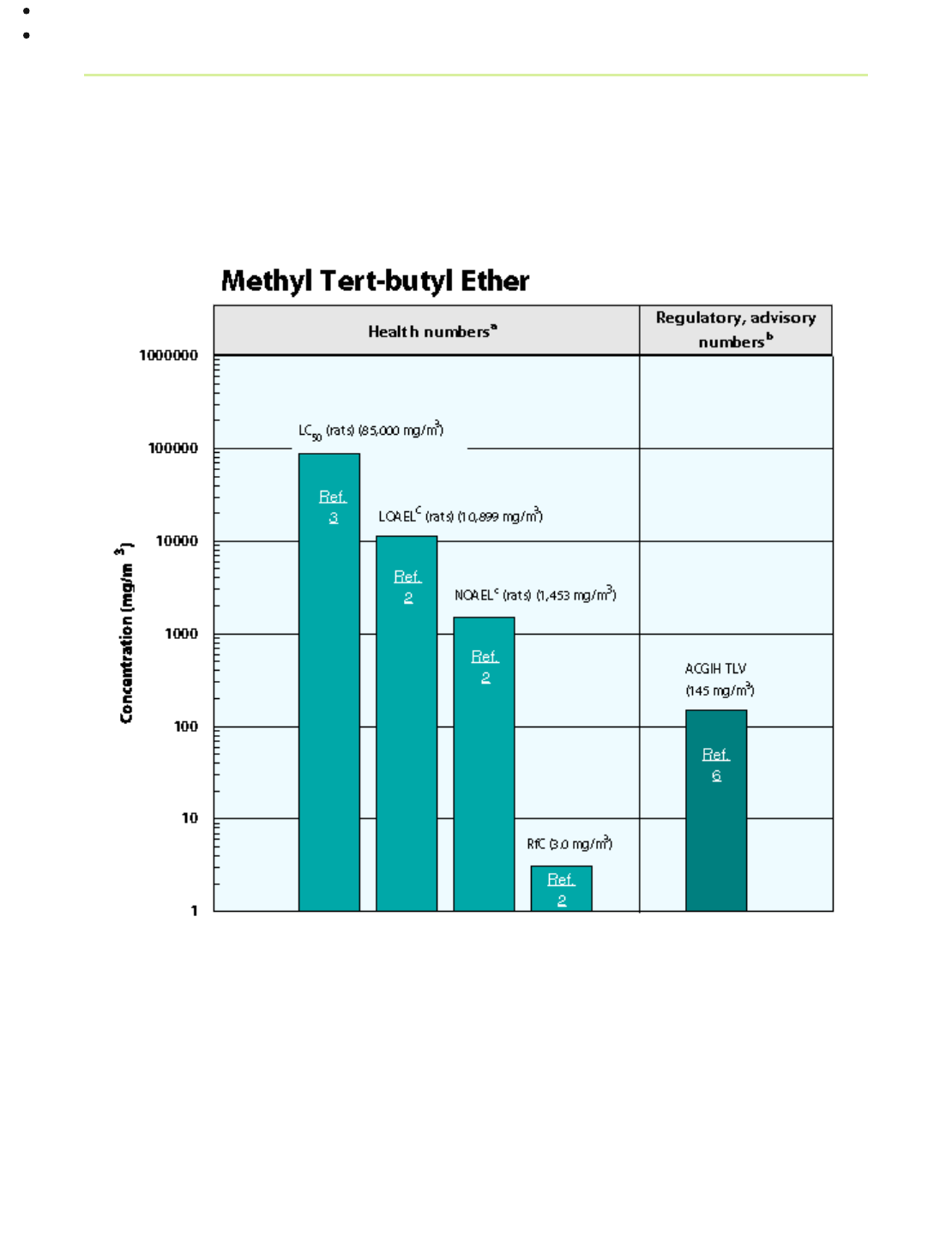
Health Data from Inhalation Exposure
ACGIH TLV --American Conference of Governmental and Industrial Hygienists' threshold limit value expressed as a
time-weighted average; the concentration of a substance to which most workers can be exposed without adverse
effects.
LC
50
(Lethal Concentration
50
)--A calculated concentration of a chemical in air to which exposure for a specific
length of time is expected to cause death in 50% of a defined experimental animal population.
The health and regulatory values cited in this factsheet were obtained in December 1999.
a
Health numbers are toxicological numbers from animal testing or risk assessment values developed by EPA.
b
Regulatory numbers are values that have been incorporated in Government regulations, while advisory numbers
are nonregulatory values provided by the Government or other groups as advice. ACGIH numbers are advisory.
c
The LOAEL and NOAEL are from the critical study used as the basis for the EPA RfC.
References
1. U.S. Department of Health and Human Services. Hazardous Substances Data Bank (HSDB, online database).
Summary created in April 1992, updated January 2000

1. U.S. Department of Health and Human Services. Hazardous Substances Data Bank (HSDB, online database).
National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993.
2. U.S. Environmental Protection Agency.Integrated Risk Information System (IRIS) on Methyl tert-Butyl Ether.
National Center for Environmental Assessment, Office of Research and Development, Washington, DC.
1999.
3. U.S. Department of Health and Human Services. Registry of Toxic Effects of Chemical Substances (RTECS,
online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD.
1993.
4. Agency for Toxic Substances and Disease Registry (ATSDR).Toxicological Profile for Methyl t-Butyl
Ether.Public Health Service, U.S. Department of Health and Human Services. Atlanta, GA. 1996.
5. The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals. 11th ed. Ed. S. Budavari. Merck and
Co. Inc., Rahway, NJ. 1989.
6. American Conference of Governmental Industrial Hygienists (ACGIH).1999 TLVs and BEIs. Threshold Limit
Values for Chemical Substances and Physical Agents. Biological Exposure Indices. Cincinnati, OH. 1999.
