
661
Ciênc. Tecnol. Aliment., Campinas, 32(4): 661-667, out.-dez. 2012
Original
Ciência e Tecnologia de Alimentos ISSN 0101-2061
Received 16/3/2010
Accepted 28/2/2012 (004730)
1
Departament of Technology and Science of Food, Universidade Federal de Santa Maria – UFSM, Bairro Camobi, CEP 97105-900, Santa Maria, RS, Brazil,
e-mail: ajcichoski@hotmail.com
2
Departament of Food Engineering, Universidade Regional Integrada do Alto Uruguai e das Missões – URI, Campus de Erechim, Av. Sete de Setembro, 1621,
CEP 99700-000, Erechim, RS, Brazil
3
EMBRAPA Pigs and Poultry, BR 153, Km 110, CP 21, CEP 89700-000, Concórdia, SC, Brazil
*Corresponding author
Investigation of glutathione peroxidase activity in chicken meat under dierent
experimental conditions
Investigação da atividade de glutationa peroxidase em carne de frango submetida a diferentes
condições experimentais
Alexandre José CICHOSKI
1
*, Renata Bezerra ROTTA
2
, Gerson SCHEUERMANN
3
,
Anildo CUNHA JUNIOR
3
, Juliano Smanioto BARIN
1
1 Introduction
Glutathione peroxidase (GSH-Px) is a selenoenzyme that is
able to slow down and prevent oxidative reactions in muscular
tissues by controlling free radicals formation from preexisting
peroxides (ARAIetal., 1994). GSH-Px is naturally found in
bones and inhibits lipid oxidation in both live tissues and
post-slaughter meat (CHAN; DECKER, 1994). Hence, GSH-Px
Resumo
Uma vez que estudos anteriores sobre a atividade enzimática da glutationa peroxidase (GSH-Px) divergem acerca da metodologia e dos
resultados, este estudo teve por objetivo investigar a inuência de diferentes condições de ensaio sobre a atividade da GSH-Px em coxas de
frangos, oriundos de diferentes dietas, nas quais se variaram a fonte e a concentração de selênio. As atividades da GSH-Px foram determinadas
seis horas após o abate e 120 dias após o armazenamento a –18 °C. As diferentes condições de ensaio envolveram a pré-incubação (0,
10 e 30minutos), o meio de reação, os tipos de substratos [H
2
O
2
(0,72 mM, 7,2 mM e 72 mM) e terc-butil hidroperóxido 15 mM] e as
concentrações diferentes de tampões [tampão 1 (fosfato de potássio 50 mM pH 7,0+EDTA 1 mM+mercaptoetanol 1 mM) e tampão
2(tris-HCl 50 mM pH 7,6+EDTA 1 mM+mercaptoetanol 5 mM)] no meio de reação. Os resultados mostraram que: i) a maior atividade
da GSH-Px foi observada quando a enzima e o substrato foram colocados em contato a 22 °C sem qualquer pré-incubação; ii) o peróxido de
hidrogênio, quando utilizado em concentrações acima de 0,72 mM, saturou a enzima GSH-Px e inibiu sua atividade; iii) a enzima GSH-Px
apresentou maior anidade para o substrato peróxido de hidrogênio, quando comparado ao peróxido de terc-butil, e iv) a adição do tampão
mercaptoetanol não promoveu aumento na atividade da enzima GSH-Px. A atividade da GSH-Px também não foi inuenciada pela fonte e
pela concentração de selênio vindo pela dieta. Os resultados obtidos permitiram denir qual a melhor temperatura de contato entre a enzima
e o substrato (22 °C) e qual a melhor concentração e o tipo de substrato e de tampão a serem utilizados. Essas informações poderão servir de
base para a execução de futuros trabalhos envolvendo a determinação da atividade da GSH-Px em carnes, pois há contradições nas poucas
informações existentes na literatura.
Palavras-chave: GSH-Px; carne de frango; atividade enzimática; selênio; tampão; substrato.
Abstract
Due to the fact that previous studies on the enzymatic activity of Glutathione peroxidase (GSH-Px) diverge widely in their methodology and
results, this study aimed to investigate the inuence of dierent analytical conditions on GSH-Px activity in chicken thighs from broilers that
were fed dierent diets with dierent sources and concentrations of selenium. GSH-Px activity was evaluated six hours aer slaughter and
120 days aer frozen storage at –18 °C. e dierent analytical conditions included time of pre-incubation (0, 10 and 30 minutes), reaction
medium, types of substrate (H
2
O
2
(0.72 mM, 7.2 mM, and 72 mM) and Terc-butil hydroperoxide 15 mM), and dierent buer concentrations
(buer 1, potassium phosphate 50 mM pH 7.0+EDTA 1 mM+mercaptoethanol 1 mM, and buer 2, tris-HCl 50 mM pH 7.6+EDTA
1mM+mercapthanol 5 mM). e results show that the highest GSH-Px activity was observed when enzyme and substrate were in contact at
22 °C without any pre-incubation, and that, when used at concentrations above 0.72 mM, hydrogen peroxide saturated the GSH-Px enzyme
and inhibited its activity. e enzyme presented higher anity to hydrogen peroxide when compared to terc-butil peroxide, and the addition
of a buer containing mercaptoethanol did not increase GSH-Px enzymatic activity. e activity of GSH-Px was not inuenced by the source
and concentration of selenium in the diet either. e obtained results allowed the determination of the best temperature of contact between
the enzyme and substrate (22 °C), the optimum concentration, and the type of substrate and buer to be used. is information is extremely
useful for future studies on GSH-Px activity in meat due to the divergence and little information found in the literature.
Keywords: GSH-Px; meat chicken; enzymatic activity; selenium; buers; substrate.
OI:D http://dx.doi.org/10.1590/S0101-20612012005000107

Ciênc. Tecnol. Aliment., Campinas, 32(4): 661-667, out.-dez. 2012
662
Investigation of glutathione peroxidase activity in chicken meat
into two stages: the rst stage comprised ve experiments, in
which the thigh meat enzymatic activity was evaluated six
hours aer slaughter; the second stage was carried out on based
on the results obtained in the rst stage. In the second stage,
the material used to measure GSH-Px activity was raw meat
frozen for 120 days at –18 °C. e samples were randomly
chosen among the chickens fed T2 and T5 diets. In both stages,
three replicates were made for each analysis. Means, standard
deviation, analysis of variance, and the Tukey’s test of means
were performed at 5% condence level using Statistica (Statso,
Tulsa, USA) soware, version 6.1.
2.2 GSH-Px determination in chicken thigh meat six hours
aer slaughter
The enzymatic extract was obtained according to the
modied method proposed by Devoreetal. (1983). Skinless and
visually fat-free chicken thighs were homogenized with tris-HCl
buer 50 mM at pH 7.6 and 5°C at the 1:5 w/v ratio. Next, the
mixture was centrifuged (27.500 g, 30 minutes, 4°C), and the
supernatant was ltered and once again centrifuged under the
same conditions.
e GSH-Px activity of enzymatic extract was determined
according to a modication of the method proposed by Paglia
and Valentine (1967). e reaction medium was composed of
potassium phosphate buer 171 mM, sodium azide 4.28 mM,
EDTA 2.14 mM, reduced glutathione 6 mM, NADPH 0.9 mM,
and glutathione reductase 2 U.mL
–1
. e reaction took place
at 22°C (±1), starting with the addition of H
2
O
2
0.72 mM.
e absorbance of the samples was measured at 340nm using
a spectrophotometer. e measurements were taken every
15seconds for 300 seconds. e GSH-Px enzymatic activity
can be expressed in enzymatic units permL of sample (U.mL
–1
),
U.L
–1
, U.g
–1
of tissue, U.g
–1
of protein, or U.mg
–1
of hemoglobin
(PUNCHARD; KELLY, 1996). In the present study, the GSH-Px
enzymatic activity was expressed as U.g
–1
of tissue and was
calculated using Equation1:
/ / minUg A F
=∆×
(1)
where F is a constant used for converting absorbance per
minute (∆A/min) into enzymatic units (U). F is calculated by
the following Equation2:
×
=
( / )5
6.22
RV SV
F
(2)
where RV is the reaction volume (inmL); SV is the sample
volume (inmL); 5 is the volume (inmL) used to dilute 1 g of
tissue during the enzyme extraction; and 6.22 is the NADPH
molar extinction coecient (in Mm.cm
–1
).
2.3 GSH-Px determination in chicken thigh aer 120 days of
storage at –18 °C
The enzymatic extract was obtained according to the
same procedure described above, except from the fact that
two dierent buers where tested in the reaction medium:
buer 1, composed of potassium phosphate 50 mM at pH of
activity has been measured in several types of meat, such as
cattle (LEE; MEI; DECKER, 1996a, 1996b; O’GRADY etal.,
2001), pork (MAHAN; PARRETT, 1996; HERNÁNDEZ; PARK;
RHEE, 2002), and poultry (MOREIRAet al., 2001; SURAI,
2002; CARRERASetal., 2004, HOACetal., 2006). GSH-Px
catalyzes the detoxication of hydrogen peroxide (H
2
O
2
) in vitro
presenting high specicity for this substrate (ROTRUCKetal.,
1973). In fact, such reaction takes place in vivo through a parallel
oxidation of the reduced form of glutathione (GSH), a tripeptide.
e resulting molecule, oxidized glutathione (GSSG), is reduced
back through a parallel reaction with the reduced form of
nicotinamide adenine dinucleotide phosphate (NADPH).
is reaction is catalyzed by the glutathione reductase enzyme
(ROVERJUNIORetal., 2001).
Considering these reactions, Paglia and Valentine (1967)
proposed an analytical method for the determination of GSH-Px
enzymatic activity in erythrocytes. According to this method, the
decrease in the NADPH absorbance at 340nm, in the presence
of H
2
O
2
, is proportional to GSH-Px enzymatic activity. Since
its proposal, this has been the standard method for measuring
GSH-Px activity in all kinds of samples due to its convenience
and reliability. (CARRERASetal., 2004; DAUN; AKESSON,
2004a, 2004b; HOACetal., 2006; HOLOVSKÁJUNIORetal.,
2003). Despite the existence of several studies on GSH-Px
activity measurement, the inuence of experimental parameters
for estimating GSH-Px activity is still not clear. e standard
technique is an indirect and complex method, and there are
some uncertainties about the reaction mechanisms involved.
In addition, the studies conducted on this topic diverge widely
in their methodology and results. Due to the little information
available about the analytical method and the divergences
concerning the determination of GSH-Px activity in the
muscle, this study aimed to investigate the inuence of dierent
experimental conditions on the activity of GSH-Px in chicken
thighs. erefore, the type and concentration of peroxide, the
buers, the inuence of reaction time and temperature at the
analytical signal, as well as the inuence of broiler’s diet and
the eect of storage were studied, and the optimized conditions
were established.
2 Material and methods
2.1 Materials
One thousand and one hundred forty male chickens
(lineage Ross) were fed ve dierent diets: T1, without selenium
supplementation; T2, supplemented with inorganic selenium
(sodium selenite) at the concentration of 0.15mg.kg
–1
; T3,
supplemented with inorganic selenium at the concentration of
0.35mg/kg; T4, supplemented with organic selenium (Sel-Plex
@
)
at the concentration of 0.15mg.kg
–1
; and T5, supplemented
with organic selenium at the concentration of 0.35mg.kg
–1
.
e diets were elaborated from basic formulations following
recommended nutritional requirements (ROSTAGNO etal.,
2005).
At the age of 42 days, ninety birds were slaughtered, and
their thigh meat was used as raw material for evaluating the
glutathione peroxidase (GSH-Px) activity. e study was divided

Ciênc. Tecnol. Aliment., Campinas, 32(4): 661-667, out.-dez. 2012
663
Cichoski et al.
Stagsted (2006) observed the same behavior when measuring
GSH-Px activity in milk using the standard Paglia and Valentine
method and also obtained dierent results from other studies.
erefore, they decided to vary the enzymatic assay conditions
in order to optimize the method.
3.2 GSH-Px activity in chicken thigh aer 120 days of
storage at –18 °C
Eect of dierent hydrogen peroxide concentrations on GSH-Px
activity
e GSH-Px enzyme reduces several reactive oxygen species
(ROS) including hydrogen peroxide (CHAUDIEREetal., 1984).
e rst step in such reduction reaction is a direct oxidation of
selenolate anion (E-Se
–
) or selenol (E-SeH), which are the two
catalytically active forms of the selenocysteine residue contained
in GSH-Px (PRABHAKARetal., 2005). is reaction is shown
in Equation3.
22 2
––
E SeH H O E SeOH H O+→ +
(3)
Aer the oxidation of the GSH-Px active center by hydrogen
peroxide, the enzyme reacts with a reduced glutathione (GSH)
molecule forming an enzyme-glutathione complex (Equation4).
Next, this complex reacts with another GSH molecule, which
reduces back the GSH-Px enzyme and releases an oxidized
glutathione molecule (Equation5). Finally, GSH-Px reduces
once again the oxidized glutathione molecule in the presence
of NADPH (PRABHAKARetal., 2005).
2
– ––E SeOH GSH E Se SG H O+→ +
(4)
–– –
E Se SG GSH E SeH GSSG
+→ +
(5)
Since hydrogen peroxide is a substrate for the GSH-Px
enzyme, its concentration controls the reaction speed. us,
lack or excess of hydrogen peroxide in the reaction medium
could aect the enzymatic kinetics. In order to evaluate the
eect of hydrogen peroxide concentration on the GSH-Px
reaction rate, three dierent hydrogen peroxide concentrations
were used in the preparation of the reaction medium: 0.72mM,
7.2 mM, and 72 mM. e results shown in Figure1 show
that the reaction rates were signicantly aected by hydrogen
peroxide concentration. According to Lehninger, Nelson and
Cox (1995), all enzymes can be saturated by a proper substrate,
7.0, EDTA 1 mM, and mercaptoethanol 1 mM; and buer 2,
composed of tris-HCl 50 mM at pH of 7.6, EDTA 1 mM and
mercaptoethanol 5 mM. In both cases, the extraction took place
at 5°C with a 1:5w/v ratio. Next, the mixture was centrifuged
as described above and the supernatant was ltered and once
again centrifuged under the same conditions.
e GSH-Px activity of enzymatic extract was determined
according to the same method described above. In this stage,
however, the reaction took place at 36 °C aer a pre-incubation
time of 10 or 30 minutes. In addition, two substrates were tested
under dierent concentrations: H
2
O
2
at the concentrations of
0.72 mM, 7.2 mM, and 72 mM; and terc-butil hydroperoxide
at the concentration of 15 mM. e absorbance was measured
under the same conditions previously described, and GSH-Px
enzymatic activity was expressed in U.g
–1
of tissue and calculated
using Equations1 and2.
3 Results and discussion
3.1 GSH-Px activity in chicken thigh meat six hours aer
slaughter
Table1 shows the GSH-Px activity values in chicken thigh
meat six hours aer slaughter considering the dierent diets of
the birds with inorganic or organic selenium.
e results show that changing the selenium source and
concentration in the diet did not inuence GSH-Px activity
in chicken thighs. HolovskáJunioretal. (2003) studied the
eect of four diets (without selenium, 0.2mg of inorganic
selenium.kg
–1
, 0.2mg of organic selenium.kg
–1
, and 0.3mg
of organic selenium.kg
–1
) on chicken liver GSH-Px activity.
ey observed that changing the birds diet did not inuence
the chicken liver GSH-Px activity. A study conducted by Daun
and Akesson (2004a) reports similar results: the dierences
in GSH-Px activity in chicken thighs were not related to the
levels of selenium in the diets. Such results could be attributed
to the fact that the selenium ingested by the birds is used
for producing several selenoproteins besides GSH-Px. us,
selenium distribution in the bird body is regulated by its
metabolic needs (DAUN; AKESSON, 2004b). For example, birds
subjected to heat or cold stress present an increase in the levels
of the selenoprotein Type I Iodothyronine Deiodinase, which
regulates body temperature (ARTHUR; NICOL; BECKETT,
1993). Since thirty selenoproteins have been previously reported,
it is quite possible that the selenium added to the birds diet in
the present study was used for other metabolic activities than
the GSH-Px synthesis. e GSH-Px activity values observed in
our study were similar to those observed by HOACetal. (2006)
in chicken breast (0.050 U.g
–1
) but lower than those observed by
Daun and Akesson (2004a) in chicken breast (0.7 to 1.0 U.g
–1
).
According to Punchard and Kelly (1996), the ideal decay
rate of absorbance for the GSH-Px enzyme is between 0.01 and
0.05 U/min. In the rst stage of the present study, the absorbance
decay rate was much lower than these values. erefore, it was
necessary to study the inuence of changing the enzymatic
assay conditions on GSH-Px activity in order to optimize the
method and also study details of the GSH-Px enzymatic kinetics.
Table 1. Inuence of dierent diets (T1, T2, T3, T4, and T5) on
GSH-Px enzymatic activity (U.g
–1
) in chicken thigh meat six hours aer
slaughter. e data are expressed as mean ± standard deviation (n = 3).
Treatment Enzymatic activity (U.g
–1
tissue)
T1 (no Se) 0.051
A
(± 0.031)
T2 (inorganic Se at 0.15mg.kg
–1
) 0.083
A
(± 0.14)
T3 (inorganic Se at 0.35mg.kg
–1
) 0.045
A
(±0.003)
T4 (organic Se at 0.15mg.kg
–1
) 0.077
A
(± 0.015)
T5 (organic Se at 0.35mg.kg
–1
) 0.085
A
(± 0.019)
A
Dierent letters in the same column indicates signicant dierence by the Tukey test
(p < 0.05).

Ciênc. Tecnol. Aliment., Campinas, 32(4): 661-667, out.-dez. 2012
664
Investigation of glutathione peroxidase activity in chicken meat
during the pre-incubation period in water bath. erefore,
when the absorbance readings were made, the NADPH was
almost fully oxidized yielding reaction rates closer to zero. is
behavior was expected, since the reactions catalyzed by cellular
GSH-Px enzyme, found in the muscles, are faster than those
catalyzed by extracellular enzymes, found in milk and blood
plasma (STAGSTED, 2006). ose two enzyme subspecies
are structurally and functionally dierent from each other.
Extracellular GSH-Px enzyme presents a higher resistance to
high temperatures when compared to cellular GSH-Px enzyme
(LINDMARK-MANSSONetal., 2001). erefore, GSH-Px
might have been inhibited by the temperatures used for pre-
incubation in the present study.
Eect of dierent substrate types on the GSH-Px activity
Hydrogen peroxide and terc-butil hydroperoxide are
substrates for GSH-Px enzyme presenting Michaelis-Menten
constants (K
m
) of 0.003 and 0.059 mM, respectively (BRENDA,
but the substrate concentration required to saturate each one of
them is variable. In this study, hydrogen peroxide concentrations
above 0.72 mM reduced the reaction rate, i.e., saturating the
enzyme and inhibiting its catalytic activity. Splittgerber and
Tappel (1979) used three types of hydroperoxides at dierent
concentrations as substrates for mice liver GSH and GSH-Px
keeping the enzyme concentration constant. ey observed that
an increase in hydroperoxides concentration caused a decrease
in the reaction rates. Lin and Hultin (1978) also reported that
the GSH-Px enzyme is easily saturated by hydrogen peroxide.
erefore, high concentrations of hydrogen peroxide reduce the
reaction rate by slowing down the initial reaction (Equation3).
Eect of dierent pre-incubation conditions of the reaction
medium on the GSH-Px activity
According to previous ndings, the GSH-Px enzymatic
activity is aected by the pre-incubation time and temperature
of the reaction medium. In the study of Carrerasetal. (2004), the
reaction medium was pre-incubated at 30°C for 5 minutes prior
to the addition of hydroperoxide and absorbance readings. On
the other hand, Moreiraetal. (2001) and Penha-Silvaetal. (2005)
pre-incubated the medium at 37°C for 10 minutes before adding
the starter. Some authors report that the immediate reaction
takes place at 37°C, without pre-incubation (LINDMARK-
MANSSONetal., 2001; HOLOVSKÁJUNIORetal., 2003;
HOACetal., 2006). Other studies report the use of temperatures
for reactions that range from 20 °C to 25 °C (LEE; MEI;
DECKER, 1997; HERNÁNDEZ; PARK; RHEE, 2002). In the
present study, the rst stage comprised essays carried out at
22°C without pre-incubation. In the second stage, dierent
times and temperatures of pre-incubation were tested.
In the reaction catalyzed by the GSH-Px enzyme, the
oxidized glutathione (GSSG) generated from glutathione
(GSH) (Equation6) is instantly and continuously reduced in
the presence of the GSH-Px enzyme (Equation7). is keeps
a constant glutathione level, preventing the redox cycle from
stopping (ROVERJUNIORetal., 2001; PENHA-SILVAetal.,
2005).
22 2
2 –2
GSH H O GSH Px GSSG H O+→ → +
(6)
2GSSG NADPH H GS RED GSH NADP
+
+ +→ → +
(7)
In this redox cycle, the NADPH molecule behaves as an
electron donor, which is oxidized. In the standard Paglia and
Valentine (1967) method, this oxidation is photometrically
monitored by the absorption decay at 340nm, which is
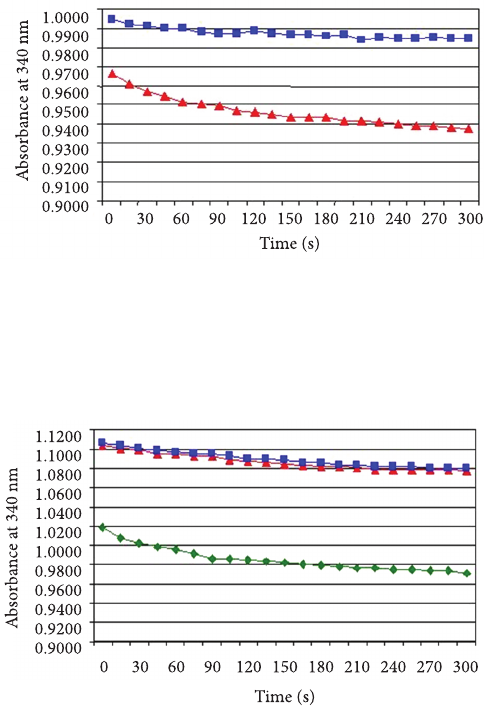
correlated with the GSH-Px enzymatic activity. Figure2 shows
that the GSH-Px enzymatic activity during the ve minutes of
reaction is inuenced by the dierent pre-incubation conditions
of the reaction medium (22 °C immediate, 36 °C/30min,
and 36 °C/10min).The reaction rate was 0.0003 abs/min
when the sample was pre-incubated at 36°C for 30 minutes;
0.0007 abs/min for pre-incubation at 36°C for 10 minutes;
and 0.0058 abs/min for the reaction at 22°C without pre-
incubation. Such behavior could be attributed to the fact that
this enzymatic reaction is rather fast, probably taking place
Figure 1. Eect of dierent hydrogen peroxide concentrations on the
reaction rates (abs.min
–1
) of the conversion of hydrogen peroxide by
the GSH-Px enzyme for the immediate reaction at 22°C in raw chicken
thigh meat frozen for 120 days at –18 °C. e data are expressed as mean
± standard deviation (n = 3).
A,B,C
Dierent letters indicates signicant
dierence by the Tukey test (p < 0.05).
Figure 2. Inuence of dierent pre-incubation conditions of the
reaction medium on NADPH absorbance at 340nm (GSH-Px
enzymatic activity) during the reaction period. Symbols: ( )
immediate reaction at 22°C; () reaction aer water bath at 36°C for
30 minutes; (■) reaction aer water bath at 36°C for 10 minutes. e
data are expressed as mean±standard deviation (n = 3).
Ciênc. Tecnol. Aliment., Campinas, 32(4): 661-667, out.-dez. 2012
665
Cichoski et al.
4 Conclusions
e data obtained in this study help to elucidate the reaction
mechanisms that take place in the method used for measuring
the GSH-Px enzymatic activity. e dierent parameters of
enzymatic assay studied showed that the activity of GSH-Px
is inuenced by some parameters related to the analytical
method. When hydrogen peroxide was added at concentrations
above 0.72 mM, the GSH-Px enzyme was saturated and its
activity was inhibited. GSH-Px decreased its activity in contact
with terc-butil hydroperoxide when compared to hydrogen
peroxide, conrming its higher anity to the latter. Other
important ndings were that the highest GSH-Px activity was
observed when enzyme and substrate were put into contact at
22°C without any pre-incubation and that the use of reaction
media containing mercaptoethanol was not associated with an
increase the GSH-Px enzymatic activity, as previous reported
in literature. e results obtained allowed to dene the best
2007). In other words, GSH-Px enzyme presents higher anity
to hydrogen peroxide. e reaction rates of the conversion of
hydrogen peroxide and terc-butil hydroperoxide by GSH-Px
enzyme at 22 °C are shown in Figure3. e GSH-Px enzymatic
activity was 0.0028 abs/min for the conversion of terc-butil
hydroperoxide and 0.0077 abs/min for the conversion of
hydrogen peroxide conrming that GSH-Px has higher anity
to the latter. Avissaretal. (1991) found similar enzymatic activity
values of milk GSH-Px when using hydrogen peroxide and terc-
butil hydroperoxide as substrates.
Eect of the presence of dierent buers in the reaction medium
on the GSH-Px activity
e determination of GSH-Px enzyme activity in animal
tissues requires a previous extraction, since the enzyme is found
inside the mitochondria and cytosol of the muscle cells (CHAN;
DECKER, 1994). During extraction, some conditions must
be controlled in order to preserve the enzyme. ose factors
include pH and electrolytes concentration in the buer solution,
extraction temperature, and solution dilution (FENNEMA,
1993).
The GSH-Px optimal pH is close to 8.0 (PAGLIA;
VALENTINE, 1967), and its activity is minimal at pH lower
than 6.0 (MILLS, 1959). erefore, the GSH-Px activity is oen
measured at the 7.0-7.6 pH range (PUNCHARDetal., 1996).
High electrolyte concentrations aect the proteins solubility
(FENNEMA, 1993). According to Chen, Lindmark-Mansson
and Akesson (2000), low buer concentrations increase the
extraction of the GSH-Px enzyme. e extraction temperature
is also critical since high temperatures can cause thermal
inactivation of the enzyme. Thus, in this study, the buffer
solution was kept at 4°C, the homogenization took place under
ice bath, and the centrifugation was performed at 4°C.
The active center of GSH-Px contains the amino acid
selenocysteine, a derivative of cysteine in which a sulfur atom is
substituted by selenium. Selenocysteine facilitates the GSH-Px
oxidation by generating a covalent bond between two atoms
of selenium. ese bonds make the protein structure more
stableimpairing GSH-Px extraction and reducing its reactivity
with the substrate (LEHNINGER; NELSON; COX, 1995).
According to Araietal. (1994), mercaptoethanol is a stabilizing
agent which forms a reversible enzyme-inhibitor (FARFÁN,
1994), a useful characteristic when one wants to prevent
spontaneous oxidation during the GSH-Px enzyme extraction.
erefore, it was expected that the presence of mercaptoethanol
in the buer solution favored the enzymatic extraction. To study
the eect of dierent buer solutions on the GSH-Px enzymatic
activity, tris-HCl buer 50 mM pH 7.6 and two other types
of extraction buers containing mercaptoethanol were used.
e results are shown in Figure4. e GSH-Px enzymatic
activity was 0.0062 abs/min using an extraction medium
with buer number 1, 0.0072 abs/min using buer number 2,
and 0.0114abs/min using buer number 3. e results show
that the highest enzymatic activity was observed when buer
number 3 was used (tris-HCl 50 mM pH 7.6), suggesting that
mercaptoethanol was not useful for improving the enzyme
extraction. Chaudiereetal. (1984) observed inhibition of the
GSH-Px enzymatic activity when adding mercaptoethanol to the
reaction medium obtaining the same results hereby reported.
Figure 3. Eect of substrate type on NADPH absorbance at 340nm
(GSH-Px enzymatic activity) during the reaction period for the
immediate reaction at 22°C. Symbols: ( ) hydrogen peroxide
at 0.72mM; (■) terc-butil hydroperoxide at 15 mM. e data are
expressed as mean ± standard deviation.
Figure 4. Inuence of the presence of buers in the reaction medium
on NADPH absorbance at 340 nm (GSH-Px enzymatic activity)
during the reaction period for the immediate reaction at 22 °C.
Symbols: (■) buer 1 (potassium phosphate 50 mM at pH 7,0+EDTA
1mM+mercaptoethanol 1 mM); () buer 2 (tris-HCl 50 mM at pH
7,6+EDTA 1 mM+mercaptoethanol 5 mM); (♦) buer 3 (tris-HCl
50 mM pH 7,6). e data are expressed as mean ± standard deviation
(n = 3).
Ciênc. Tecnol. Aliment., Campinas, 32(4): 661-667, out.-dez. 2012
666
Investigation of glutathione peroxidase activity in chicken meat
HOLOVSKÁ JUNIOR, K.etal. Antioxidant enzyme activities in liver
tissue of chickens fed diets supplemented with various forms and
amounts of selenium. Journal of Animal and Feed Sciences, v.12,
p.143-152,2003.
LEE, S. K.; MEI, L.; DECKER, E. A. Lipid oxidation in cooked turkey
as eect by added antioxidant enzymes. Journal of Food Science,
v.61, p.726-728,795,1996a.
LEE, S. K.; MEI, L.; DECKER, E. A. Role of antioxidant enzymes in
the development of oxidative rancidity in cooked and salted muscle
foods. Meat Focus International, v.5, p.310-311,1996b.
LEE, S. K.; MEI, L.; DECKER, E. A. Inuence of sodium chloride on
antioxidant enzyme activity and lipid oxidation in frozen ground
pork. Meat Science, v.46, n. 4, p.349- 355,1997. http://dx.doi.
org/10.1016/S0309-1740(97)00029-6
LEHNINGER, A. L.; NELSON, D. L.; COX, M. M. Princípios de
bioquímica.2.ed. São Paulo: Sarvier,1995. cap.8, p.147-176.
LIN, T. S.; HULTIN, H. O. Glutathione peroxidase of skeletal muscle.
Journal of Food Biochemistry, v.2, p.39-47,1978. http://dx.doi.
org/10.1111/j.1745-4514.1978.tb00602.x
LINDMARK-MANSSON, H.et al. e eect of storage and heat
treatment on glutathione peroxidase in bovine milk and whey.
International Dairy Journal, v.11, p.71-81,2001. http://dx.doi.
org/10.1016/S0958-6946(01)00034-6
MAHAN, D. C.; PARRETT, N. A. Evaluating the ecacy of selenium-
enriched yeast and sodium selenite on tissue selenium retention
and serum glutathione peroxidase activity in grower and nisher
swine. Journal of Animal Science, v. 74, p.2967-2974,1996.
PMid:8994911.
MILLS, G. C. e purication and properties of glutathione peroxidase
of erythrocytes. Journal of Biological Chemistry, v.234,
p.502-506,1959. PMid:13641249.
MOREIRA, J.etal. Efeito de fonte e níveis de selênio na atividade
enzimática da glutationa peroxidase e no desempenho de frangos
de corte. Ciência e Agrotecnologia, v.25, n.3, p.645-649,2001.
O’GRADY, M. N.etal. Eects of dietary supplementation with vitamin
E and organic selenium on the oxidative stability of beef. Journal of
Animal Science, v.79, p.2827-2834,2001. PMid:11768111.
PAGLIA, D. E.; VALENTINE, W. N. Studies on the quantitative and
qualitative characterization of erythrocyte glutathione peroxidase.
Journal of Laboratory and Clinical Medicine, v.70, n.1,
p.158-169,1967. PMid:6066618.
PENHA-SILVA, N.etal. Determinação da faixa de referência de
glutationa peroxidase no soro de bovinos sadios. Bioscience
Journal, v.21, n.2, p.89-93,2005.
PRABHAKAR, R.etal. Elucidation of the mechanism of selenoprotein
glutathione peroxidase catalysed hydrogen peroxide reduction
by two glutathione molecules: a density functional study.
Biochemistry, v.44, p.11864-11871,2005. PMid:16128588. http://
dx.doi.org/10.1021/bi050815q
PUNCHARD, N. A.; KELLY, F. J. Glutathione peroxidase: activity and
steady-state level of mRNA. In: DARET, K.etal. Free radicals: a
pratical approach. London: Oxford University Press,1996. cap.16,
p.227-240.
ROSTAGNO, H. S.et al. Tabelas brasileiras para aves e suínos:
composição de alimentos e exigências nutricionais.2.ed. Viçosa:
Universidade Federal de Viçosa,2005.186 p.
ROTRUCK, J. T.et al. Selenium: biochemical role as component
of glutathione peroxidase. Science, v.179, p.588-590,1973.
PMid:4686466. http://dx.doi.org/10.1126/science.179.4073.588
ROVER JUNIOR, L.etal. Sistema antioxidante envolvendo o ciclo
metabólico da glutationa associado a métodos eletroanalíticos
temperature of contact between the enzyme and the substrate,
the best concentration, and type of substrate and buer to be
used.
References
ARAI, T. et al. Glutathione peroxidase activity in tissues of chickens
supplemented with dietary selenium. Comparative Biochemistry
and Physiology, v. 107A, p. 245-248, 1994.
ARTHUR, J. R.; NICOL, F.; BECKETT, G. J. Selenium deciency, thyroid
hormone metabolism, and thyroid hormone deiodinases. American
Journal of Clinical Nutrition Supply, v.57, p.236-239,1993.
AVISSAR, N.et al. Partial sequence of human plasma glutathione
peroxidase and immunological identication of milk glutathione
peroxidase as the plasma enzyme. Journal of Nutrition, v. 121,
p.1243-1249,1991. PMid:1861172.
BRENDA. The Comprehensive Enzyme Information System.
Disponível em: <http://www.brenda.uni-koeln.de>. Acesso em:02
maio2007.
CARRERAS, I. F.etal. Inuence of enrooxacin administration and
?-tocopheryl acetate supplemented diets on oxidative stability of
broiler tissues. Poultry Science, v.83, p.1-7,2004. PMid:14761077.
CHEN, J.; LINDMARK-MANSSON, H.; AKESSON, B. Optimisation
of a coupled enzymatic assay of glutathione peroxidase activity
in bovine milk and whey. International Dairy Journal, v. 10, p.
347-351, 2000.
CHAN, K. M.; DECKER, E. A. Endogenous skeletal muscle
antioxidants. Food Science and Nutrition, v.34, n.4,403-426,1994.
PMid:7945896.
CHAUDIERE, J.; WILLHELMSEN, E. C.; TAPPEL, A. L. Mechanism
of selenium-glutathione peroxidase and its inhibition by
mercaptocarboxylic acids and other mercaptans. The Journal
of Biological Chemistry, v.259, n.2, p. 1043-1050,1984.
PMid:6693375.
DAUN, C.; AKESSON, B. Comparison of glutathione peroxidase
activity, and of total and soluble selenium content in two
muscles from chicken, turkey, duck, ostrich and lamb. Food
Chemistry, v. 85, p.295-303,2004a. http://dx.doi.org/10.1016/j.
foodchem.2003.07.009
DAUN, C.; AKESSON, B. Glutathione peroxidase activity, and content
of total and soluble selenium in ve bovine and porcine organs used
in meat production. Meat Science, v.66, p.801-807,2004b. http://
dx.doi.org/10.1016/S0309-1740(03)00178-5
DEVORE, V. R.etal. iobarbituric acid values and glutathione
peroxidase activity in meat from chickens fed a selenium-
supplemented diet. Journal of Food Science, v.48, p.300-301,1983.
http://dx.doi.org/10.1111/j.1365-2621.1983.tb14860.x
FARFÁN, J. A. Modicação química. Química de proteínas aplicada
à ciência e tecnologia dos alimentos.2.ed. Campinas: Editora
Unicamp,1994. cap.4, p.65-105.
FENNEMA, O. R. Química de los alimentos.2.ed. Zaragoza:
Acribia,1993. cap.6, p.416-536.
HERNÁNDEZ, P.; PARK, D.; RHEE, K. S. Chloride salt type/ionic
strength, muscle site and refrigeration effects on antioxidant
enzymes and lipid oxidation in pork. Meat Science, v.61,
p.405-410,2002. http://dx.doi.org/10.1016/S0309-1740(01)00212-1
HOAC, T.et al. Influence of heat treatment on lipid oxidation
and glutathione peroxidase activity in chicken and duck meat.
Innovative Food Science & Emerging Technologies, v. 7,
p.88-93,2006. http://dx.doi.org/10.1016/j.ifset.2005.10.001
Ciênc. Tecnol. Aliment., Campinas, 32(4): 661-667, out.-dez. 2012
667
Cichoski et al.
na avaliação do estresse oxidativo. Química Nova, v.24, n.1,
p.112-119,2001.
SPLITTGERBER, A. G.; TAPPEL, A. L. Steady state and pre-steady state
kinetic properties of rat liver selenium-glutathione peroxidase. e
Journal of Biological Chemistry, v.254, n.19, p.9807-9813,1979.
PMid:489570.
STAGSTED, J. Absence of both glutathione peroxidase activity and
glutathione in bovine milk. International Dairy Journal, v.16,
p.662-668,2006. http://dx.doi.org/10.1016/j.idairyj.2005.08.013
SURAI, P. F. Selenium in poultry nutrition- antioxidant properties,
deciency and toxicity. World’s Poultry Science Journal, v.58,
p.333-347,2002. http://dx.doi.org/10.1079/WPS20020026
