
Calculation of chemical elimination half-life from blood with an ongoing
exposure source: The example of perfluorooctanoic acid (PFOA)
Mark H. Russell
a,
⇑
, Robert L. Waterland
b
, Fiona Wong
c
a
DuPont Haskell Global Centers for Health and Environmental Sciences, S320/214, P.O. Box 50, Newark, DE 19714-0050, United States
b
DuPont Central Research & Development, Experimental Station, E320/314, Rt 141 and Henry Clay, Wilmington, DE 19880, United States
c
Department of Applied Environmental Science, Stockholm University, Svante Arrhenius väg 8, SE-10691 Stockholm, Sweden
article info
Article history:
Received 14 March 2014
Received in revised form 17 July 2014
Accepted 20 July 2014
Available online 20 August 2014
Handling Editor: I. Cousins
Keywords:
Elimination
Perfluorooctanoic acid
Half-life
Background exposure
abstract
Determination of the chemical clearance rate from human blood is a critical component of toxicokinetic
exposure assessment. Analysis of temporal biomonitoring data without consideration of ongoing expo-
sure results in calculation of apparent elimination half-life values that are longer than the intrinsic value.
The intrinsic elimination half-life is solely a function of the rate of elimination while the apparent elim-
ination half-life reflects the processes of both elimination and ongoing exposure. Confusion between
intrinsic and apparent half-life values can lead to misinterpretation of biomonitoring data and can result
in exaggerated predictions in subsequent modeling efforts. This work provides a review of the first-order
equations that have been developed to calculate intrinsic and apparent half-life values and the potential
bias that can result from confusing these two values. Published human biomonitoring data for perfluoro-
octanoic acid (PFOA) are analyzed using these equations to provide examples of low, medium and high
bias in determination of the intrinsic elimination half-life from plasma or serum, the components of
blood typically analyzed for PFOA. An approach is also provided to estimate the extent of exposure
reduction that is indicated by declining longitudinal or cross-sectional biomonitoring data. Based on
the evaluation methodology presented in this work, the intrinsic elimination half-life of PFOA in humans
is 2.4 years, representing the average of independent estimates of 2.5 years (95% CI, 2.4–2.7) and 2.3 years
(95% CI, 2.1–2.4). The declining concentration of PFOA in blood of the general USA adult population
represents an estimated exposure reduction of 20–30% over the period 1999–2008.
Ó 2014 Elsevier Ltd. All rights reserved.
1. Introduction
One of the critical parameters in toxicokinetic assessment of
chemical exposure is determination of the rate of clearance from
the body via a combination of metabolism, conjugation and
physical elimination. In biomonitoring studies, internal chemical
concentrations are routinely determined as a longitudinal or
cross-sectional series of samples from a readily accessible tissue
such as blood, plasma or serum. After the cessation of exposure,
the rate of elimination can then be determined from mathematical
analysis of the time course of the samples. However, in many situ-
ations there is some level of ongoing exposure which results in an
‘apparent’ rather than the ‘intrinsic’ elimination half-life.
Researchers have previously pointed out that apparent half-life
values are functions of both the ongoing rate of uptake as well as
the rate of elimination, resulting in biased estimation of the intrin-
sic elimination half-life (Shirai and Kissel, 1996). However, failure
to explicitly account for ongoing exposure is common in the
published literature. The rate of intrinsic elimination can be deter-
mined if the influence of ongoing exposure and changes in physi-
ology (such as body weight) are accounted for. Population-based
pharmacokinetic models have been developed (Ritter et al., 2011)
to calculate the intrinsic elimination half-lives of polychlorinated
biphenyls in the U.K. population and perfluorooctane sulfonic acid
in the U.S. population (Wong et al., 2014). The following discourse
provides a review of simple first-order equations for analysis of
apparent and intrinsic half-life values, similar to the relationships
http://dx.doi.org/10.1016/j.chemosphere.2014.07.061
0045-6535/Ó 2014 Elsevier Ltd. All rights reserved.
Abbreviations: PFOA, perfluorooctanoic acid; Intrinsic elimination half-life, the
first-order elimination half-life obtained from the depuration of a chemical from
biota when the effects of ongoing exposure are not present or are negligible;
Apparent elimination half-life, the first-order elimination half-life obtained from
the depuration of a chemical from biota when the effects of ongoing exposure are
neglected; Bias, the difference between the apparent elimination half-life and the
intrinsic half-life, typically expressed as a normalized percent of the intrinsic value.
⇑
Corresponding author. Tel.: +1 (302) 366 6020; fax: +1 (302) 451 4531.
Chemosphere 129 (2015) 210–216
Contents lists available at ScienceDirect
Chemosphere
journal homepage: www.elsevier.com/locate/chemosphere
originally developed by Shirai and Kissel. These equations are then
used to assess the potential bias in using apparent half-life values
to represent intrinsic half-life and to estimate the extent of
exposure reduction from temporal biomonitoring data. Similar
equations are applicable to environmental systems such as lakes
and soil which have simultaneous chemical inputs (i.e., inflow or
deposition) and outputs (i.e., outflow, degradation or leaching)
(Schnoor, 1996).
The application of these equations is illustrated through exam-
ination of three sets of published biomonitoring results for perflu-
orooctanoic acid (PFOA, CAS 000335-67-1) in human plasma and
serum samples. The developed kinetic equations are equally valid
for analysis of biomonitoring data for concentrations in blood,
plasma or serum. Human biomonitoring for PFOA currently repre-
sents one of the most robust datasets available and provides an
excellent example of the issue of potential bias in the calculation
of elimination rates.
2. Methods: Derivation of equations
2.1. Calculation of blood concentration in response to a constant
exposure source
The following equations are similar to mathematical relation-
ships that were originally developed to clarify differences between
the intrinsic (‘true’) and apparent elimination half-lives of PCBs
from humans (Shirai and Kissel, 1996). Continuous exposure to a
chemical contaminant and subsequent uptake and distribution of
that chemical into body tissues commonly leads to an increasing
concentration C of the chemical in human blood (or plasma or
serum). When chemical exposure is constant or nearly constant
and elimination is first order, the rate of change of concentration
with time (ng L
1
d
1
) is given by:
dC=dt ¼ I
0
E
a
=ðV
d
MÞk
e
C ð1Þ
where I
0
is the rate of chemical exposure (ng d
1
), E
a
is a dimen-
sionless chemical uptake fraction which accounts for the fraction
of the chemical exposure that is absorbed into blood, V
d
is the vol-
ume of distribution (L kg
1
) of the chemical and M is body mass
(kg). k
e
is the intrinsic elimination rate constant (d
1
) which
describes the underlying rate of chemical loss due to the combina-
tion of physical elimination and metabolism. This equation
accounts for the difference between constant chemical exposure
and first order chemical loss from blood.
Approximate values of V
d
include 0.08 L kg
1
for a chemical that
is distributed solely to human blood and 0.20 L kg
1
for distribu-
tion to extracellular fluid (i.e. blood, lymph and other fluids)
(Wagner, 1975; Brown et al., 1997). Integration of Eq. (1) yields:
CðtÞ¼C
0
e
k
e
t
þ C
ss
ð1 e
k
e
t
Þð2Þ
where C
ss
= I
0
E
a
/(k
e
V
d
M) and C
0
is the chemical concentration in
blood at any given initial time taken to be t = 0 for convenience.
2.2. Calculation of steady-state blood concentration for initial chemical
exposure
If no chemical is initially present in blood, C
0
= 0 and the blood
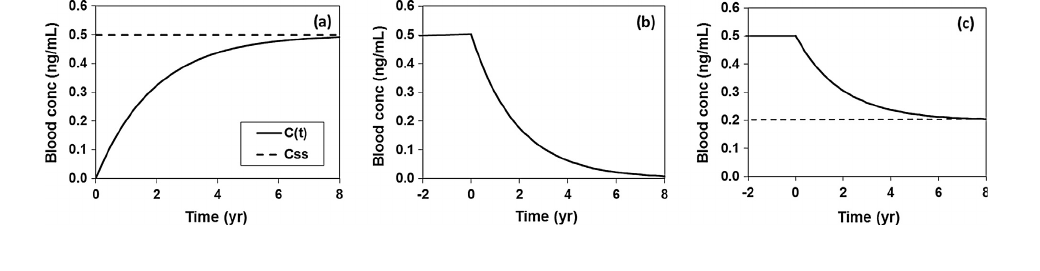
concentration increases with time, asymptotically approaching a
steady-state value C
ss
after an extended period of constant expo-
sure (Fig. 1a). Eventually, the rate of chemical elimination
approaches the rate of chemical uptake and the blood concentra-
tion stabilizes at the steady-state concentration C
ss
:
lim
t!1
CðtÞ¼C
ss
¼ I
0
E
a
=ðk
e
V
d
MÞð3Þ
Eq. (3) is a simple, one-compartment toxicokinetic (TK) model
that is routinely used to calculate the chemical concentration in
blood in response to a constant or chronic chemical exposure rate
I
0
. This model can be applied if the fractional chemical uptake (E
a
),
the rate of elimination and metabolism (k
e
), the volume of distribu-
tion (V
d
) and body mass (M) are known or can be reliably
estimated. One-compartment TK models have been applied to
estimate human blood concentrations of a wide variety of environ-
mental contaminants that result in chronic, constant or near-con-
stant exposure including pesticides (Timchalk, 2010), brominated
flame retardants (Quinn and Wania, 2012; Bjerregaard et al.,
2013), perfluoroalkyl substances (Egeghy and Lorber, 2011),
dioxin-like chemicals (Olsen, 2012) and polycyclic aromatic hydro-
carbons (Li et al., 2012).
2.3. Calculation of blood concentration when constant exposure ceases
When constant chemical exposure has continued for a consider-
able amount of time, the chemical concentration in blood C(t)
approaches its steady state concentration, C
ss
. Now consider the
transient response in blood concentration if chemical exposure
suddenly stops or is very significantly reduced. This situation
may occur for a number of reasons. A factory worker with high
occupational exposure may retire or move to a new assignment
with little or no exposure. A person may move from a city with
significant local exposure to a different city with no further
exposure or they may remain in the same location but exposure
is suddenly eliminated by a treatment method (e.g. a carbon filter
is placed on drinking water).
If chemical uptake abruptly ceases, the subsequent elimination
of the contaminant from blood is given by:
dC=dt ¼k
e
C ð4Þ
The solution of this first-order differential equation is:
CðtÞ¼C
0
e
k
e
t
ð5Þ
Fig. 1. Example profiles of blood concentrations in response to (a) a constant exposure source, (b) cessation of steady-state exposure and (c) fractional reduction of steady-
state exposure. Dashed line is steady-state concentration and solid line is transient concentration.
M.H. Russell et al. / Chemosphere 129 (2015) 210–216
211

where C
0
is defined as the chemical concentration in blood at the
time when exposure ends, again taken to be t = 0 for convenience.
This behavior is illustrated in Fig. 1b where C is constant during
the period of exposure but then follows a first order decline towards
zero when exposure ceases.
2.4. Calculation of blood concentration when exposure is reduced but
not fully eliminated
Suppose as before that constant exposure has produced a con-
stant blood concentration C
ss0
. At time t
0
exposure is markedly
reduced but not completely eliminated. When chemical exposure
is reduced from an initial constant value (I
0
) to a lower but non-
zero constant value (I
1
), the following expression can easily be
derived from Eq. (2):
CðtÞ¼C
ss1
þðC
ss0
C
ss1
Þe
k
e
t
ð6Þ
where C(t) is the chemical concentration in blood at any time t sub-
sequent to t
0
and C
ss1
is the new asymptotic steady-state blood con-
centration associated with the reduced constant exposure input I
1
.
C
ss0
and C
ss1
are easily obtained from Eq. (3) if I
0
and I
1
are known.
If the exposures are not known, C
ss0
can be taken to be the initial
blood concentration C(t
0
) of the more highly exposed sub-popula-
tion being studied and C
ss1
as the background blood concentration
measured in the general public. Uncertainty in the values of C
ss0
and/or C
ss1
results in uncertainty in the calculated concentration
values, C(t), especially when the magnitude of change is small.
Eq. (6) shows that reducing the chemical dose rate from I
0
to I
1
leads to an exponential decrease in blood concentration from an
initial steady-state value C
ss0
to a new, lower steady-state value
C
ss1
. This behavior is illustrated in Fig. 1c. Solving Eq. (6) for k
e
yields the following expression:
k
e
¼ ln
C
ss0
C
ss1
CðtÞC
ss1
=t ð7Þ
k
e
is the intrinsic elimination rate constant for blood and reflects the
actual rate at which chemical is removed from blood via the combi-
nation of metabolism and physical elimination. The corresponding
intrinsic elimination half-life is t
e
1=2
¼ ln ð2Þ=k
e
.
In many studies, the rate of elimination is evaluated without
considering the potential impact of any ongoing source of expo-
sure. Therefore, a conventional first-order equation analogous to
Eq. (5) is typically applied:
CðtÞ¼C
ss0
e
k
a
t
ð8Þ
where k
a
is the apparent elimination rate constant, the best
estimate of the chemical elimination rate constant when ongoing
exposure is neglected. Solving Eq. (8) for k
a
gives
k
a
¼ ln
C
ss0
CðtÞ
=t ð9Þ
and a corresponding apparent elimination half-life t
a
1=2
¼ ln ð2Þ=k
a
.
Values of k
a
are frequently estimated from only two data points:
an initial blood concentration (i.e. C
ss0
) and a concentration at some
later time, i.e. C(t). If there is no ongoing source of exposure, C
ss1
=0
and k
a
= k
e
. However, if exposure is decreased but not eliminated
then k
a
< k
e,
that is, the apparent rate of elimination is slower than
the intrinsic rate of elimination. As a result, the apparent elimina-
tion half-life is always longer than the intrinsic half-life. Proof of
this relationship is provided in the Supplementary Data (SD).
2.5. Bias in calculation of intrinsic elimination half-life
When the contribution of an ongoing source of exposure is
ignored, the bias in calculating accurate values of the intrinsic
blood elimination half-life can range from minor to extreme. The
bias can be estimated from the following relationship:
Biasð%Þ100
k
e
k
a
k
e
¼ 100 1
t
e
1=2
t
a
1=2
!
ð10Þ
Estimated values of k
a
and the percent bias depend on the
sampling time t. In contrast, k
e
is an intrinsic quantity and can gen-
erally be assumed to be invariant with time (see later discussion
for possible longer-term variation in k
e
). Estimation of k
e
using k
a
values from the flawed model described by Eq. (9) leads to two dif-
ficulties: k
a
systematically underestimates k
e
, and the degree of
underestimation depends on the times at which blood samples
are taken.
For highly exposed individuals who are subsequently removed
from any further significant exposure (i.e. C
ss0
C
ss1
), the calcu-
lated percent bias in the elimination half-life is low throughout
the initial period of depuration. When the initial steady-state con-
centration C
ss0
is 100 times greater than the final steady-state con-
centration C
ss1
, the percent bias varies in the range of 1.4–2.2% for
sampling times which range over 1–2 intrinsic half-lives (i.e. for
the first 50–75% of the change to the new steady-state) (Calcula-
tion provided in SD). When the initial to final steady-state ratio
is small, the percent bias increases rapidly: if C
ss0
is 5 times larger
than C
ss1
, the bias increases to 29–50% for the same time frame. In
each case where there is an ongoing source, the intrinsic elimina-
tion half-life is shorter than the apparent elimination half-life.
This analysis shows that the most accurate estimations of blood
elimination half-lives for xenobiotic chemicals will be obtained by
examining highly exposed individuals who are subsequently
exposed to only minor background concentrations, or from more
marginally exposed individuals who are completely removed from
any further exposure. The bias in these half-life calculations is gen-
erally negligible or is within experimental error. In contrast,
attempts to determine intrinsic chemical elimination half-lives
from evaluations of moderate to minor reductions in the blood
concentrations of the general population will be highly biased
and will result in excessively long estimates of intrinsic elimina-
tion half-lives.
3. Results: Applying the bias equations to human biomonitoring
of PFOA
Bias in calculation of intrinsic elimination half-life values is best
illustrated with actual examples. Blood monitoring studies can
provide datasets useful for estimation of intrinsic elimination
half-lives, especially if information on the relative contribution of
ongoing exposure sources is also available. The extensive human
biomonitoring data for PFOA provide a unique set of results to
demonstrate the potential bias in estimation of intrinsic elimina-
tion half-lives inherent in different types of temporal biomonitor-
ing studies.
3.1. Example of minimal bias in intrinsic half-life calculation of PFOA
The results summarized in Table 1 are from a study of recently
retired workers who were occupationally exposed to PFOA and
other fluorinated chemicals (Olsen et al., 2007). The initial serum
concentrations of these individuals ranged between 72 and
5100 ng mL
1
PFOA and many workers had initial concentrations
greater than 100 times the population background concentration
of 4.06 ng mL
1
estimated from NHANES data for the general pop-
ulation in the USA during the same time frame (CDC, 2012). For
workers with initial serum concentrations >500 ng mL
1
, the
estimated calculation bias between the apparent and intrinsic
elimination half-lives is less than 1.2% indicating that correction
212 M.H. Russell et al. / Chemosphere 129 (2015) 210–216

of the observed serum concentrations for background exposure has
a negligible effect. However, for those individuals with lower initial
serum concentrations, the calculation bias ranges up 13%. It should
be noted that minimal bias values (i.e. <10–15%) are likely to be
similar to the uncertainty in analytical measurements.
The apparent geometric mean elimination half-life for all work-
ers reported by Olsen et al. was 3.5 years (95% CI, 3.0–4.1) (Olsen
et al., 2007). However, if the calculations are restricted to only
those workers with initial serum concentrations greater than
500 ng mL
1
, a less-biased estimate of the intrinsic half-life of
PFOA is 3.0 years (95% CI, 2.4–3.8). Due to the highly elevated ini-
tial concentration and the extended sampling duration (almost two
half-lives), the intrinsic elimination half-life determined from
these highly exposed individuals is expected to provide an accurate
estimate of the elimination rate of PFOA from this population of
male adults.
3.2. Example of moderate bias in intrinsic half-life calculation of PFOA
An example of moderate calculation bias in elimination
half-lives can be found in the recent study of residents of Arnsberg,
Germany who were environmentally exposed to PFOA through
drinking water during the period 2006–2008 (Brede et al., 2010).
The geometric mean PFOA apparent elimination half-life for the
adult participants in Arnsberg was 3.2 years (95% CI, 2.9–3.5)
(Table 2). Adjusting for background exposure from the reported
control population provides a geometric mean intrinsic elimination
half-life of 2.5 years (95% CI, 2.4–2.7) for adults. In this case the
initial plasma PFOA concentrations were 5–8 times higher than
the estimated background plasma concentrations in the control
population and the sampling interval was less than one half-life.
For the Arnsberg study, neglecting to correct for the observed back-
ground concentrations resulted in a mean calculation bias of 21%
for the adult population.
In a similar study of adult residents in the Mid-Ohio Valley of
the USA who were exposed to PFOA in drinking water, the appar-
ent elimination half-life was observed to vary with both time
and concentration, leading to an initial conclusion that the clear-
ance of PFOA may be concentration dependent or the result of
ongoing background exposure (Seals et al., 2011). When the
observed biomonitoring results were analyzed using a statistical
model that accounted for ongoing exposure, the mean elimination
half-life was estimated to be 2.3 years (95% CI, 2.1–2.4) (Bartell
et al., 2010). This half-life result is in excellent agreement with
the value determined above for the Arnsberg population. An aver-
age intrinsic elimination half-life of 2.4 years from the results from
the Arnsberg and Ohio Valley studies provides a reasonable
estimate to be used for comparison with other temporal biomoni-
toring studies of PFOA for the general population.
3.3. Example of high bias in intrinsic half-life calculation of PFOA
An example of high bias in estimated PFOA elimination
half-lives is shown in Table 3. These cross-sectional data were
Table 1
Example of negligible calculation bias between apparent and intrinsic elimination half-life values for PFOA (data from Olsen et al., 2007).
Population and sample
collection period
Serum concentration (ng mL
1
) Elapsed
time (yr)
t
a
1=2
, apparent
elimination
half-life, (yr)
t
e
1=2
, intrinsic
elimination
half-life (yr)
à
Estimated
calculation
bias (%)
§
Initial Final Bkgd
*
Retired workers with
occupational exposure to
PFOA, 1998–2004
5100 2435 4.06 4.78 4.48 4.47 0.12
1833 486 4.06 5.33 2.78 2.77 0.46
1622 577 4.06 5.33 3.57 3.56 0.44
1180 145 4.06 4.74 1.57 1.55 1.18
1077 404 4.06 4.74 3.35 3.33 0.64
883 266 4.06 5.33 3.08 3.05 0.89
702 248 4.06 4.18 2.78 2.75 1.02
549 235 4.06 4.74 3.87 3.83 1.17
496 284 4.06 5.27 6.56 6.48 1.10
490 129 4.06 5.27 2.74 2.69 1.74
474 162 4.06 5.33 3.44 3.39 1.54
430 108 4.06 5.33 2.67 2.62 2.04
425 162 4.06 5.33 3.83 3.77 1.61
390 61 4.06 4.18 1.56 1.51 3.05
356 244 4.06 5.33 9.78 9.64 1.39
306 188 4.06 5.33 7.58 7.45 1.71
254 150 4.06 3.12 4.11 4.02 2.11
247 104 4.06 5.33 4.27 4.16 2.62
212 84 4.06 5.33 3.99 3.86 3.16
183 50 4.06 5.33 2.85 2.72 4.58
181 65 4.06 5.33 3.61 3.47 3.92
167 78 4.06 5.33 4.85 4.67 3.65
142 51 4.06 5.33 3.61 3.43 5.00
131 45 4.06 5.33 3.46 3.26 5.57
74 26 4.06 5.33 3.53 3.19 9.78
72 17 4.06 5.33 2.56 2.23 12.96
Workers with initial serum concentration > 500 ng mL
1
- Geomean: 3.06 3.04} 0.6
95% CI: (2.46–3.81) (2.44–3.79)
All workers - Geomean: 3.56 3.46} 2.8
95% CI: (3.06–4.16) (2.96–4.05)
*
Background PFOA plasma values are geomean averages of NHANES data for 1999–2004: Geomean of adults 60+, 1999–2004 = 4.06 ng mL
1
.
Calculated with Eq. (9). Values differ slightly from Olsen et al. values which were determined by linear regression with multiple data points.
à
Calculated with Eq. (7).
§
Calculated with Eq. (10).
M.H. Russell et al. / Chemosphere 129 (2015) 210–216
213

compiled by the U. S. Centers for Disease Control and Prevention
(CDC) as a part of the National Health and Nutritional Evaluation
Survey (NHANES) (Kato et al., 2011). NHANES data from the
years of 1999–2008 showed a general downward trend in PFOA
serum concentrations for the U.S. general population. Geometric
mean PFOA concentrations in males 12 years of age or older fell
from 5.71 ng mL
1
in 1999–2000 to 4.47 ng mL
1
in 2003–2004
but remained largely unchanged from 2003 to 2008. Serum PFOA
concentrations in females 12 years of age or older were lower
than those found for males but followed the same general trend.
These changes reflect a general reduction in exposure to poly-
fluoroalkyl chemicals which is most likely due to changes in
manufacturing practices and product formulation that began in
2002.
PFOA is a persistent chemical and there is direct, ongoing
exposure to legacy PFOA in the environment. In addition, ongoing
indirect sources of PFOA have been identified such as formation
from precursor chemistry (Prevedouros et al., 2006). The NHANES
data indicate that PFOA uptake has been reduced since 1999 but
ongoing exposure to legacy and indirect sources remains. As a
result, calculation of the apparent elimination half-life of PFOA
from the gradual decline observed in the general population pro-
vides a misleading estimate of the intrinsic elimination half-life
with a mean calculation bias of 91% (95% CI, 87–95) (Table 3).
The Mexican–American (MA) sub-population shows a PFOA con-
centration decline of less than 10% (geomean values of
3.89 ng mL
1
declining to 3.53 ng mL
1
) which leads to a highly
biased (and highly inaccurate) value of the intrinsic elimination
half-life for this sub-population. The calculation biases summa-
rized in Table 3 result almost entirely from neglecting ongoing
PFOA exposure as concentrations in the general population are
observed to slowly decline.
3.4. Using biomonitoring data to estimate chemical exposure reduction
Eq. (6) describes how a partial reduction in chemical exposure
leads to a reduction in blood concentration. In the derivation of
Eq. (6) it was noted that the initial and final steady state concentra-
tions (C
ss0
and C
ss1
) can be readily obtained from the generic
expression C
ss
= IE
a
/(k
e
V
d
M) when the initial and final chemical
exposure rates (I
0
and I
1
) are known. The inverse proposition also
holds: initial and final chemical exposures can be obtained if initial
and final steady state blood concentrations are known. In fact, the
ratio of the initial and final exposure rates is equal to the ratio of
the initial and final steady state concentrations, that is
I
1
=I
0
¼ C
ss1
=C
ss0
ð11Þ
The ratio of C
ss1
to C
ss0
can be obtained from Eq. (6) and the
resulting percentage exposure reduction (ER) is then given by:
ER ð%Þ100 1 I
1
=I
0
ðÞ¼100 1
CðtÞ
C
ss0
= 1 e
k
e
t
ð12Þ
In Eq. (12), t is the time that has elapsed since exposure was
reduced. Eq. (12) shows that, within the limitations of this simple
model, exposure reduction can be calculated directly from mea-
surements of C(t) if the intrinsic elimination rate constant, k
e
, the
initial steady state concentration, C
ss0
, and the elapsed time since
exposure reduction, t, are known. In practice, C
ss0
and t can be esti-
mated by examination of the time history of blood concentration
to find the onset of reduction in C(t): C
ss0
can be taken as the mean
value of C(t) before the blood concentration changed and t can be
taken as the elapsed time since the onset of reduction of C(t).
The derivation of Eqs. (11) and (12) is given in the SD. Eq. (12)
can be used to estimate the exposure reduction of many additional
environmental contaminants such as lead, cotinine and benzene
Table 2
Example of moderate calculation bias between apparent and intrinsic elimination half-life values for PFOA (from Brede et al., 2010 ).
Population and sample
collection period
Sub-population
*
Plasma concentration (ng mL
1
) Elapsed
time (yr)
Apparent
elimination
half-life, (yr)
Intrinsic
elimination
half-life (yr)
Calculation
bias (%)
Initial Final Bkgd
Arnsberg residents, 2006–2008 Children (20) 23.4 13.2 5.0 2.00 2.42 1.72 29.2
Mothers (22) 23.6 13.3 2.9 2.00 2.42 2.01 16.7
Men (23) 30.3 21.7 6.3 2.00 4.15 3.13 24.6
All sub-populations: {Geomean: 2.93 2.24 23.5
95% CI: (2.75–3.12) (2.10–2.39)
Adults only: {Geomean: 3.19 2.52 20.9
95% CI: (2.94–3.45) (2.36–2.69)
*
Number of individuals in the population given in parentheses.
Background PFOA plasma values are the geomean of the control population in Siegen Germany.
Table 3
Example of high calculation bias between apparent and intrinsic elimination half-life values for PFOA (from Kato et al., 2011).
Population and sample collection period Sub-Population
*
Serum concentration (ng mL
1
) Elapsed
time (yr)
Apparent
elimination
half-life (yr)
Intrinsic
elimination
half-life
(yr)
Calculation
bias (%)
Initial Final
USA general population, 1999–2008 Males 5.71 4.80 8.0 31.9 2.4 92
Females 4.80 3.56 8.0 18.6 2.4 87
nHW
à
5.60 4.38 8.0 22.6 2.4 89
MA
à
3.89 3.53 8.0 57.1 2.4 96
nHB
à
4.80 3.86 8.0 25.4 2.4 91
Mean bias: 91
95% CI: (87–95)
*
All individuals in the study were >12 years old.
The intrinsic elimination half-life was determined to be 2.4 years, the average of the general population data of Bartell et al. (2010) and Brede et al. (2010).
à
Key: nHW (non-Hispanic Whites); MA (Mexican–Americans); nHB (non-Hispanic Blacks).
214 M.H. Russell et al. / Chemosphere 129 (2015) 210–216
for which extensive biomonitoring data have been collected and
reported in studies such as NHANES.
3.5. Estimating exposure reduction of PFOA in USA general population
As discussed above, the intrinsic PFOA half-life for the general
population is 2.4 years resulting in k
e
= ln(2)/2.4 = 0.289 yr
1
. The
NHANES data summarized for males and females in Table 3 were
collected over an eight year period (i.e. t = 8). When these values
are substituted into Eq. (12), the estimated exposure reduction
for the general US male population is 18% and the corresponding
final steady-state serum concentration is 4.7 ng mL
1
. For
females 12 and older, a similar calculation results in an esti-
mated exposure reduction of 29% and a corresponding final
steady state serum concentration of 3.4 ng mL
1
. Apparent
sex-based differences in PFOA exposure reduction may reflect
differences in exposure scenarios, limitations in the modeling
assumptions, uncertainty and bias in the monitoring data as well
as actual differences in intrinsic elimination rates for men and
women. Based on these inverse calculations, it is reasonable to
conclude that PFOA exposure in the general US adult population
decreased by 20–30% over the period 1999–2008. The estimated
exposure reduction is similar to the change in male and female
body burdens of PFOA over this period, as reflected in the
decrease in serum concentrations, i.e. 16% and 26% for males
and females, respectively. Due to the multi-year intrinsic elimi-
nation half-life of PFOA, the observed decline in serum concen-
tration lags the estimated reduction in external exposure over
the nine period of biomonitoring.
4. Discussion
As demonstrated in the examples described above, accurate
determination of intrinsic chemical elimination half-life values
is a function of the magnitude of observed concentration decline
as well as the level of ongoing exposure (Eq. (7)). Accurate cal-
culation of intrinsic elimination half-life values requires both
an extended period of sampling (i.e. more than 1–2 half-lives)
and appropriate correction for ongoing exposure. Alternatively,
intrinsic elimination half-life values can be derived from multi-
ple cross-sectional biomonitoring and intake data by using pop-
ulation-level pharmacokinetic modeling as demonstrated for
human exposure to polychlorinated biphenyls (Ritter et al.,
2011).
The intrinsic elimination half-life is not necessarily the same in
all sub-populations. For chemicals such as PFOA which are elimi-
nated almost exclusively via urinary excretion, it is important to
consider the age and gender of the population being studied. The
glomerular filtration rate (GFR) of the kidney typically decreases
with age (Sun et al., 2009; Musso and Oreopoulos, 2011). In addi-
tion, differences in the elimination half-life of PFOA have been
noted between males and females. Enhanced elimination of PFOA
in menstruating women is possible as several studies have
reported lower PFOA levels in women than men (Harada et al.,
2005; Yeung et al., 2006; Kato et al., 2011). Furthermore, mean
elimination half-lives of PFOA have been estimated to be slower
for young females (2.1 yr) than for males or older females
(2.6 yr) (Zhang et al., 2013). These factors may help explain the dif-
ferences observed between the intrinsic elimination half-lives cal-
culated for PFOA in older, predominantly male workers (Table 1)
and for the general population (Table 2). Thus, for chemicals such
as PFOA, it is important to consider potential confounding factors
such as age and gender when evaluating bioelimination half-lives
from biomonitoring data to ensure appropriate application in
subsequent toxicokinetic modeling.
5. Conclusions
Perfluorooctanoic acid (PFOA) is a persistent and widely dis-
persed environmental pollutant that has been detected in biota
and humans worldwide. Based on evaluation of two biomonitoring
studies of the general population, the intrinsic plasma elimination
half-life of PFOA in humans is estimated to be 2.4 years, represent-
ing the average of the results from Brede et al. (2.5 years, 95% CI
2.4–2.7) and Bartell et al. (2.3 years, 95% CI 2.1–2.4). The accurate
determination of intrinsic chemical elimination half-lives from
longitudinal or cross-sectional studies of human blood, plasma or
serum requires careful consideration of the magnitude of the
observed concentration changes as well as evaluation of the extent
of ongoing levels of exposure. Calculation of intrinsic chemical
elimination half-life values clarifies the actual rate of metabolism
and elimination and permits improved understanding and predic-
tion of the fate of xenobiotic chemicals in humans.
Determination of the bioelimination rate of xenobiotics is a crit-
ical component of toxicokinetic modeling and subsequent risk
assessments. Failure to account for ongoing exposure results in
calculation of biased elimination rates and produces exaggerated
estimates of chemical elimination half-lives. Care should be taken
in calculating and reporting elimination data to minimize misun-
derstanding and potential misuse of experimental kinetic results.
For some chemicals, factors such as age or gender may impact
intrinsic elimination half-life values for specific subpopulations
and additional values may be needed to enable accurate toxicoki-
netic modeling of subpopulations.
Evaluation of elimination kinetics from biomonitoring data
without explicitly compensating for the effects of ongoing
exposure can lead to speculation involving nonlinear elimination
kinetics and novel retention mechanisms, especially when moni-
tored concentrations approach background values (Costa et al.,
2009; Yali and Yaqi, 2014). Evaluation of accurate intrinsic elimi-
nation rates for PFOA from humans can contribute to improved
exposure assessments, more reliable risk assessments and more
complete understanding of the behavior of persistent chemicals
in the environment. Kinetic equations similar to those described
in this study can be developed to differentiate between intrinsic
and apparent rates of chemical decline in environmental media
such as lakes and soil where input sources occur simultaneously
with various elimination mechanisms.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in
the online version, at http://dx.doi.org/10.1016/j.chemosphere.
2014.07.061.
References
Bartell, S.M., Calafat, A.M., Lyu, C., Kato, K., Ryan, P.B., Steenland, K., 2010. Rate of
decline in serum PFOA concentrations after granular activated carbon filtration
at two public water systems in Ohio and West Virginia. Environ. Health
Perspect. 118, 222–228
.
Bjerregaard, P., Pedersen, H., Nielsen, N., Dewqilly, E., 2013. Population surveys in
Greenland 1993–2009: temporal trend of PCBs and pesticides in the general
Inuit population by age and urbanization. Sci. Total Environ. 454
.
Brede, E., Wilhelm, M., Goen, T., Muller, J., Rauchfuss, K., Kraft, M., Holzer, J., 2010.
Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC
plasma levels after PFOA reduction in public water system in Arnsberg,
Germany. Int. J. Hyg. Environ. Health 213, 217–223
.
Brown, R.P., Delp, M.D., Lindstedt, S.L., Rhomberg, L.R., Beliles, R.P., 1997.
Physiological parameter values for physiologically based pharmacokinetic
models. Toxicol. Ind. Health 13, 407–484
.
CDC. Fourth National Report on Human Exposure to Environmental Chemicals,
Updated Tables, February 2012. Department of Health and Human Services,
Center for Disease Control; 2012.
M.H. Russell et al. / Chemosphere 129 (2015) 210–216
215
Costa, G., Sartori, S., Consonni, D., 2009. Thirty years of medical surveillance in
perfluorooctanoic acid production workers. J. Occup. Environ. Med. 51, 364–
372
.
Egeghy, P.P., Lorber, M., 2011. An assessment of the exposure of Americans to
perfluorooctane sulfonate: a comparison of estimated intake with values
inferred from NHANES data. J. Expos. Sci. Environ. Epidemiol. 21, 150–168
.
Harada, K., Inoue, K., Morikawa, A., Yoshinaga, T., Saito, N., Koizumi, A., 2005. Renal
clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and
their species-specific excretion. Environ. Res. 99, 253–261
.
Kato, K., Wong, L.-Y., Jia, L.T., Kuklenyik, Z., Calafat, A.M., 2011. Trends in exposure to
polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ. Sci.
Technol. 45, 8037–8045
.
Li, Z., Romanoff, L., Bartell, S., Pittman, E.N., Trinidad, D.A., McClean, M., Webster,
T.F., Sjodin, A., 2012. Excretion profiles and half-lives of ten urinary polycyclic
aromatic hydrocarbon metabolites after dietary exposure. Chem. Res. Toxicol.
25, 1452–1461
.
Musso, C.G., Oreopoulos, D.G., 2011. Aging and physiological changes of the kidneys
including changes in glomerular filtration rate. Nephron Physiol. 119, 1–5
.
Olsen, J., 2012. Pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-dioxin and related
compounds. In: Schecter, A. (Ed.), Dioxins and Health. John Wiley & Sons,
Hoboken, NJ
.
Olsen, G.W., Burris, J.M., Ehresman, D.J., Froehlich, J.W., Seacat, A.M., Butenhoff, J.L.,
Zobel, L.R., 2007. Half-life of serum elimination of perfluorooctanesulfonate,
perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical
production workers. Environ. Health Perspect. 115, 1298–1305
.
Prevedouros, K., Cousins, I.T., Buck, R.C., Korzeniowski, S.H., 2006. Sources, fate and
transport of perfluorocarboxylates. Environ. Sci. Technol. 40, 32–44
.
Quinn, C.L., Wania, F., 2012. Understanding differences in the body burden-age
relationships of bioaccumulating contaminants based on population cross
sections versus individuals. Environ. Health Perspect. 120, 554–559
.
Ritter, R., Scheringer, M., MacLeod, M., Moeckel, C., Jones, K.C., Hungerbühler, K.,
2011. Intrinsic human elimination half-lives of polychlorinated biphenyls
derived from the temporal evolution of cross-sectional biomonitoring data
from the United Kingdom. Environ. Health Perspect. 119, 225–231
.
Schnoor, J.L., 1996. Environmental Modeling: Fate and Transport of Pollutants in
Water, air and Soil. John Wiley & Sons, Inc., New York
.
Seals, R., Bartell, S.M., Steenland, K., 2011. Accumulation and clearance of
perfluorooctanoic acid (PFOA) in current and former residents of an exposed
community. Environ. Health Perspect. 119, 119–124
.
Shirai, J., Kissel, J., 1996. Uncertainty in estimated half-lives of PCBs in humans:
impact on exposure assessment. Sci. Total Environ. 187, 199–210
.
Sun, X., Chen, Y., Chen, X., Wang, J., Xi, C., Lin, S., Liu, X., 2009. Change of glomerular
filtration rate in healthy adults with aging. Nephrology 14, 506–513
.
Timchalk, C., 2010. Biomonitoring of pesticides: pharmacokinetics of
organophosphorus and carbamate insecticides. In: Satoh, T., Gupta, R. (Eds.),
Anticholinesterase Pesticides: Metabolism, Neurotoxicity and Epidemiology.
John Wiley & Sons, Hoboken, NJ
.
Wagner, J.G., 1975. Fundamentals of Clinical Pharmacokinetics. Drug Intelligence
Publications, Inc., Hamilton, IL
.
Wong, F., MacLeod, M., Mueller, J.F., Cousins, I.T., 2014. Enhanced elimination of
perfluorooctane sulfonic acid by menstruating women: evidence from
population-based pharmacokinetic modeling. Environ. Sci. Technol. 48, 8807–
8814
.
Yali, S., Yaqi, C., 2014. Study of per- and polyfluoroalkyl substances related
environmental problems. Prog. Chem. 26, 665–681
.
Yeung, L.W.Y., So, M.K., Jiang, G., Taniyasu, S., Yamashita, N., Song, M., Wu, Y., Li, J.,
Giesy, J.P., Guruge, K.S., Lam, P.K.S., 2006. Perfluorooctanesulfonate and related
fluorochemicals in human blood samples from China. Environ. Sci. Technol. 40,
715–720
.
Zhang, Y., Beesoon, S., Zhu, L., Martin, J.W., 2013. Biomonitoring of perfluoroalkyl
acids in human urine and estimates of biological half-life. Environ. Sci. Technol.
47, 10619–10627
.
216 M.H. Russell et al. / Chemosphere 129 (2015) 210–216
