
Shulin packages axonemal outer dynein arms for
ciliary targeting
Girish R. Mali
1,
, Ferdos Abid Ali
1,
, Clinton K. Lau
1,
, Farida Begum
1
, Mark Skehel
1
, and Andrew P. Carter
1,*
1
Structural Studies Division, MRC Laboratory of Molecular Biology, Cambridge, United Kingdom
The main force generators in eukaryotic cilia and flagella
are axonemal outer dynein arms (ODAs). During cilio-
genesis, these ~1.8 MDa complexes are assembled in the
cytoplasm and targeted to cilia via an unknown mecha-
nism. Here we use the ciliate Tetrahymena to identify two
novel factors (Q22YU3 and Q22MS1) which bind ODAs
in the cytoplasm and are required for their delivery to
cilia. We show that Q22YU3, which we name Shulin,
locks the ODA motor domains into a closed conformation
and inhibits motor activity. Cryo-EM reveals how Shulin
stabilizes this compact form of ODAs by binding to the
dynein tails. Our findings provide a molecular explana-
tion for how newly assembled dyneins are packaged for
delivery to the cilia.
Motile cilia play essential roles that range from setting up
the left-right body axis to clearing mucus from the lungs (1).
These slender cellular projections contain an axoneme built
of microtubule doublets. Ciliary beating is powered by ar-
rays of inner and outer dynein arm motors that slide adjacent
doublets past each other (2). The outer dynein arms (ODAs)
are the main force generators in cilia and the most frequently
mutated components in human motile ciliopathies (3). ODAs
are multi-subunit complexes (4), which are pre-assembled in
the cytoplasm by a collection of nine dynein axonemal as-
sembly factors and associated chaperones (4, 5). Following
assembly, ODAs are targeted to cilia, where the intraflagel-
lar transport (IFT) machinery carries them to their docking
sites (6, 7). However, the mechanism of ODA delivery to the
cilia and whether any additional factors are required for this
process are both unknown.
To identify potential ciliary targeting factors, we purified
newly-assembled ODAs from the cytoplasm of the protozoan
ciliate Tetrahymena thermophila. We de-ciliated Tetrahy-
mena to remove pre-existing axonemes and trigger new ODA
synthesis (8) (Figure 1A). ODA complexes, containing a
FLAG-tagged copy of the intermediate chain IC3, were ex-
tracted and separated from other assembly intermediates by
size exclusion chromatography (SEC). Two factors co-eluted
with the new fully-assembled ODAs and were identified by
mass spectrometry (MS) as Q22MS1 and Q22YU3 (Figure
1B, Figure S1A). We also performed label-free quantitative-
MS on the immunoprecipitated IC3 subunit and detected an
equivalent fold enrichment of both factors relative to other
ODA subunits (Figure S1B, Table S1). Taken together,
these data suggest Q22MS1 and Q22YU3 interact tightly
Co-first authors
with ODAs in the cell body.
These novel factors lack known functions and have not
previously been linked to motile cilia. Q22YU3 shares
24% identity to human C20ORF194 (Figure S2) whereas
Q22MS1 has a unique domain architecture and no ortholog
outside of Tetrahymena. To investigate their roles, we gener-
ated Tetrahymena knockout strains for each factor (Figure
S3A). Both strains showed an ~2-fold decrease in swim-
ming speed compared to wildtype (Q22YU3: 10.6 ± 12
µm/s, Q22MS1: 11.88 ± 12.6 µm/s, WT: 22.5 ± 16.9 µm/s;
mean ± SD) suggesting defects in cilia movement (Figure
1C). Mutants also had decreased accumulation of food vac-
uoles and a higher frequency of cytokinetic defects, which are
hallmarks of defective cilia motility in Tetrahymena (9, 10)
(Figure S3B, C). We found the lengths and numbers of cilia
in our knockouts were similar to wildtype suggesting our ob-
servations were not due to defects in ciliogenesis (Figure
S3D, E). However, high-speed imaging showed mutant cilia
beat slowly (movie S1), similar to a temperature-sensitive
mutant with reduced ODAs in cilia (11) (movie S2). We
therefore used immunofluorescence to test if loss of Q22YU3
and Q22MS1 affects ODA targeting to cilia. Staining with an
antibody against ODAs showed marked reductions in their
levels in cilia of mutant strains compared to the wildtype
(Figure 1D, E, Figure S3F). Together, our data suggest that
loss of Q22YU3 or Q22MS1 result in defective ciliary move-
ment due to reduced delivery of ODAs to the cilia.
It has been proposed that ODAs need to be held in an inac-
tive state during transport into the axoneme (4). We there-
fore tested whether Q22YU3 and Q22MS1 inhibit dynein
motor activity. We expressed both factors recombinantly
and assayed their effect on the microtubule gliding activity
of ODAs purified from axonemes (Figure 2A). In the ab-
sence of Q22YU3 and Q22MS1, ODAs translocated micro-
tubules at 1.39 ± 0.6 µm/s (mean ± SD). Microtubule gliding
was severely compromised in the presence of both factors to-
gether (0.15 ± 0.15 µm/s) or with Q22YU3 alone (0.1 ± 0.15
µm/s). Q22MS1 reduced microtubule gliding velocities to a
lesser extent (0.56 ± 0.76 µm/s). In contrast, addition of both
factors to cytoplasmic dynein-1 did not significantly alter its
microtubule gliding velocity (0.76 ± 0.47 µm/s for dynein-
1 alone vs 0.69 ± 0.27 µm/s with both factors). These data
show Q22YU3 is sufficient to specifically inhibit ODA activ-
ity.
Tetrahymena ODAs contain three dynein heavy chains and,
Mali et al. | bioRχiv | August 20, 2020 | 1–28
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure 1. Q22MS1 and Q22YU3 deliver ODAs from cytoplasm to cilia. (A) Scheme used to identify novel interactors of ODAs assembled in the cell body. (B) SDS-PAGE of
ODA purified from the cell body by IP-SEC showing co-elution with Q22MS1 and Q22YU3, HC: Heavy chains, IC: Intermediate chains. (C) Cell swimming velocity comparing
wildtype (WT n=108) and mutant strains (Q22YU3∆ n=102 and Q22MS1∆ n=110). (D) Ratio of ODA/Tubulin immunofluorescence intensity along individual cilia (WT n=118,
Q22YU3∆ n=104, Q22MS1∆ n=129, 3-10 cilia from 14-17 cells/genotype). (E) Representative cells showing immunofluorescence for ODA and tubulin (quantified in D). Scale
bars:10 µm. Error bars show standard deviation; ns=not significantly different, ****p≤0.0001 (ANOVA with Tukey’s test for multiple comparisons).
when purified from axonemes, show an open bouquet confor-
mation with the heavy chain motor domains separated (12).
However, when we used negative stain electron microscopy
(EM) to image ODAs purified from the cytoplasm, we no-
ticed ~40% of intact particles displayed a ’closed’ conforma-
tion where the motor domains are clustered, and the tails are
compacted (Figure S4). This closed conformation resembles
a form previously observed only after cross-linking (12). To
identify the factor responsible, we reconstituted ODAs ex-
tracted from axonemes with Q22MS1 and Q22YU3. Both
factors form stable complexes with ODAs either together or
individually (Figure 2B, Figure S5A). Whereas ODAs on
their own were entirely in the open conformation (Figure
2C, D), in the presence of both factors 50 ± 8.3% (mean
± SD) of particles were closed, similar to the fraction ob-
served for ODAs purified from the cytoplasm. ODAs bound
to Q22MS1 alone were closed only 7.7 ± 5.0% of the time.
In contrast, in the presence of Q22YU3 alone, 60 ± 11.4%
of ODAs were closed (Figure 2C, D, Figure S5B-F). Col-
lectively, our findings suggest that Q22YU3 inhibits ODAs
by holding the three heavy chains together into a closed con-
formation. We therefore propose to name this novel protein
Shulin (Sanskrit: one that holds the trident).
To elucidate how Shulin closes ODAs at a molecular level,
we determined the structure of the reconstituted Tetrahymena
ODA-Shulin complex by cryo-EM. The resolution of our
overall structure is limited to 8.8 Å due to its flexibility. How-
ever, focused classification and local refinement produced a
series of sub-region maps ranging from 4.3-5.9 Å in global
resolution (Figure 3A, Figure S6). The central portion of
the Shulin region map has a local resolution range of 3.2-4.2
Å (Figure S7) enabling de novo building of Shulin and its in-
teractions (Figure 3A). In combination with MS to identify
the ODA subunit composition (Figure S8), our maps allowed
us to assign the positions of the three heavy chains (Dyh3,
Dyh4, Dyh5), two essential intermediate chains (ICs: Dic2,
2 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
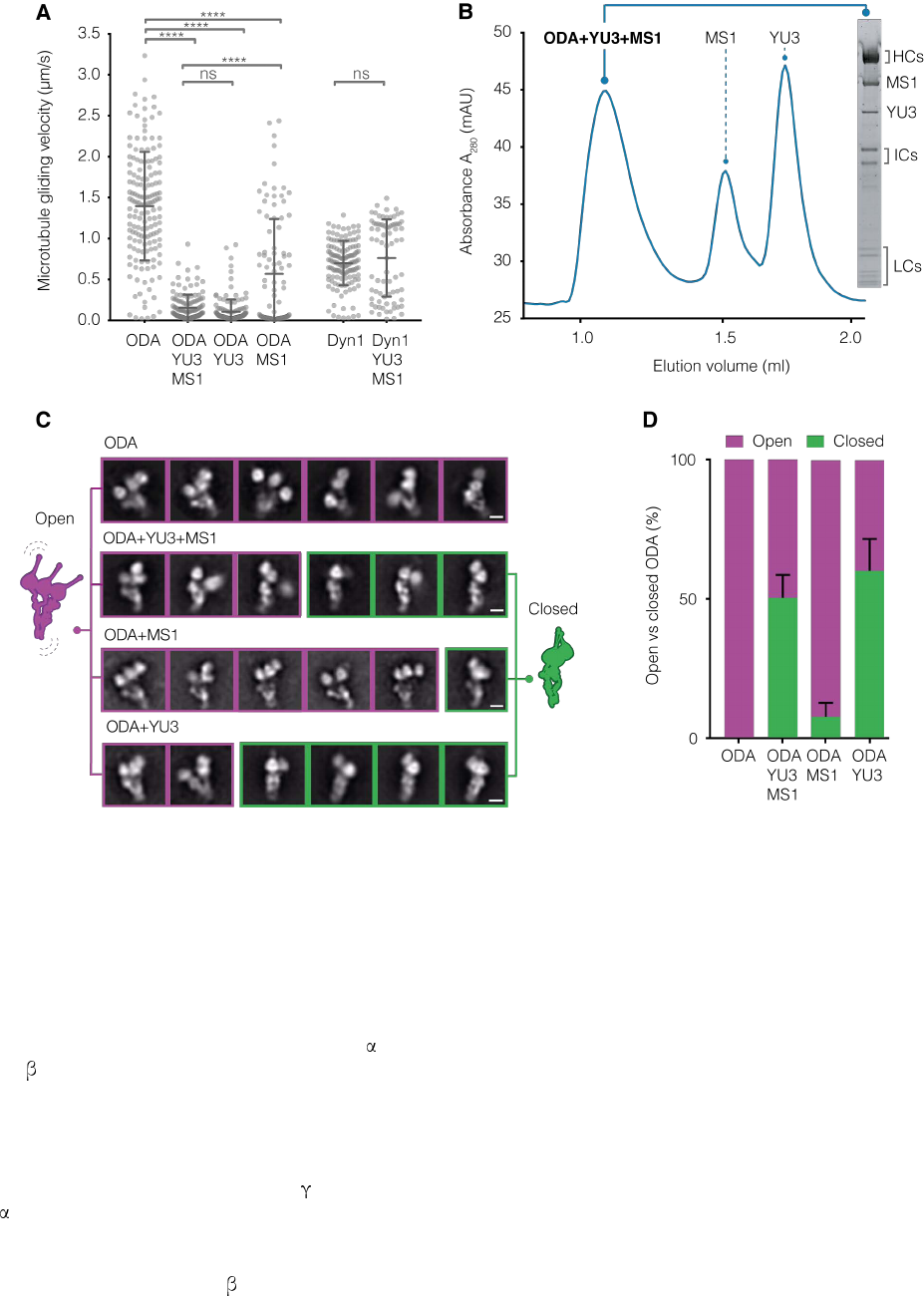
Figure 2. Q22YU3 binding inhibits ODAs by clustering motors. (A) Microtubule gliding velocities. Individual gliding events from three technical replicates/sample are
plotted (ODA n=159, ODA+YU3+MS1 n=136, ODA+YU3 n=146, ODA+MS1 n=94, Dyn1 n=136, Dyn1+YU3+MS1 n=76). YU3: Q22YU3, MS1: Q22MS1, Dyn1: human
cytoplasmic dynein 1. Error bars show standard deviation; ns=not significantly different, ****p≤0.0001 (ANOVA with Tukey’s test for multiple comparisons). (B) Axonemal-
purified ODA reconstituted with recombinant Q22YU3 and Q22MS1. SDS-PAGE gel of SEC peak fraction. (C) Representative 2D class averages showing the distribution of
open (purple) and closed (green) ODA particles from ODAs alone and reconstituted with factors. Scale bars = 10 nm. (D) Quantification of closed and open particles shown
in (C) represented as percentage. Error bars show standard deviation.
Dic3) and 11 light chains (LCs) (Figure 3B, Figures S6-S8).
In our structure, the motor domains are clustered in a closed
conformation (Figure 3B) consistent with the negative stain
data. At the core of the structure are the Dyh3 (-HC) and
Dyh4 (-HC) heavy chains, which are conserved across all
eukaryotes with motile axonemes (13). They form a het-
erodimer held together by an N-terminal dimerization do-
main in an arrangement that is similar to cytoplasmic dyneins
(14, 15). The third heavy chain, which is only found in ciliate
and algal ODAs, is Dyh5 in Tetrahymena (-HC equivalent to
the -HC in the alga Chlamydomonas). Our structure shows
that Dyh5 is much shorter than the other heavy chains and is
anchored to Dyh4 halfway along its length. The N-terminus
of Dyh5 contains a Kelch-type -propeller domain that sits
on the helical bundles of Dyh4 (Figure 3C). The peripheral
attachment of this third heavy chain explains why its loss in
Chlamydomonas is largely tolerated (16).
In their tail regions, Dyh3 and Dyh4 wrap round the globu-
lar WD40 domains of the intermediate chains and Dyh4 also
binds to a small density consistent with the thioredoxin-like
Lc3 light chain (Figure 3D, Figure S9A). The intermediate
chains have long N-terminal extensions which are held to-
gether by a tower of light chains consisting of a Lc7/Lc7b
heterodimer, three dimers of Lc8 orthologs and at the end,
bent over to one side, a Tctex like heterodimer of Lc2a/Lc9
(Figure 3D, Figure S9B). Based on side-chain density we as-
signed the positions of Lc8 and its orthologs: Lc8d, Lc8e and
an unnamed Lc8-like protein (UniProt ID: Q22R86) which
we call Lc8f. Our MS analysis showed the additional pres-
ence of Lc10 and Lc8b which we tentatively assigned to
the remaining two positions. The bent arrangement of the
Lc2a/Lc9 heterodimer is stabilized by the Dic2 N-terminus,
which loops out from where it contacts Lc2a and wedges be-
tween Lc8d and Lc8e (Figure S9B).
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 3
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
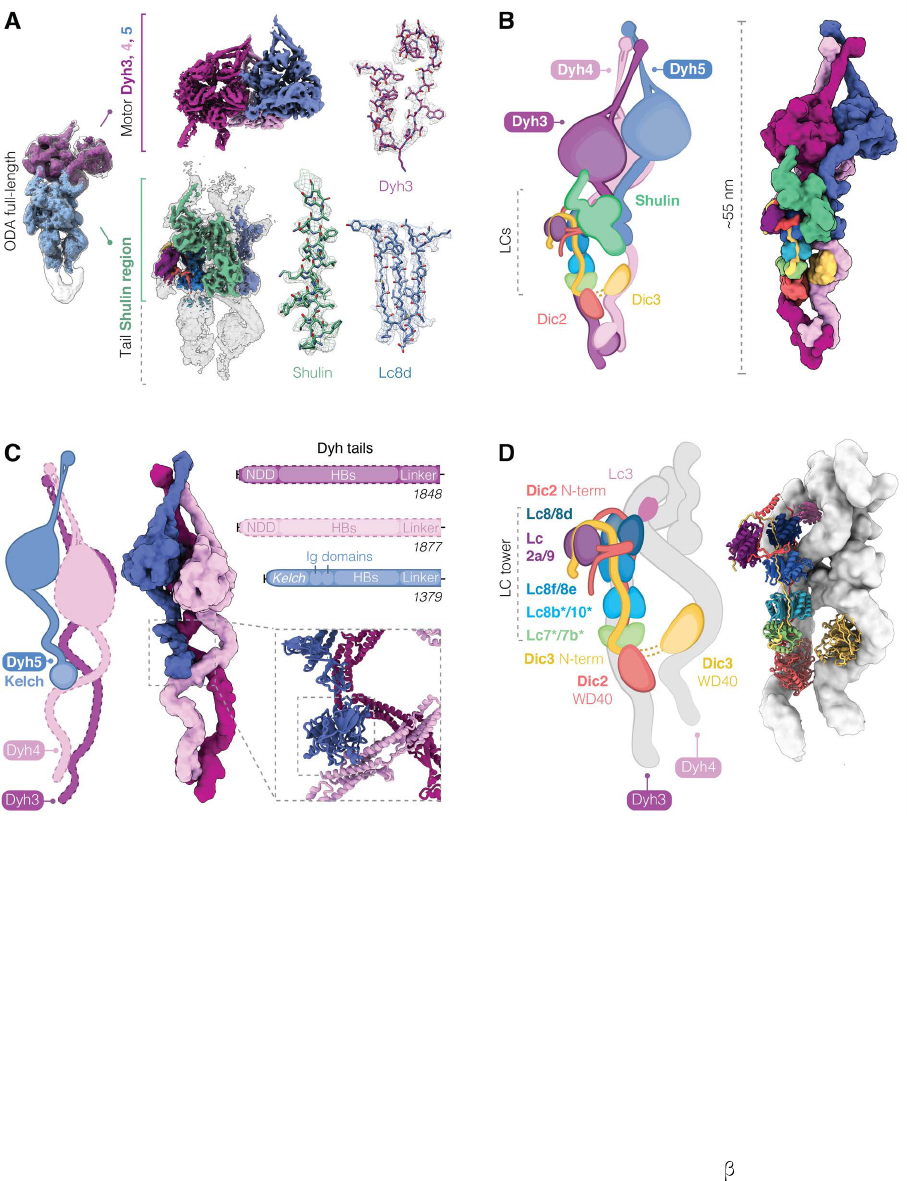
Figure 3. Cryo-EM reconstruction shows architecture of closed ODA. (A) Overview of the closed ODA bound by Shulin with head (purple) and tail (blue) maps docked in
an overall map (grey). Maps obtained after masked refinements are shown for the head region containing densities for Dyh3,4 and 5 motor domains and the tail map contains
a docked Shulin-region map (green). Representative cryo-EM densities are shown. (B) Cartoon and filtered surface representation of all modeled subunits. (C) Dyh5 binds
Dyh4 via its N-terminal Kelch domain (inset). HB: Helical bundles, NDD: N-terminal dimerization domain. (D) DIC N-terminal extensions bind dimers of LCs forming a LC
tower and followed by globular WD40 domains that contact Dyh3 and Dyh4. Heterodimers of Lc7/7b and Lc8b/Lc10 are tentatively assigned (*). Lc3 sits on Dyh4 and is not
part of the LC tower.
In the motor region, all three heavy chains are locked in the
pre-power stroke conformation of their catalytic cycle (17)
with their force producing linker domains bent through 90°
(Figure S10A, B). The density suggests that the coiled-coil
stalks of each motor domain are angled to interact with each
other close to their microtubule binding domains (Figure 3A,
B, Figure S10B). Clustering of motor domains is further sta-
bilized by interactions between the Dyh3 and Dyh4 linkers,
between the Dyh4 AAA4 and the elbow of the Dyh3 linker
and between the Dyh5 AAA3 and Dyh3 AAA4 (Figure
S10C, D). This clustered conformation is distinct to the one
ODAs adopt upon docking onto ciliary doublets where their
motor domains are stacked parallel to each other and free to
undergo their catalytic cycle (18). Thus, the closed confor-
mation is an inactive state of ODAs prior to their final incor-
poration into cilia.
Our Shulin structure shows that it contains N-terminal do-
mains (N1, N2) which are related to the aminopeptidase P
domain of Spt16, a core component of the histone chaper-
one FACT (Figure 4A). We built the middle (M) domain de
novo, revealing that it adopted a pleckstrin homology (PH)
fold akin to the Spt16 C-terminus. Therefore, the N-terminal
half of Shulin bears high structural homology to Spt16 (19).
Shulin’s C-terminal domains (C1 and C2) are homologous to
the bacterial GTPase YjiA (20). C1 has a Ras-like fold and
C2 comprises of a five-stranded -sheet. There is a nucleotide
between C1 and C2, which plays a structural role holding
the two domains together (Figure S11A). Shulin ends with
a long C-terminal finger with a helix-loop arrangement that
projects away from the core of the molecule (C3) (Figure
4A).
Shulin stabilizes the closed conformation of ODAs by bind-
ing all three heavy chains and the LC tower (Figure 4B). It
makes its most extensive interactions with Dyh3 (3216 Å
2
4 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure 4. Characterization of Shulin structure and its mechanism of ODA inhibition. (A) Domain architecture of Shulin. N-terminal (N1, N2) and Middle (M) domains
bear homology to FACT complex core subunit Spt16. C-terminal (C1, C2) domains are similar to GTPase YjiA and are followed by a C-terminal finger (C3). (B) Cartoon and
filtered surface representation with all contacts between Shulin and ODA subunits highlighted in green spheres. (C) Shulin’s N1 domain contacts helical bundles proximal to
the linker in Dyh3 tail. The C3 finger projects out to contact Dyh3 AAA1(S) (insets). (D) Shulin’s N1 domain contacts Dyh5 helical bundles and its N2 domain touches the
Kelch domain. Shulin contacts Dyh4 just below Dyh5 Kelch-domain (insets).
surface area). Shulin’s N1 domain contacts multiple sites in
the Dyh3 tail and its C3 finger binds AAA1S of the Dyh3
motor domain (Figure 4C). These contacts bridge the Dyh3
tail and motor holding them in a rigid conformation (Figure
S7). Shulin can only make these connections if the motor is
in its pre-power stroke conformation, suggesting it directly
locks Dyh3 into its closed state (Figure S11B).
Shulin holds the other two motor domains in their closed
conformation indirectly by stabilizing the contacts they make
with Dyh3 and each other. Its N2 domain makes a small con-
nection (313 Å
2
) to Dyh4 close to where the Dyh5 Kelch
domain is docked (Figure 4D). This interaction holds Dyh3
and Dyh4 together and reinforces a contact between the heli-
cal bundles in their tail regions (Figure S11B). This, in turn,
supports the previously-described connections between their
motor domains (Figure S10). Shulin’s connection to Dyh5,
via its N1 domain, is also small (292 Å
2
), but sufficient to
stabilize the Dyh5 linker binding to the Dyh3 motor domain
and motor-motor contacts between Dyh4 and Dyh5 (Figure
S10E). In the light chain tower the M-domain of Shulin con-
tacts Lc8e and its C1-domain contacts Lc8d and an -helix in
the Dic2 N-terminus (Figure S11C). These contacts stabi-
lize packing of the LC tower against the Dyh3 tail (Figure
S11D). Taken together, Shulin makes contacts with multiple
ODA subunits and stabilizes the interactions between them
that hold the motors in a closed conformation.
Here, we identify two proteins, Q22MS1 and Shulin, which
co-purify with ODAs in the cytoplasm and are required for
their delivery to cilia. Q22MS1 is a 222 kDa protein con-
taining a catalytically inactive nucleoside diphosphate kinase
(NDK) domain. A homologous NDK domain is found in the
recently identified Xenopus protein DAAP1 (21). DAAP1
has also been implicated in ODA delivery to cilia and lo-
calizes to membrane-less organelles involved in dynein as-
sembly. Interestingly however, there is no homology be-
tween Q22MS1 and DAAP1 outside the NDK domain and
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 5
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

the mammalian orthologs of DAAP1 lack the NDK-domain
completely.
Shulin shuts down ODA motor activity, suggesting it is the
proposed inhibitor (4) required during targeting of ODAs to
the cilia. Unlike the cytoplasmic dyneins which are auto-
inhibited (14, 15) ODAs rely on Shulin to enforce inhibition.
Inactivating ODAs may be important to prevent them from
escaping prematurely from cilia. Using immunostaining we
found that Shulin localizes to regenerating motile cilia 30-
minutes after deciliation (Figure S12A, C). This is the stage
of ciliogenesis when ODAs are being actively imported and
incorporated (8). In contrast, Shulin is predominantly cyto-
plasmic in cells with fully assembled cilia (Figure S12B, D).
This suggests, Shulin travels with ODAs during ciliogenesis
until they reach their final axonemal location.
In addition to its inhibitory role, Shulin may directly tar-
get ODAs to cilia. A candidate for the delivery process is
the small G-protein Arl3 which regulates targeting of nu-
merous ciliary cargo (22). The human ortholog of Shulin,
C20ORF194, is reported to bind the GTP-bound form of
Arl3, which specifically localizes to cilia (23). Thus, Shulin
may serve dual purposes of packaging and targeting ODAs to
cilia.
Materials and methods
Tetrahymena thermophila strain engineering and pheno-
typic analyses
Tetrahymena thermophila wildtype CU428 strain (Tetrahymena
Stock Center) was used in this study. Cultures were maintained in
SPP medium (1% bacto proteose peptone (Difco), 0.2% glucose,
0.1% yeast extract, 33 µM FeCl
3
). Transgenic lines were gen-
erated using biolistic transformation as previously described (24).
For tagging IC3 polypeptide with tandem ZZ and FLAG tags, a
construct bearing homology arms to the gene region upstream of
the stop codon and the 3’ UTR of DIC3 (TTHERM_00079230)
flanking a Neomycin resistance cassette, with codon optimized se-
quences for the tags was used. DNA was precipitated onto 10-
micron gold carriers for biolistic bombardment of the macronucleus
using a Gene Gun (Bio-Rad). For disruption of Q22YU3/SHULIN
(TTHERM_00122270) and Q22MS1 (TTHERM_00030520), con-
structs with homology arms to the 5’ UTR and 3’ UTR of the respec-
tive genes flanking the resistance cassette were generated. Trans-
formants were transferred to SPP medium for recovery and the pro-
moter driving the Neomycin resistance gene was switched on by ad-
dition of cadmium chloride (1 µM). After recovery, bombarded cells
were transferred into 96-well plates to isolate transformed clones.
Cultures were passaged every few days into medium containing in-
creasing concentrations of Paromomycin for phenotypic assortment
of transformants. Successful generation of tagged transgenic strains
was assayed by performing genomic PCRs spanning the Neo3 cas-
sette after several generations. Only transgenic strains would am-
plify this resistance gene. Immunoblotting and immunofluorescence
with a monoclonal FLAG antibody (Flag M2 Sigma, 1:100 dilution)
further confirmed the endogenous knock-in of the epitope tag at the
carboxy terminus of the IC3 polypeptide.
The knockout mutant strains tolerated up to 20-50 mg/ml of Paro-
momycin concentration. Genomic PCRs spanning Exons 1 to 3
of Q22YU3 (Exon 1 forward primer: atgaatttaaattttgcatgtcttcaataag,
Exon 3 reverse primer: ttatacatcatgaactgtacaatcacttgg) and Q22MS1
(Exon 1 forward primer: atgtttggatttgaagatatccattactaacc, Exon 3
reverse primer: attagaggcttagtgaacatgtcttcgtc) confirmed disruption
of both loci as only wildtype controls resulted in a robust ampli-
con whereas both mutants failed to generate a strong PCR product
(fig. S3A). A control PCR for -heavy chain gene was performed
to verify the integrity of the genomic DNA. Additionally, immunos-
taining Q22YU3/SHULIN cells with a custom polyclonal anti-
body against Shulin (Eurogentec, 1:100 dilution) confirmed loss of
protein as well as serving as antibody validation (Figure S12C, D).
For assessing ciliary defects in Q22YU3/SHULIN and
Q22MS1 mutant strains, three main phenotypic assays were per-
formed. Cell images or movies were acquired using a QIClick cam-
era with QCapture software mounted onto a Leica DM IL LED
microscope. Imaging was performed at room temperature. Im-
munofluorescence images were acquired using LSM710 confocal
microscope. Videos of cells swimming close to the plane of imag-
ing (closest to the slide) were acquired at 10 frames per second for
20-40 seconds using a 20x objective. High speed videos to visualize
cilia beating were acquired by digitally magnifying on individual
cells using a 100x objective at 10 frames per second. All cellular
phenotyping was done using FIJI (25). Cell velocity was measured
using MTrackJ plugin (26). Cell paths were manually traced, and
cell velocity was calculated as a function of distance traversed in a
given time frame. Food vacuoles were manually counted in phase
contrast images. Cilia numbers were counted using central confo-
cal slices in the plane of both the macro and micronucleus. Images
were thresholded to segment cilia around the cell circumference for
counting using FIJI. Cilia lengths were measured by drawing a line
along an individual cilium and measuring its distance. Lengths of
5-15 randomly selected cilia from 14-22 cells, from replicate stain-
ing experiments were measured. Cilium lengths per cell were av-
eraged and plotted. Cytokinesis defects were scored in images of
cells stained with acetylated -tubulin (SantaCruz, sc-23950, 1:250
dilution) to mark the cilia and demarcate the cell shape and DAPI
to stain for the nuclei. Cells with more than one oral apparatus
and/or macronucleus were counted for each genotype. To quan-
tify ciliary loss of ODAs in mutants, indirect immunofluorescence
was performed using a custom generated polyclonal antibody raised
against the ODA holocomplex (Eurogentec, 1:150 dilution) and an
acetylated -tubulin antibody (SantaCruz, sc-23950, 1:250 dilution)
to mark the ciliary axoneme. The ODA antibody was validated by
the manufacturer with ELISA tests against the ODA holocomplex
antigen. Additional validation of specificity was performed using a
temperature sensitive OAD1 C11 mutant strain (Tetrahymena Stock
Center) with reduced ciliary ODA staining when grown at the re-
strictive temperature of 39°C (Attwell et al. 1992). Fluorescence
intensity values along the cilium were measured using plot profile
tool, averaged and plotted in GraphPad Prism7.
Purification of ODA complexes and interactors from cell
body
Large scale cultures of IC3: ZZ: 3xFLAG strains were grown to a
high cell density in SPP medium typically for 72 hours. Cultures
were starved in Tris-Acetate buffer for 2 hours to reduce numbers
of phagocytic vacuoles containing proteases. Cells were deciliated
with dibucaine hydrochloride (0.5 M). The extent of deciliation was
carefully monitored by visual inspection under a stereomicroscope
to ensure minimal cell lysis. Typically, within 5 minutes of adding
dibucaine most cells appeared to have lost their cilia. Dibucaine
concentration was diluted three-fold by adding more medium and
6 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
cells were pelleted. Cell pellets were washed in Tris-Acetate buffer
and lysed in Lysis Buffer (20 mM HEPES NaOH, pH 8.0, 50 mM
NaCl, 1 mM EDTA, 5 mM DTT, and 10% glycerol supplemented
with 0.1% Triton X-100, 0.2% IGEPAL CA-630, 1 mM PMSF, 5
µM proteasome inhibitor MG-132, and 3x Complete protease in-
hibitor tablets (Roche). Lysates were clarified by ultracentrifuga-
tion at 70,000 rpm in a Ti70 rotor (Beckman) and flown multi-
ple times over FLAG-M2 affinity beads (Sigma) which were pre-
equilibrated in lysis buffer and packed in a gravity flow column.
The beads were washed for at least 5 column volumes times in Wash
buffer (20 mM HEPES, pH 7.4, 50 mM NaCl, 1 mM MgCl
2
, 1 mM
TCEP, 10% glycerol and 0.1% IGEPAL). IC3-ZZ-3xFLAG contain-
ing complexes were eluted into 5 fractions by sequentially flowing 5
bed volumes of elution buffer containing FLAG peptide. Efficiency
of elution was assessed by running SDS-PAGE gels and staining
with Instant Blue (Expedeon). Elution of desired ODA complexes
was deemed successful upon detection of high molecular weight
bands corresponding to dynein heavy chains. Eluted fractions con-
taining the highest concentration of complexes were further frac-
tionated over a Superose 6 increase 3.2/300 size exclusion column
(GE Healthcare) in GF50 gel filtration buffer (25 mM HEPES pH
7.4, 50 mM NaCl, 1 mM MgCl
2
, 1 mM DTT, 0.1 mM ATP). FLAG
eluates and peak fractions from gel filtration run were analysed by
mass spectrometry.
Purification of ODA from cilia axonemes
Axonemal ODAs were purified as previously described (12). Large
scale wildtype Tetrahymena cultures were deciliated using dibu-
caine (as above). Deciliated cell pellets were discarded and cilia in
the supernatant were centrifuged at 13,500 x g at room temperature.
Cilia pellets were washed in Cilia Isolation Buffer (CIB: 20 mM
HEPES pH7.4, 100 mM NaCl, 4 mM MgCl
2
, 0.1 mM EDTA) and
centrifuged at 600 x g several times to remove cell bodies and mu-
cus. A final high-speed spin at 13500 x g at 4°C yielded a pure cilia
pellet with a white fluffy appearance. Cilia were de-membranated
by resuspending the pellet in CIB containing 0.25% Triton-X de-
tergent, freshly added protease inhibitors, 1 mM DTT and 200 mM
PMSF followed by a 30-minute incubation on ice. De-membranated
cilia axonemes were isolated by centrifugation at 17000 x g at 4°C.
Axonemes were washed in CIB buffer to remove residual deter-
gent and re-pelleted. Axoneme pellets were resuspended in high
salt buffer (20 mM HEPES pH7.4, 600 mM NaCl, 4 mM MgCl
2
,
0.1 mM EDTA, 1 mM DTT, 0.1 mM ATP, 200 mM PMSF) and in-
cubated for 30 minutes on ice to isolate dynein arms. The dynein
containing high salt extract was loaded onto 6 identical 5-25% su-
crose density gradients made in CIB and ODA arms were separated
from other axonemal complexes over 16 hours by centrifugation at
33,000 rpm in an SW40 rotor at 4°C. The following day, sucrose
gradients were manually fractionated into 500 µl fractions. Every
alternate fraction was resolved on an SDS-PAGE gel and stained
with instant blue. ODA isolation was deemed successful upon de-
tection of characteristic high molecular weight bands corresponding
to ODA heavy chain polypeptides over several of the denser frac-
tions towards the bottom of the gradient. Sucrose gradient fractions
containing ODA complexes were further purified over a MonoQ
5/50 anion exchange column (GE Healthcare) to separate out other
axonemal dynein species. ODA complexes eluting at ~300 mM salt
off the MonoQ column were verified for intactness and presence of
subunits by a further gel filtration step, negative stain electron mi-
croscopy and mass spectrometry analyses. ODA complexes purified
as above were used in all biochemical reconstitution experiments
and subsequent cryo-EM studies.
Insect cell expression and purification of Q22MS1 and
Q22YU3/Shulin
Gene sequences coding for Tetrahymena thermophila Q22MS1
(TTHERM_00030520) and Q22YU3 (TTHERM_00122270;
C20ORF194-like) were codon-optimized and synthesized (Epoch)
for expression in Spodoptera frugiperda derived Sf 9 cells. Codon
optimized sequences were sub-cloned into pACEBac1-derived
vectors containing C-terminal 2xStrep tag. The following con-
structs were generated pACEBac1-Q22MS1-Psc-2ŒStrep and
pACEBac1-Q22YU3-Psc-2ŒStrep. Baculoviruses for individual
expression of Q22MS1 and Q22YU3/Shulin were prepared using
the insect cell-baculovirus system. Cells expressing recombinant
proteins were harvested 48 hours after infection and lysed in
50 ml cell lysis buffer (20 mM Hepes-NaOH pH 7.2, 100 mM
NaCl, 2 mM MgAc, 1 mM EDTA, 10% (v/v) glycerol, 1 mM
DTT). Cells were mechanically lysed in a 40 ml Dounce-type
homogenizer (Wheaton) using 15-25 strokes. Lysates were clarified
by ultracentrifugation at maximum speed in a Ti70 rotor (503,000
x g) for 45 min, 4°C (Beckman Coulter). Clarified lysates were
poured several times over 0.5-1 ml Streptactin resin (IBA) which
was applied into a gravity flow column and pre-equilibrated in lysis
buffer. The resin was washed for 20 column volumes to remove
non-specifically bound contaminants. Recombinant proteins were
eluted off the resin in five fractions over sequential incubations in
an elution buffer (lysis buffer containing 3 mM D-desthiobiotin).
Eluates were resolved on an SDS-PAGE and stained with Instant
Blue to assess the purity of the recombinant proteins. Recombinant
proteins eluting off the Streptactin beads were further cleaned over
gel filtration using GF150 (25 mM HEPES pH 7.4, 150 mM NaCl,
1mM MgCl
2
, 1 mM DTT, 0.1 mM ATP) buffer. Proteins were
snap frozen in liquid nitrogen and stored at -80°C for use in all
biochemical reconstitutions which were performed at 4°C.
Reconstitution of ODA with Shulin and/or Q22MS1
ODA complexes (~0.5-1 mg/ml) purified over a MonoQ col-
umn were mixed with 10-25x molar excesses of purified
Q22YU3/Shulin, Q22MS1 (~1-1.5 mg/ml) or both. Complexes
formed by overnight dialysis into 50 mM or 150 mM NaCl buffer
were used for negative stain EM analyses and complexes reconsti-
tuted by dialysis into 150 mM salt buffer were used for cryo-EM
grid freezing and analyses. Complex formation was assessed by
fractionating dialysates over a Superose 6 increase 3.2/300 size ex-
clusion column (GE) in GF50 (25 mM HEPES NaOH pH7.4, 50
mM NaCl, 1mM MgCl
2
, 1 mM DTT, 0.1 mM ATP) or GF150
(25 mM HEPES NaOH pH 7.4, 150 mM NaCl, 1mM MgCl
2
, 1
mM DTT, 0.1 mM ATP) buffer. Fractions spanning the entire col-
umn volume were resolved on an SDS-PAGE gel and stained with
Coomassie or SYPRO Ruby gel stain (Bio-Rad). The primary peak
consisted of ODA subunits with bound factors confirming success-
ful reconstitution. Secondary peaks contained molar excesses of
individual proteins unbound to ODAs.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 7
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
Microtubule gliding assays and quantification
Gliding assays were performed as previously described (27). Micro-
tubules were polymerized at 37°C using Alexa-647 and unlabeled
tubulin at 3 µm and 11 µm concentration respectively in polymer-
ization mix (BRB80: 80mM potassium PIPES pH6.8, 1mM MgCl
2
and 1mM EGTA with 10mM GTP). Polymerized microtubules were
stabilized with 2 µM Taxol in BRB80 (Sigma T1912). 10-20 µl of
freshly prepared or freshly thawed ODA at ~100 µg/ml concentra-
tion was applied to a flow chamber and allowed to adhere to glass
for 2 min at room temperature. Flow chamber was washed in buffer
(50 mM KAc, 10 mM HEPES pH 7.4, 4 mM MgAc, 1 mM EGTA)
containing 1% BSA. Taxol stabilized microtubules were flowed in
with a motility mix (20 mM HEPES pH 7.4, 5 mM MgSO
4
, 1 mM
DTT, 1 mM EGTA) and allowed to bind to motors. Finally, motil-
ity mix containing 1mM ATP was flowed in prior to TIRF imaging.
Imaging was performed at room temperature using a Nikon micro-
scope with a 100x oil-immersion objective (Nikon, 1.49 NA Oil,
APO TIRF).
The imaging system used the 100 mW 641 nm (Coherent Cube)
laser. Images were acquired with a back illuminated EMCCD cam-
era (iXonEM+ DU897E, Andor, UK) controlled with µManager
software. Imaging was performed with 100 ms exposures taken at 2
s intervals, one pixel = 0.16 x 0.16 µm with a pixel size of 160 nm.
For testing effect of factors, ODAs were pre-incubated on ice for
30 minutes with Q22YU3/Shulin, Q22MS1 or both in 10-25x mo-
lar excess and these complexes were applied to the flow chamber as
above. These molar ratios resulted in successful reconstitutions as
above and enforced closure of ODAs (by Shulin) and were therefore
also used in gliding assays. As control, full-length human cytoplas-
mic dynein-1 (Schlager et al. 2014) was used at 100 µg/ml with or
without both factors. Microtubule gliding velocities for each condi-
tion tested were calculated by manually tracking the leading edge of
moving microtubules using the FIJI plugin MTrackJ. The average
velocity for the track of each microtubule was used to calculate the
average velocity for the entire population of microtubules recorded.
Experiments were performed in triplicate technical repeats.
Negative stain electron microscopy analysis
Negative stain microscopy analyses were performed on ODAs from
axonemes, ODAs from cell bodies and ODA complexes reconsti-
tuted with Q22YU3/Shulin, Q22MS1 or both. In each case, 3 µl of
sample at ~0.05-0.1 mg/ml concentration were applied for 1 minute
to freshly glow-discharged 400 mesh copper grids coated with a
continuous carbon support layer (Agar Scientific) followed by ap-
plication of 2% uranyl acetate for a minute and air-dried after wick-
ing away excess liquid. For statistical analysis of open versus closed
ODA bouquets, triplicate datasets per condition were collected man-
ually on a FEI Spirit T12 microscope (equipped with Gatan 2K Œ
2K CCD (model 984) operated at 120 kV with a 1-1.5 second expo-
sure and a pixel size of 3.64-3.5 Å/pix. For initial structural analysis
of cell body ODA complexes, large datasets were collected using
EPU on a FEI F20 microscope operated at 200 kV with 1s expo-
sure; 3.4 Å/pix. A typical dose of ~20 e
1
per Åand a range of defoci
between 0.5-1.5 µm were used.
Statistical analysis of open and closed ODA conformations
To quantify effect of factors on ODA structure, ODAs alone, ODAs
reconstituted with Q22YU3/Shulin, Q22MS1 or with both factors
were each freshly prepared in triplicates and applied to negative
stain grids. Micrographs were collected manually from random re-
gions of grids and processed using RELION 3 (28). CTF was es-
timated using GCTF (29). A small subset of manually picked par-
ticles from each dataset yielded class averages that were used to
autopick particles for that dataset.
Several rounds of 2D classification were performed to remove
ambiguous particles representing ODAs which had fallen apart
or could not be assigned into closed or open classes. Only
intact three headed ODAs were further sub-classified. From
each of the three datasets per sample, a total of 13299 (ODA),
9118 (ODA+Q22YU3+Q22MS1), 9966 (ODA+Q22YU3) and 3610
(ODA+Q22MS1) intact ODA particles were used. These particles
were sub-classified and assigned into open versus closed conforma-
tions. Open conformation refers to ODAs with heads (motor do-
mains) far apart and tails open in a V-shape kinked to one side. In
contrast, a closed state is characterized by a tightly clustered ap-
pearance of heads and a compact tail straight in line with the heads.
Further rounds of sub-classification were done, and raw particles
were visually inspected to assess that particles were being correctly
assigned into an open or closed class according to their conforma-
tion. All four datasets were analysed in triplicates (triplicate datasets
acquired from triplicate reconstitutions per sample) and for each set
mean frequency of open versus closed and standard deviations were
calculated.
For cell body ODAs, a large dataset of 279,106 particles acquired
from a cell body purification of ODAs was sub-classified as above.
Non-ODA particles such as ribosomes, other cellular complexes and
broken ODAs were classed in an ambiguous class. Intact ODA parti-
cles were sub-classified as above until closed and open classes were
clearly distinguishable ( Figure S4).
Cryo-EM grid preparation
ODA were reconstituted with Q22YU3/Shulin using 1:10-1:25 mo-
lar ratios (ODA:Shulin; ODA at ~0.5-1 mg/ml and Shulin at ~1-1.5
mg/ml) as described above. Reconstituted complexes were purified
over a Superose 6 increase 3.2/300 size exclusion column SEC in
GF150 buffer and immediately crosslinked in 0.025% Glutaralde-
hyde (Sigma-Aldrich) for 30 minutes on ice followed by quench-
ing with 1 mM Tris-HCl (pH 7.4). This mild crosslinking with
0.025% glutaraldehyde minimized complex dissociation during grid
freezing. To assess that crosslinking did not cause gross artefacts
to reconstituted complexes, crosslinked complexes were negatively
stained and had an appearance indistinguishable to non-crosslinked
complexes freshly applied to EM grids.
ODA:Shulin complexes were applied at a concentration of ~0.1-
0.2 mg/ml to graphene oxide (GO) grids. GO grids were pre-
pared a day prior to freezing as previously described (30). Briefly,
gold 300 mesh Quantifoil R2/2 holey carbon grids (Quantifoil Mi-
cro Tools) were glow discharged using an Edwards Sputter Coater
305B. Graphene oxide (GO) dispersion (Sigma-Aldrich; 2 mg/mL
in H2O) was diluted ten-fold with ddH2O to a final concentration
of 0.2 mg/ml and subsequently spun down at 600 x g for ~15 sec
to remove large aggregates of GO flakes. Three microliters of GO
flake solution from the top was applied to grids. After incubation
for one minute with graphene oxide dispersion, the GO solution
was removed by blotting briefly with Whatman No.1 filter paper
and washed by absorbing 20 µl ddH2O onto the GO coated side
twice and once on the back side of the grid with blotting steps in
between. Grids were air-dried and used the next day for cryo-EM
grid freezing without further glow discharging as GO grids were
already hydrophilic. Freezing was performed at 4°C with 100% hu-
midity. 3 µl of sample were applied to the GO side of the grids.
8 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
After a wait time of 45 seconds, excess liquid was blotted away for
2-2.5 seconds with Whatman filter papers pre-equilibrated in the hu-
midity chamber. Grids were immediately plunge-frozen into liquid
ethane using a Vitrobot IV (ThermoFisher Scientific). Grids were
transferred into grid-boxes and stored in liquid nitrogen for future
screening and data collection.
Cryo-EM data collection and initial processing of whole ODA
molecule
Electron micrograph movies were recorded using a Titan Krios
(Thermo Fisher Scientific) equipped with an energy filtered K3
detector (Gatan) at 81,000x magnification in EFTEM mode. Six
datasets were collected at the LMB using Serial EM at a pixel size of
1.11 Å/pixel, 300 kV, 66 frames, 2.6 s exposure, ~52 e
-
/Å
2
. A script
was used to collect data in a 3x3 hole pattern, 3 images/hole, using
beam-tilt to speed up data collection. A further dataset was col-
lected at the Electron Bio-Imaging Centre (eBIC), Diamond Light
Source, UK using EPU v2.7 at 0.53 Å/pixel, 300 kV, 54 frames, 3 s
exposure, 54 e
-
/Å
2
(Table S2). Aberration-free image shift (AFIS)
collection was used to speed up data collection (4 images/hole).
Cryo-EM image processing
All image processing was performed using RELION-3.1 and soft-
ware wrapped within ( 28). Inter-frame motion in each movie was
corrected using RELIONs own implementation of motion correc-
tion as described above, using 5x5 patches and a B-factor of 150
Å applied to the micrographs (31). Defocus parameters were esti-
mated on non-dose weighted micrographs either using GCTF v1.18
(29) or CTFFIND4 v4.1.13 (32). For each individual dataset, par-
ticles were picked on non-dose weighted micrographs using Gau-
tomatch v0.56 using permissive picking parameters (cc_cutoff=0.1,
400 Å diameter) and projections from an initial structure of full-
length ODA. This initial model was obtained from a preliminary
round of processing of the eBIC and first two LMB datasets where
the phi-dynein structure was used as a reference (EMD-3705).
Particles were extracted from dose weighted micrographs with bin
4 parameters (768-pixel box size re-scaled to 192, yielding a final
pixel size of 4.24-4.44 Å/pixel). 2D classification was subsequently
performed with 75-100 classes, T=4, 750 Å circular mask, limit-
ing resolution E-step to 15 Å and ignoring CTFs until first peak.
A further 2-3 rounds of 2D classification without alignment (50-70
classes) was performed to remove graphene oxide layer artifacts,
ice contamination and aggregated particles refractory to averaging.
Particles from 2D classes showing projections of recognizable ODA
views were selected and joined from all datasets (1,300,000 parti-
cles).
The combined particles were subjected to global auto-refinement
with a loose mask around the whole ODA and an initial model of
the whole molecule filtered to 50 Å giving a 15 Å structure. Con-
siderable flexibility was observed in this overall structure (hereafter
referred to as overall-1), particularly the Dyh4 and Dyh5 motors
and the lower tail section. To resolve the tail and motors of ODA
separately, we employed a combination of focused classification,
masked refinement and signal subtraction as outlined in Figure S6.
All masks used were created using volumes of sub-regions gener-
ated in Chimera (UCSF) using ’volume eraser’ or ’color zone’ (33).
These sub volumes were low pass filtered to 15 Å with a soft edge
and binary map extension (both 6-8 pixels).
Processing of full-length and Dyh4/Dyh5 motors
To improve upon the resolution of the full-length ODA structure,
the overall-1 refinement data.star was used as input for masked 3D
classification without alignment (5 classes, T=4). Particles from the
class showing the most complete density (evidence for Dyh4 and
Dyh5 at lower threshold levels) were selected for masked global
3D refinement, yielding a 9.7 Åstructure. At this point particles
were re-extracted to their bin by 2 and unbinned parameters in par-
allel: 384-pixel box size at 2.22 Å/pixel and 768-pixel box size at
1.11 Å/pixel, respectively. These particles were used for local re-
finements, yielding full-length ODA structures that resolved to 8.9
Å (bin by 2) (overall-2) and 8.8 Å (unbinned) (overall-3; EMD-
11576). A mask was applied to the tail of the overall-3 map and
a local refinement was performed resulting in a 6.7 Å map of the
entire ODA tail (EMD-11577). The overall-2 map provided higher
signal to noise ratio for the flexible Dyh4 and Dyh5 motor domains
and was thus used to resolve these regions through signal subtrac-
tion and focused refinements. To this end, particle subtraction was
performed by centering the subtracted images on a mask encom-
passing both Dyh4 and Dyh5 motors and re-boxing to 256 pixels
(2.22 Å/pixel). Subtracted particles were subjected to a local refine-
ment, giving a an overall 12.9 Å structure of Dyh4-Dyh5 motors.
This reconstruction was used as the basis for signal subtraction of
Dyh4 and Dyh5 individually. Each motor was subsequently locally
refined (Dyh4, 10.5 Å and Dyh5, 11.8 Å with no post processing)
and 3D classified without alignment (5 classes, T=50). A final local
refinement with particles from the best 3D classes (selection criteria:
ordered motor, presence of stalk and limited noise) was performed,
resolving to 5.0 Å (57,761 particles) and 5.6 Å (49,756 particles)
for Dyh4 (EMD-11582) and Dyh5 (EMD-11583, EMD-11584), re-
spectively.
Processing of the tail and Shulin region
To get high resolution information on the Shulin region, a mask for
the full tail was first generated based on the overall-1 structure. Us-
ing this mask, a round of 3D classification without alignment was
performed on bin by 4 particles (8 classes, T=8) with the data.star
from overall-1 as input. Particles from the class containing clear
density for the Shulin finger, mid- and low tail region were selected
for global refinement, resolving to 9.0 Å. This was subsequently
used as an input for 3D classification without alignment with a mask
focusing on the Shulin region (8 classes, T=100). Six classes con-
taining clear density for the Shulin region were selected for a lo-
cal refinement that produced an 8.9 Å reconstruction. At this point,
123,484 particles were unbinned and re-boxed to a smaller 384-pixel
box size (1.11 Å/pixel). A more specific mask of Shulin was created
encompassing its core N-terminal and C-terminal domains, the C3
finger as well as contacts to the uppermost LC tower and portions of
the contacting Dyh3-5 tails. Masked local refinement of this region
produced a 4.8 Å structure. Finally, the Shulin region refinement
parameters were used for a round of 3D classification without align-
ment to sort out remaining heterogeneity (5 classes, T=50). 43,338
particles from two overlapping classes were combined for two sep-
arate masked local refinements of the Shulin region: Shulin region
and the DYH3 tail contact (4.6 Å; EMD-11580), and Shulin region
excluding C3 finger (4.3 Å; EMD-11579). A larger mask of the tail
was also applied to the earlier 123,484 subset of particles to get an
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 9
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
overview of the lower tail region (refined to 5.9 Å after signal sub-
traction, re-centering and masked 3D classification; EMD-11578).
Processing of the Dyh3 region
The overall-1 map indicates that Dyh3 is rigid relative to the two
other motors, enabling direct masked 3D classification without
alignment of this motor and region of the upper tail (based on
overall-1 data.star, 8 classes, T=10). Selection of particles from the
best class and global 3D refinement resulted in a 9.0 Å structure, at
which point particles were unbinned and re-boxed (512-pixel box
size). A tighter Dyh3 motor only mask was generated and used for
a round of local 3D refinement, producing a 4.8 Å structure. This
was used as the basis for 3D classification without alignment to sep-
arate out conformational heterogeneity (5 classes, T=50), resulting
in only one class showing complete density. 49,397 particles from
this class were selected for a final round of local 3D refinement and
re-centering in the box, yielding a 4.4 Å resolution structure (Dyh3
region; EMD-11581).
Model building and refinement
Homology models were generated for ODA subunits using
PHYRE2 (34), using the sequences of chains found in mass spec-
trometry. These were supplemented by homology models from se-
quences for all ODA light chain subunits. Initially models were fit
into density using rigid body fitting, followed by jiggle fitting in
Coot (35). For the dynein heavy chain, density in the motor do-
mains allowed for the distinction between Dyh3 and Dyh4. For
the LC tower, side-chain density allowed us to confidently assign
four of the LC8-like light chains (W7XJB1_Lc8, Q24CE5_Lc8d,
Q24DI9_Lc8e, Q22R86 (named Lc8f), and the Tctex-like light
chains (A4VEB3_Lc9 and Q1HGH8_Lc2a). The roadblock light-
chains Lc7 and Lc7b were tentatively assigned based on their dif-
fering C-terminal sequence lengths. The final two LC8-like chains
identified in the MS data were assigned to the remaining positions
based on their N-terminal sequence lengths. Models in higher reso-
lution density were manually refined into maps using Coot (EMD-
11579, EMD-11581), rebuilding regions when necessary. Side-
chain resolution density also allowed us to distinguish the N-termini
of the two intermediate chains, Dic2 and Dic3. The orientation of
the two N-termini allowed us to distinguish the IC WD40 domains,
which were modeled. We could also assign the registry of Dyh3 and
Dyh4 in the tail regions that contacts Shulin (EMD-11580).
For Shulin, PHYRE2 initially generated two models. The N-
terminus residues (21-500 aligned with c5ce6A) were predicted to
adopt a fold similar to Spt16 from the FACT complex, whilst the
residues (726-1104 aligned with c1nijA) were predicted to fold sim-
ilar to the GTPase YjiA. These two domains were fit into EMD-
11579 in Coot (35). We then rebuilt sections of both domains into
our map and built the middle domain of Shulin de novo. The C ter-
minal finger was fit into lower resolution density, with the loops that
contact the Dyh3 motor domain built using Dyh3 region map EMD-
11581. Density for nucleotide between the C1 and C2 domains was
fitted with a GTP analogue from PDB 2HF8, changed to GTP and
manually refined in Coot. Once all the subunits were modeled or
built, the structure was split, with subunits refined against the high-
est resolution maps using PHENIX (36) and REFMAC5 (37) (table
S3). Regions were refined until their model validation statistics,
calculated using PHENIX, no longer improved. In other regions,
homology models were docked into density (EMD-11577, EMD-
11578, EMD-11582, EMD-11583, EMD-11584) then refined using
PHENIX, including secondary structure restraints. For all three mo-
tor domains, microtubule binding domains were tentatively placed
based on the angles and registries of the stalks. All regions were
then re-assembled into one model, with boundaries refined using
PHENIX. For the overall model, all side chains were removed for
deposition.
Mass spectrometry
Protein identification by mass-spectrometry was done either using
in-gel or in-solution tryptic digestion. Three separate types of MS
analyses were performed. 1) For identifying novel interactors of cell
body ODAs, IC3-ZZ-FLAG pulldowns were performed on decili-
ated cells (as described above) in quadruplicates. Eluates were run
on SDS-PAGE gels, Coomassie stained and excised gel slices were
analysed by mass spectrometry. 2) For identifying proteins in the
cell body ODA peak fraction following pulldowns and gel filtra-
tion, samples were resolved on SDS-PAGE and stained with SYPRO
ruby protein gel stain (Bio-Rad). Polyacrylamide gel slices contain-
ing the bands for ODA holocomplex and bound factors were pre-
pared for mass spectrometric analysis. 3) For precisely identifying
subunit composition of ODAs in cilia, IP/MS experiments as above
were performed on the isolated ciliary fraction of the IC3-ZZ-FLAG
strains. Additionally, MonoQ fractions containing ODAs purified
from cilia of wildtype strains (as described above) were tryptically
digested in-solution for mass spectrometric analysis. Both these lat-
ter mass spectrometry experiments identified the same subunit com-
position for ODAs.
For polyacrylamide gel slices (1-2 mm) containing the purified pro-
teins were prepared for mass spectrometric analysis by manual in
situ enzymatic digestion. Briefly, the excised protein gel pieces were
placed in a well of a 96-well microtitre plate and destained with
50% v/v acetonitrile and 50 mM ammonium bicarbonate, reduced
with 10 mM DTT, and alkylated with 55 mM iodoacetamide. After
alkylation, proteins were digested with 6 ng/µL Trypsin (Promega,
UK) overnight at 37
ˇ
rC. The resulting peptides were extracted in
2% v/v formic acid, 2% v/v acetonitrile. Protein samples in solution
were reduced with 10 mM DTT and alkylated with 50 mM iodoac-
etamide. Following alkylation, the proteins were digested with
trypsin (Promega, UK) at an enzyme-to-substrate ratio of 1:100, for
1 hour at room temperature and then further digested overnight at 37
ˇ
rC following a subsequent addition of trypsin at a ratio of 1:20. The
digests were analysed by nano-scale capillary LC-MS/MS using a
Ultimate U3000 HPLC (ThermoScientific Dionex, San Jose, USA)
to deliver a flow of approximately 300 nL/min. A C18 Acclaim
PepMap100 5 µm, 100 µm x 20 mm nanoViper (ThermoScientific
Dionex, San Jose, USA), trapped the peptides prior to separation
on a C18 Acclaim PepMap100 3 µm, 75 µm x 150 mm nanoViper
(ThermoScientific Dionex, San Jose, USA). Peptides were eluted
with a gradient of acetonitrile. The analytical column outlet was
directly interfaced via a modified nano-flow electrospray ionization
source, with a hybrid dual pressure linear ion trap mass spectrometer
(Orbitrap Velos, ThermoScientific, San Jose, USA). Data dependent
analysis was carried out, using a resolution of 30,000 for the full
MS spectrum, followed by ten MS/MS spectra in the linear ion trap.
MS spectra were collected over a m/z range of 3002000. MS/MS
scans were collected using a threshold energy of 35 for collision
induced dissociation. LC-MS/MS data were then searched against
an in-house protein sequence database, containing Swiss-Prot and
the protein constructs specific to the experiment, using the Mascot
search engine program (Matrix Science, UK) (38).
10 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
Database search parameters were set with a precursor tolerance of
5 ppm and a fragment ion mass tolerance of 0.8 Da. Two missed
enzyme cleavages were allowed and variable modifications for ox-
idized methionine, carbamidomethyl cysteine, pyroglutamic acid,
phosphorylated serine, threonine, tyrosine, tert-butyloxycarbonyl-
lysine, norbornene-lysine and prop-2-yn-1-yloxycarbonyl-lysine
were included. MS/MS data were validated using the Scaffold pro-
gram (Proteome Software Inc., USA) (39). All data were addi-
tionally interrogated manually. For quantitative analysis of repli-
cate runs, NSAF (Normalized Spectral Abundance Factor) values
for each protein hit were used as a proxy of protein abundance ( 40).
NSAF values were used to calculate significance and fold-changes
(i.e. consistent enrichment of a given protein in test sample over
control sample).
Bioinformatics
Predicted homology models were generated using Phyre2 (34). For
ortholog searches and sequence alignments PSI-BLAST and align
tools embedded in UniProt, NCBI, ENSEMBL and Tetrahymena
Genome Databases were used. Sequence alignments were visual-
ized using ESPript (41).
Image processing and structure representation tools
FIJI (25) used for all phenotyping and gliding assays. RELION-
3.1 (42) was used for all EM data processing. Cryo-EM
maps were resampled using EMDA (https://www2.mrc-
lmb.cam.ac.uk/groups/murshudov/content/emda/emda.html).
Chimera (33) and ChimeraX (43) used for model fitting and
density map figure making. Manuscript formatting for Biorxiv
used a modified LaTeX template from the Henriques Lab
(https://www.overleaf.com/latex/templates/henriqueslab-biorxiv-
template/nyprsybwffws#.Wp8hF1Cnx-E)
Statistical tests and data representation
Usage of one-way ANOVA with Tukey’s test for multiple compar-
isons is indicated wherever applied. High speed videos of cells to
visualize cilia movement were composited using the kapwing online
tool. All graphical representations were generated using GraphPad
Prism 7.
Data and materials availability
Atomic coordinates and cryo-EM maps have been deposited in the
Protein Data Bank under accession code 6ZYW, 6ZYX, 6ZYY and
in the Electron Microscopy Data Bank under accession codes EMD-
11576, EMD-11577, EMD-11578, EMD-11579, EMD-11580,
EMD-11581, EMD-11582, EMD-11583, EMD-11584.
Author contributions
G. R. M. discovered Shulin, performed all cell biological, biochem-
ical and negative stain EM analyses, optimized and prepared ODA-
Shulin cryo-EM samples. C. K. L collected all cryo-EM datasets.
G. R. M., F. A. A. and C. K. L. determined the cryo-EM structure.
A. P. C., C. K. L. and F. A. A. built and refined models. M. S. and F.
B. performed mass spectrometry. A. P. C. and G. R. M. conceived
the project, oversaw its implementation, and with F. A. A. prepared
figures and wrote the manuscript. All authors contributed to aspects
of manuscript writing and editing.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Funding sources
Medical Research Council, UK (MRC_UP_A025_1011) and Well-
come Trust (210711/Z/18/Z) to A.P.C. and (218653/Z/19/Z) to
F.A.A.
ACKNOWLEDGEMENTS
We thank B. Santhanam for help with comparative genomic analyses, J. Grimmett
and T. Darling for data storage and high-performance computing support, MRC
LMB EM Facility (G. Sharov, G. Cannone) and Diamond (eBIC: proposal bi23268)
for microscopy data acquisition support, K. Nguyen and V. Chandrasekaran for mi-
croscopy time, F. Coscia for GO grid assistance, S. Bullock and members of the
Carter lab for comments on the manuscr ipt, C. Stone for figure scripting and S.
Utekar for suggesting the name Shulin.
Bibliography
1. Iain A. Drummond. Cilia functions in development. Current Opinion in Cell Biology, 24(1):
24–30, 2012. ISSN 09550674. doi: 10.1016/j.ceb.2011.12.007.
2. Ursula W. Goodenough and John E. Heuser. Outer and inner dynein arms of cilia and
flagella. Cell, 41(2):341–342, 1985. ISSN 00928674. doi: 10.1016/s0092-8674(85)80003-9.
3. Margaret W. Leigh, Jessica E. Pittman, Johnny L. Carson, Thomas W. Ferkol, Sharon D.
Dell, Stephanie D. Davis, Michael R. Knowles, and Maimoona A. Zariwala. Clinical and
genetic aspects of primary ciliary dyskinesia/kartagener syndrome. Genetics in Medicine,
11(7):473–487, 2009. ISSN 10983600. doi: 10.1097/GIM.0b013e3181a53562.
4. Stephen M. King. Axonemal dynein arms. Cold Spring Harbor Perspectives in Biology, 8
(11):a028100, 2016. ISSN 19430264. doi: 10.1101/cshperspect.a028100.
5. Mary Elizabeth Fowkes and David Rees Mitchell. The role of preassembled cytoplasmic
complexes in assembly of flagellar dynein subunits. Molecular Biology of the Cell, 1998.
ISSN 10591524. doi: 10.1091/mbc.9.9.2337.
6. Noveera T Ahmed, Chunlei Gao, Ben F Lucker, Douglas G Cole, and David R Mitchell.
ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflag-
ellar transport machinery. Journal of Cell Biology, 183:313–322, 2008. ISSN 00219525.
doi: 10.1083/jcb.200802025.
7. Jin Dai, Francesco Barbieri, David R. Mitchell, and Karl F. Lechtreck. In vivo analy-
sis of outer arm dynein transport reveals cargo-specific intraflagellar transport proper-
ties. Molecular Biology of the Cell, 29(21):2553–2565, 2018. ISSN 19394586. doi:
10.1091/mbc.E18-05-0291.
8. J L Rosenbaum, J E Moulder, and D L Ringo. Flagellar elongation and shortening in
Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and as-
sembly of flagellar proteins. Journal of Cell Biology, 41:600–619, 1969. ISSN 00219525.
doi: 10.1083/jcb.41.2.600.
9. Norman E. Williams, Che Chia Tsao, Josephine Bowen, Gery L. Hehman, Ruth J. Williams,
and Joseph Frankel. The actin gene ACT1 is required for phagocytosis, motility, and
cell separation of Tetrahymena thermophila. Eukaryotic Cell, 5(3):555–567, 2006. ISSN
15359778. doi: 10.1128/EC.5.3.555-567.2006.
10. Christopher R. Wood, Robert Hard, and Todd M. Hennessey. Targeted gene disruption of
dynein heavy chain 7 of Tetrahymena thermophila results in altered ciliary waveform and
reduced swim speed. Journal of Cell Science, 120(17):3075–3085, 2007. ISSN 00219533.
doi: 10.1242/jcs.007369.
11. Gwilym J. Attwell, Connie S. Bricker, Anita Schwandt, Martin A. Gorovsky, and David G.
Pennock. A TemperatureSensitive Mutation Affecting Synthesis of Outer Arm Dyneins
in Tetrahymena thermophila. The Journal of Protozoology, 39(2):261–266, 1992. ISSN
15507408. doi: 10.1111/j.1550-7408.1992.tb01312.x.
12. Ursula Goodenough and John Heuser. Structural comparison of purified dynein proteins
with in situ dynein arms. Journal of Molecular Biology, 180(4):1083–1118, 1984. ISSN
00222836. doi: 10.1016/0022-2836(84)90272-9.
13. Bill Wickstead and Keith Gull. Dyneins across eukaryotes: A comparative genomic analysis.
Traffic, 8(12):1708–1721, 2007. ISSN 13989219. doi: 10.1111/j.1600-0854.2007.00646.x.
14. Kai Zhang, Helen E. Foster, Arnaud Rondelet, Samuel E. Lacey, Nadia Bahi-Buisson,
Alexander W. Bird, and Andrew P. Carter. Cryo-EM Reveals How Human Cytoplasmic
Dynein Is Auto-inhibited and Activated. Cell, 169(7):1303–1314, 2017. ISSN 10974172.
doi: 10.1016/j.cell.2017.05.025.
15. Katerina Toropova, Ruta Zalyte, Aakash G. Mukhopadhyay, Miroslav Mladenov, Andrew P.
Carter, and Anthony J. Roberts. Structure of the dynein-2 complex and its assembly with in-
traflagellar transport trains. Nature Structural and Molecular Biology, 26(9):823–829, 2019.
ISSN 15459985. doi: 10.1038/s41594-019-0286-y.
16. H. Sakakibara, D. R. Mitchell, and R. Kamiya. A Chlamydomonas outer arm dynein mutant
missing the α heavy chain. Journal of Cell Biology, 113(2):615–622, 1991. ISSN 00219525.
doi: 10.1083/jcb.113.3.615.
17. Helgo Schmidt, Ruta Zalyte, Linas Urnavicius, and Andrew P Carter. Structure of human
cytoplasmic dynein-2 primed for its power stroke. Nature, 518(7539):435–438, feb 2015.
ISSN 1476-4687. doi: 10.1038/nature14023.
18. Jianfeng Lin and Daniela Nicastro. Asymmetric distribution and spatial switching of dynein
activity generates ciliary motility. Science, 360(6387):aar1968, 2018. ISSN 10959203. doi:
10.1126/science.aar1968.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 11
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
19. Yang Liu, Keda Zhou, Naifu Zhang, Hui Wei, Yong Zi Tan, Zhening Zhang, Bridget Car-
ragher, Clinton S. Potter, Sheena D’Arcy, and Karolin Luger. FACT caught in the act of
manipulating the nucleosome. Nature, 577(7790):426–431, 2020. ISSN 14764687. doi:
10.1038/s41586-019-1820-0.
20. Andrew M. Sydor, Marco Jost, Katherine S. Ryan, Kaitlyn E. Turo, Colin D. Douglas, Cather-
ine L. Drennan, and Deborah B. Zamble. Metal binding properties of escherichia coli YjiA, a
member of the metal homeostasis-associated COG0523 family of GTPases. Biochemistry,
52(10):1788–1801, 2013. ISSN 00062960. doi: 10.1021/bi301600z.
21. Chanjae Lee, Rachael Cox, Ophelia Papoulas, Amjad Horani, Kevin Drew, Caitlin Devitt,
Steven L Brody, Edward M Marcotte, and John B. Wallingford. Functional partitioning of a
liquid-like organelle during assembly of axonemal dyneins. bioRxiv Cell Biology, 2020. doi:
10.1101/2020.04.21.052837.
22. Sumaya Alkanderi, Elisa Molinari, Ranad Shaheen, Yasmin Elmaghloob, Louise A. Stephen,
Veronica Sammut, Simon A. Ramsbottom, Shalabh Srivastava, George Cairns, Noel Ed-
wards, Sarah J. Rice, Nour Ewida, Amal Alhashem, Kathryn White, Colin G. Miles, David H.
Steel, Fowzan S. Alkuraya, Shehab Ismail, and John A. Sayer. ARL3 Mutations Cause
Joubert Syndrome by Disrupting Ciliary Protein Composition. American Journal of Human
Genetics, 103(4):612–620, 2018. ISSN 15376605. doi: 10.1016/j.ajhg.2018.08.015.
23. Kevin J. Wright, Lisa M. Baye, Anique Olivier-Mason, Saikat Mukhopadhyay, Liyun Sang,
Mandy Kwong, Weiru Wang, Pamela R. Pretorius, Val C. Sheffield, Piali Sengupta, Diane C.
Slusarski, and Peter K. Jackson. An ARL3-UNC119-RP2 GTPase cycle targets myristoy-
lated NPHP3 to the primary cilium. Genes and Development, 25(22):2347–2360, 2011.
ISSN 08909369. doi: 10.1101/gad.173443.111.
24. Douglas L. Chalker. Transformation and Strain Engineering of Tetrahymena. Methods in Cell
Biology, 109:327–345., 2012. ISSN 0091679X. doi: 10.1016/B978-0-12-385967-9.00011-6.
25. Johannes Schindelin, Ignacio Arganda-Carreras, Erwin Frise, Verena Kaynig, Mark Longair,
Tobias Pietzsch, Stephan Preibisch, Curtis Rueden, Stephan Saalfeld, Benjamin Schmid,
Jean Yves Tinevez, Daniel James White, Volker Hartenstein, Kevin Eliceiri, Pavel Toman-
cak, and Albert Cardona. Fiji: An open-source platform for biological-image analysis. Nature
Methods, 9(7):676–682, 2012. ISSN 15487091. doi: 10.1038/nmeth.2019.
26. Erik Meijering, Oleh Dzyubachyk, and Ihor Smal. Methods for cell and particle track-
ing. Methods in Enzymology, 504:183–200, 2012. ISSN 15577988. doi: 10.1016/
B978-0-12-391857-4.00009-4.
27. Ronald D. Vale and Yoko Yano Toyoshima. Rotation and translocation of microtubules in vitro
induced by dyneins from Tetrahymena cilia. Cell, 52(3):459–469, 1988. ISSN 00928674.
doi: 10.1016/S0092-8674(88)80038-2.
28. Sjors H.W. Scheres. RELION: Implementation of a Bayesian approach to cryo-EM structure
determination. Journal of Structural Biology, 180(3):519–530, 2012. ISSN 10478477. doi:
10.1016/j.jsb.2012.09.006.
29. Kai Zhang. Gctf: Real-time CTF determination and correction. Journal of Structural Biology,
193(1):1–12, 2016. ISSN 10958657. doi: 10.1016/j.jsb.2015.11.003.
30. Radosav S. Pantelic, Jannik C. Meyer, Ute Kaiser, Wolfgang Baumeister, and Jürgen M.
Plitzko. Graphene oxide: A substrate for optimizing preparations of frozen-hydrated
samples. Journal of Structural Biology, 170(1):152–156, 2010. ISSN 10478477. doi:
10.1016/j.jsb.2009.12.020.
31. Jasenko Zivanov, Takanori Nakane, and Sjors H.W. Scheres. A Bayesian approach to beam-
induced motion correction in cryo-EM single-particle analysis. IUCrJ, 6(1):5–17, 2019. ISSN
20522525. doi: 10.1107/S205225251801463X.
32. Alexis Rohou and Nikolaus Grigorieff. CTFFIND4: Fast and accurate defocus estimation
from electron micrographs. Journal of Structural Biology, 192(2):216–221, 2015. ISSN
10958657. doi: 10.1016/j.jsb.2015.08.008.
33. Eric F. Pettersen, Thomas D. Goddard, Conrad C. Huang, Gregory S. Couch, Daniel M.
Greenblatt, Elaine C. Meng, and Thomas E. Ferrin. UCSF Chimera - A visualization sys-
tem for exploratory research and analysis. Journal of Computational Chemistry, 25(13):
1605–1612, 2004. ISSN 01928651. doi: 10.1002/jcc.20084.
34. Lawrence A. Kelley, Stefans Mezulis, Christopher M. Yates, Mark N. Wass, and Michael J.E.
Sternberg. The Phyre2 web portal for protein modeling, prediction and analysis. Nature
Protocols, 10(6):845–858, 2015. ISSN 17502799. doi: 10.1038/nprot.2015.053.
35. P. Emsley, B. Lohkamp, W. G. Scott, and K. Cowtan. Features and development of Coot.
Acta Crystallographica Section D: Biological Crystallography, 66(4):486–501, 2010. ISSN
09074449. doi: 10.1107/S0907444910007493.
36. Paul D. Adams, Pavel V. Afonine, Gábor Bunkóczi, Vincent B. Chen, Ian W. Davis, Nathaniel
Echols, Jeffrey J. Headd, Li Wei Hung, Gary J. Kapral, Ralf W. Grosse-Kunstleve, Air-
lie J. McCoy, Nigel W. Moriarty, Robert Oeffner, Randy J. Read, David C. Richardson,
Jane S. Richardson, Thomas C. Terwilliger, and Peter H. Zwart. PHENIX: A compre-
hensive Python-based system for macromolecular structure solution. Acta Crystallograph-
ica Section D: Biological Crystallography, 66(2):213–221, 2010. ISSN 09074449. doi:
10.1107/S0907444909052925.
37. Garib N. Murshudov, Alexei A. Vagin, and Eleanor J. Dodson. Refinement of macro-
molecular structures by the maximum-likelihood method. Acta Crystallographica Sec-
tion D: Biological Crystallography, 53(3):240–255, 1997. ISSN 09074449. doi: 10.1107/
S0907444996012255.
38. David N. Perkins, Darryl J.C. Pappin, David M. Creasy, and John S. Cottrell. Probability-
based protein identification by searching sequence databases using mass spectrometry
data. Electrophoresis, 20(18):3551–3567, 1999. ISSN 01730835. doi: 10.1002/(SICI)
1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2.
39. Andrew Keller, Alexey I. Nesvizhskii, Eugene Kolker, and Ruedi Aebersold. Empirical
statistical model to estimate the accuracy of peptide identifications made by MS/MS and
database search. Analytical Chemistry, 74(20):5383–5392, 2002. ISSN 00032700. doi:
10.1021/ac025747h.
40. Laurence Florens, Michael J. Carozza, Selene K. Swanson, Marjorie Fournier, Michael K.
Coleman, Jerry L. Workman, and Michael P. Washburn. Analyzing chromatin remodeling
complexes using shotgun proteomics and normalized spectral abundance factors. Methods,
40(4):303–311, 2006. ISSN 10462023. doi: 10.1016/j.ymeth.2006.07.028.
41. Patrice Gouet, Emmanuel Courcelle, David I. Stuart, and Frédéric Métoz. ESPript: Analysis
of multiple sequence alignments in PostScript. Bioinformatics, 15(4):305–308, 1999. ISSN
13674803. doi: 10.1093/bioinformatics/15.4.305.
42. Jasenko Zivanov, Takanori Nakane, and Sjors H.W. Scheres. Estimation of high-order aber-
rations and anisotropic magnification from cr yo-EM data sets in RELION-3.1. IUCrJ, 7(2):
253–267, 2020. ISSN 20522525. doi: 10.1107/S2052252520000081.
43. Thomas D. Goddard, Conrad C. Huang, Elaine C. Meng, Eric F. Pettersen, Gregory S.
Couch, John H. Morris, and Thomas E. Ferrin. UCSF ChimeraX: Meeting modern chal-
lenges in visualization and analysis. Protein Science, 27(1):14–25, 2018. ISSN 1469896X.
doi: 10.1002/pro.3235.
44. David E. Wilkes, Vidyalakshmi Rajagopalan, Clarence W.C. Chan, Ekaterina Kniazeva,
Alice E. Wiedeman, and David J. Asai. Dynein light chain family in Tetrahymena ther-
mophila. Cell Motility and the Cytoskeleton, 64(2):82–96, 2007. ISSN 08861544. doi:
10.1002/cm.20165.
45. Deborah Penque, Lisete Galego, and Claudina RodriguesPousada. Multiple αtubulin
isoforms in cilia and cytoskeleton of Tetrahymena pyriformis generated by posttransla-
tional modifications: Studies during reciliation. European Journal of Biochemistry, 195(2):
487–494, 1991. ISSN 14321033. doi: 10.1111/j.1432-1033.1991.tb15729.x.
12 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S1. Purification and identification of novel interactors of cell body ODAs. (A) SEC trace showing major peak of cell body
ODA complex. Co-eluting proteins were identified by MS. Molecular weight (MW) in kDa and peptide counts per protein are shown
in the table. (B) SDS-PAGE gel showing IC3-ZZ-FLAG immunoprecipitates from deciliated Tetrahymena cell bodies. Protein bands
were identified by mass spectrometry. Plot summarizes top hits enriched >3 fold (dotted line) in IC3 (bait protein encircled in red)
over untagged control immunoprecipitates from 4 replicate experiments. Q22MS1 and Q22YU3 are highlighted (blue circles) as novel
interactors.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 13
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S2. Alignment between Q22YU3 and human C20ORF194. Sequence alignment between Tetrahymena Q22YU3
(Q22YU3_TETTS: UNIPROT ID: Q22YU3) and human C20ORF194 (CT194_HUMAN, UNIPROT ID: Q5TEA3) shows 24% identity
highlighting evolutionary conservation. Conserved residues are highlighted in red and similar residues are colored in red with blue
boxes around.
14 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S3. Phenotypic characterization of Q22YU3∆ and Q22MS1∆ mutant strains. (A) Genomic PCRs show amplicons generated
using primers spanning exons 1-3 of Q22YU3 and Q22MS1 in wildtype (WT) and mutant strains. Control PCRs for DYH4 (ODA β-HC)
spanning exon 7 and 3’ untranslated region (UTR) of the gene are shown. (B) Cell images and graph show mutant cells have a
near absence or highly reduced number of food vacuoles (arrowhead) compared to wildtype cells. N=59 cells per genotype. Scale
bar = 10 µm. (C) Immunofluorescence images of wildtype and mutant cells with quantification of cytokinesis defects show Q22YU3∆
and Q22MS1∆ cells have a higher frequency of binucleated cells and multiple oral apparatuses compared to wildtype cells. N=25
cells/genotype. Two-way ANOVA was used to calculate p-values; ns=0.07, *p≤0.04, ****p≤0.0001. Scale bar = 10 µm. (D) Cilia
numbers show mutants have similar numbers of cilia to wildtype cells (WT n=31, Q22YU3∆ n=25, Q22MS1∆ n=24). (E) Average cilia
lengths are similar between mutant and wildtype cells (WT n=18 cells, 146 cilia; Q22YU3∆ n=16 cells, 147 cilia; Q22MS1∆ n=22 cells,
238 cilia). (F) A larger field of view showing representative cells immunostained for ODA and acetylated α-tubulin (related to Figure
1E). Scale bar = 50 µm. Error bars where used show standard deviation. One-way ANOVA was used unless otherwise indicated to
calculate p-values; ns=not significantly different, ****p ≤0.0001.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 15
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S4. ODAs purified from cell body have a closed conformation. Representative micrograph showing negatively-stained ODA
particles purified from deciliated Tetrahymena cell bodies. 2D classification scheme shows ~270,000 closed ODA (green), open ODA
(pur ple) and ambiguous particles (orange) sorted into respective class averages. Representative 2D class averages corresponding to
40% closed intact ODA particles (green), 60% open intact ODA particles (purple box).
16 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S5. Single particle 2D classification scheme. (A) Representative gel filtration traces from reconstitution experiments. (B)
2D classification strategy used to sort ODA particles into three conformational states, closed (green), open (purple) and ambiguous (or-
ange). (C-F) SDS-PAGE gel images showing fractions corresponding to traces in (A). Peak fractions were used for negative staining and
particles were counted after several rounds of 2D classification. Representative 2D class averages from a final round of classification
used for final subset selection and particle counting are shown. Note: ODA only sample has no closed particles.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 17
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S6. Overview of ODA image processing. A consensus 3D refinement was used as the basis for three main arms of processing
in RELION-3.1: (1) Full-length ODA, Dyh3 and Dyh4 (2) Tail and Shulin region (3) Dyh3 motor.
18 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S7. Validation of map and model quality. (A) Representative micrograph acquired with a K3 camera operated in counting
mode. (B) Selection of some of the ODA-Shulin 2D class averages obtained from the combined datasets. (C) Gold standard Fourier
shell correlation (FSC) curves for the different maps used in this study (FSC=0.143). (D) Angular distribution of particles contributing to
the overall ODA structure. (E-F) Local resolution as determined by RELION-3.1’s local resolution implementation.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 19
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S8. Mass spectrometry guided assignment of ODA subunits. Schematic summarizing mass spectrometry (MS) data for
determination of ODA subunit composition. ODAs purified from IC3-ZZ-FLAG ciliary immunoprecipitations and isolated from wildtype
cilia, comprise the subunits in bold. Peptide counts from both MS runs are shown in brackets next to protein. These data guided subunit
assignment and accurate model building of the LC-tower region. For assignment, all paralogous LC sequences for Tctex-like, LC8-like
and roadblock-like LC subunits (Tetrahymena Genome Database and reported previously (44)) are shown in purple, blue and green
boxes respectively. LCs with best fits and confirmed via MS are in bold. Exclusive presence of Lc7, Lc7b, Lc8b and Lc10 subunits in
the MS dataset allowed tentative assignment for these chains. Chains in black are not in the structure.
20 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S9. Architecture and organization of the LC tower. (A) The lower and upper regions of the LC tower are stacked against the
Dyh3 chain whereas Lc3 contacts Dyh4 behind the main tower. (B) Focus on DIC N-termini and the interactions they make with the
LCs. LC subunits with an * are tentatively assigned based on mass spectrometry data. The zoom inset highlights the looping of the
Dic2 N-terminus around the upper LC tower. This figure depicts atomic model of this region with the heavy chains shown as filtered
surface representation (white) and LCs shown as cryo-EM density in (B) and ribbon in (B, inset).
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 21
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S10. ODA motors are locked in an inactive state. (A-B) Shulin stabilizes the closed conformation of ODA indirectly through
several contacts with the tail. Each motor domain has its force-producing linker bent at a 90° angle, indicating an inactive pre-power
stroke conformation. The trajectory of the stalks suggests that they converge at their microtubule binding domains. (C-E) Motor-motor
contacts are shown as spheres. Figure shows filtered surface representation of the atomic model.
22 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S11. Shulin binds GTP and contacts the LC tower. (A) Nucleotide binds at the interface of the Shulin C1 and C2 domains.
Residues in the nucleotide pocket are shown. (B) Shulin’s contacts with Dyh3 tail and motor, holds the linker in a pre-powerstroke
conformation. (C) Contacts between Dyh3 and Dyh4 tail helical bundles, stabilized by Shulin, are shown. (D) Shulin contacts with Lc8e,
Lc8d and Dic2 are shown. Dic2 N-terminal domain (NTD) loop wedged between the two LC subunits is shown. (E) Shulin bridges Dyh3
and LC tower, packing the latter against the Dyh3 tail. Shulin domains making contacts with ODA subunits are in filled circles.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 23
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Figure S12. Shulin delivers ODAs during de novo ciliogenesis (A) Immunostaining of cells regenerating motile cilia after deciliation
shows Shulin enters actively extending cilia marked by acetylated α-tubulin. Insets highlight ciliary staining for Shulin. (B) Immunos-
taining of cells with fully assembled cilia shows Shulin localizes to the cytoplasm. Insets highlight residual ciliary staining for Shulin.
(C-D) SHULIN∆ cells stained as above, lacking Shulin signal serve as immunostaining controls. Note: differences in tubulin staining
between regenerating cilia and fully assembled cilia reflect differences in tubulin acetylation which occurs after microtubule assembly
in Tetrahymena axonemes (45).
24 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
UNIPROT ID
PROTEIN ID
MW (kDa)
P-value
FC
I7MHB1_TETTS
Lc7
15
0.53
4.85
I7M9J2_TETTS
Dyh4/ODA β-HC
530
1.82
4.57
Q22A67_TETTS
Dyh3/ODA γ-HC
534
2.23
4.51
Q22MS1_TETTS
NDK-domain Protein
222
0.58
4.26
Q22YU3_TETTS
Shulin
140
1.03
4.12
I7LX00_TETTS
Haemoglobin-like Protein
14
0.70
4.03
I7M1N7_TETTS
Lc1
23
1.55
4.03
A4VD75_TETTS
Lc3
14
0.62
4.01
Q231E4_TETTS
Ttp95-like Protein
126
1.67
3.88
I7M008_TETTS
Dic2/ ODA IC
78
1.27
3.87
Q1HGH8_TETTH
Lc2a
16
0.46
3.63
I7M6H4_TETTS
Dyh5/ODA
⍺
-HC
475
2.98
3.54
Q23FU1_TETTS
Dic3/ ODA IC (Bait)
77
4.55
3.52
W7X3J8_TETTS
Na/Ca Exchanger Protein
52
1.06
3.51
Q1HFX4_TETTH
Lc4a
18
0.46
3.42
Q23QY3_TETTS
Uba1
117
0.46
3.40
Q22AU5_TETTS
SLEI-family Protein
283
0.44
3.34
I7M9Z0_TETTS
Copg2
283
0.46
3.33
W7XJB1_TETTS
Lc8
13
0.46
3.32
I7MFH3_TETTS
Cytochrome C-like Protein
12
0.46
3.31
Q23AC8_TETTS
Dnajb6
30
0.60
3.28
Q24FD6_TETTS
Dnaja2
47
1.32
3.24
W7XEU3_TETTS
Cystatin-domain Protein
10
0.46
3.14
Table S1. Quantitative mass spectrometry hits of novel ODA cell body interactors. Label-free quantitative MS list of high abun-
dance proteins specifically co-precipitating with IC3-ZZ-FLAG from quadruplicate runs. Shulin and Q22MS1 are highlighted. Proteins
in bold letters are part of the preassembled ODA complex in the cell body. MW = molecular weight, FC = log
2
fold-change, P-value
significance is in -log
10
scale.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 25
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

ODA-S_1
ODA-S_2
Data collection and processing
Microscope
TFS Titan Krios
(LMB)
TFS Titan Krios (eBIC)
Detector
K3
K3
Magnification (nominal)
81,000x
81,000x
Pixel size (Å)
1.11
0.53 (super- res)
Energy filter slit width (eV)
20
20
Voltage (kV)
300
300
Number of frames
66
54
Exposure (s)
2.6
3
Voltage (kV)
300
300
Electron exposure on sample (e–/Å
2
)
52
52
Target defocus range (µm)
1.2-3
1.5-3
Number of sessions
6
1
Number of collected movies
40,158
10,463
Particle numbers after removing graphene oxide
artifacts/ice contamination
1,300,000
Final particle numbers and map resolution at
FSC=0.143 (Å)
Full-length: 131,142; 8.8 (EMD-11576)
Tail:
131,142; 6.7 (EMD-11577)
Ordered tail: 56,667; 5.9 (EMD-11578)
Shulin region: 43,338; 4.3 (EMD-11579)
Extended Shulin region: 43,338; 4.6 (EMD-11580)
Dyh3 motor region: 49,397; 4.4 (EMD-11581)
Dyh4 motor: 57,761; 5.0 (EMD-11582)
Dyh5 motor: 49,756; 5.6 (EMD-11583)
Extended Dyh5 motor: 49,756;
9.3 (EMD-11584)
Table S2. Cryo-EM data collection and processing
26 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint

Shulin
region
Dyh3 region
Dyh4
Dyh5
Ordered tail
NDD tail*
Map
EMD-11579
EMD-11581
EMD-11582
EMD-11583
EMD-11577
EMD-11577
Map resolution (Å)
FSC threshold
4.3
0.143
4.4
0.143
5.0
0.143
5.6
0.143
5.9
0.143
6.7
0.143
Map sharpening B
factor (Å
2
)
-200
-200
-131
-148
-100
n/a
Map CC (around
atoms)
0.74
0.72
0.68
0.63
0.61
0.19*
Model composition
Non-hydrogen
atoms
Protein residues
Ligands
(ATP/ADP/GTP)
16,594
2,041
0/ 0/ 1
22,561
2,988
1/ 3/ 0
14,072
2,842
0/ 0/ 0
14,264
2,880
0/ 0/ 0
27,251
5,501
0/ 0/ 1
4,879
983
0/ 0/ 0
R.M.S. deviations
Bond lengths (Å)
Bond angles (°)
0.007
1.275
0.008
1.380
0.016
1.630
0.006
1.506
0.013
1.943
0.011
2.064
Validation
MolProbity score
Clashscore
Poor rotamers (%)
1.97
6.57
0.06
2.05
7.63
0.00
1.90
4.96
0.00
1.83
4.32
0.00
1.70
2.99
0.00
1.60
2.58
0.00
Ramachandran plot
Favored (%)
Disallowed (%)
Cβ deviations (%)
87.82
0.00
0.05
86.33
0.20
0.18
86.22
0.58
0.07
87.12
0.25
0.11
87.29
0.35
0.30
89.76
0.41
0.32
Table S3. Refinement and validation statistics. * The flexible NDD region of the tail was modeled into low resolution density at low
threshold.
Mali et al. | Shulin locks ODAs for delivery to the cilia bioRχiv | 27
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
Movie. S1. Movie shows slow moving cilia (arrows) in Q22YU3 and Q22MS1 mutant cells compared to the wildtype cells
which have fast-moving cilia (arrows).
Movie. S2. Movie shows slow moving cilia (arrows) in the temperature sensitive OAD1 C11 mutant cell, which has reduced
ODAs in cilia compared to the wildtype cell which has normal ciliary ODA levels and fast-moving cilia (arrows).
Movie. S3. Movie depicting the model of ODA bound by Shulin (green). Subunits are listed and contacts made by Shulin with
the various ODA subunits are shown as green spheres.
28 | bioRχiv Mali et al. | Shulin locks ODAs for delivery to the cilia
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted September 4, 2020. ; https://doi.org/10.1101/2020.09.04.282897doi: bioRxiv preprint
