
IfU TECOTHERM NEO TN300 EN-18.docx
TEC COM GmbH
TECOTHERM NEO
MEDICAL EQUIPMENT for
THERMOREGULATION of NEONATE and
INFANTS
Instructions for Use
Revision November 2015
Applicable for software version 063/02.17 and higher
Applicable for Serial numbers 2015/24/01 and higher

Page 2 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Contents and abbreviations
page
1.
Intended Use ............................................................................................ 4
1.1
Indications for Use ................................................................................... 4
1.2
Contraindications for Use ........................................................................ 4
1.3
Operators Profile ...................................................................................... 4
2.
Information for Customers, Service & Technical support ....................... 5
3.
Device Description ................................................................................... 6
4.
Symbols, Indications ................................................................................ 8
5.
Warnings, precaution guidelines ............................................................. 9
6.
TECOTHERM NEO operating functions ............................................... 14
6.1
The physiologic closed-loop circuit (PCLC) .......................................... 14
6.2
Fallback mode ........................................................................................ 16
6.2.1
Plausibility limitations in rectal temperature measurement ................... 17
6.2.2
Operations during fallback mode ........................................................... 18
7.
TECOTHERM NEO System .................................................................. 21
7.1
TECOTHERM NEO operating modes ................................................... 23
7.1.1
Complete Treatment Mode (Servo controlled), Treatment profiles .... .. 23
7.1.2
Servo Control Mode (constant rectal temperature) .............................. 27
7.1.3
Constant Mattress Temperature Mode ................................................. 29
7.2
TECOTHERM NEO Hypothermia System Information ......................... 31
7.3
Indicators and Operation Key elements, Display screen ...................... 35
7.4
External Temperature Probes ............................................................... 38
7.5
Hoses, Hydraulic lines ........................................................................... 39
7.6
Fill- up set for Filling / Refilling Sterile Water ........................................ 40
7.7
Thermalizing Fluid .................................................................................. 40
7.8
Mattresses, Cool Wraps and protective layers ..................................... 41
7.9
MENU and the User Interface ............................................................... 43
7.10 Display and export of treatment data .................................................... 45
8.
TECOTHERM NEO Hypothermia System Putting into operation ........ 46
8.1
Initial Set up / Initial Operation .............................................................. 46
8.2
Pre- operation Check up ........................................................................ 46
8.3
Initial Operation by the customer ........................................................... 47
8.4
Stop operation / Turn off device ............................................................ 52
8.5
TECOTHERM NEO System: Filling / Refilling Procedures ................ 54
8.6
Draining a used mattress ....................................................................... 56
8.7
Application of mattresses to patients .................................................... 57
9.
Hygienic Requirements .......................................................................... 59
9.1
Cleaning and Disinfecting TECOTHERM NEO .................................... 59
9.2
Mattresses, thermally insulated hoses, tubing ...................................... 59
9.3
Temperature Probes .............................................................................. 60
10.
Storage and Transport ........................................................................... 61
10.1
Storage of the TECOTHERM NEO device ............................................ 61
10.2
Storage of Mattresses ............................................................................ 62
10.3
Transport ................................................................................................ 62

Page 3 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
11.
Alarm system, malfunctions, incident management ............................. 63
11.1
System Alarm, System failure ............................................................... 66
11.2
Temperature Alarm ................................................................................ 68
11.3
Flow rate alarm ...................................................................................... 71
11.4
Alarm Fluid level low ............................................................................ 74
11.5
Alarm No Mains Power ........................................................................ 76
11.6
Fluid escapes from the TECOTHERM NEO System ............................ 78
12.
Training & Qualification of personnel .................................................... 79
13.
Service, preventive maintenance, Software Update ............................. 79
13.1
Service & Maintenance .......................................................................... 79
13.2
Cleaning the ventilation hole structure (device bottom) ........................ 79
13.3
Substitution of sterile water in the device .............................................. 80
13.4
Substitution of sterile water in mattresses and cool wraps .................. 81
13.5
Check / calibration of temperature probes ............................................ 81
13.6
Software Update .................................................................................... 82
14.
Technical Data, TECOTHERM NEO Specification ............................... 83
15.
Declaration of Conformity ...................................................................... 84
16.
Disposal .................................................................................................. 84
17.
EMC guidance for TECOTHERM NEO ................................................. 85
Abbreviations
IfU
Instruction for Use
BCT
Body Core Temperature, as measured via the Rectum
using appropriate rectal temperature probes, see 7.4
SF System Failure

Page 4 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
1 Intended Use
This Instructions for Use (IfU) presents a detailed introduction into the operation modes of
TECOTHERM NEO from TEC COM GmbH.
The thermoregulation system, TECOTHERM NEO is designed for controlled comfortable cold
& heat treatment procedures. By means of mattresses or aqua wraps, cold and heat is applied
to the total body, body parts or specific areas of neonate depending on the therapy objective.
One main application is induced hypothermia treatment of neonate affected with Hypoxic
Ischemic Encephalopathy.
The IfU contains a Technical Description and technical data.
TECOTHERM NEO exhibits SERVO CONTROL operation as a modern excellence feature
using MENU assistance.
TECOTHERM NEO has been equipped with two microcomputers and with numerous
monitoring and alarm features which guarantee a high standard of treatment and operation
safety.
Note: The Manufacturer carries responsibility for basic safety, reliability and capability of
the TECOTHERM NEO system only when
local electrical installation fully meets the requirements of the IfU.
initial operation is performed according prescribed Instruction procedure by
authorized personnel.
TECOTHERM NEO is operated according to the instructions and statements of
said IfU.
1.1 Indications for Use
The TECOTHERM NEO is a temperature management system for pediatric patients, indicated for
controlling and monitoring patient’s temperature through conductive heat transfer.
1.2
Contraindications for Use
No general contraindications are known. For possible adverse effects study the relevant
treatment and therapy protocols.
Avoid direct contact of mattress or cool wrap with patient’s skin!
Avoid direct contact of mattress or cool wrap with fresh or non- closed wounds, infectious
areas, areas with ulceration and abscesses, rash and burns.
1.3
Operators Profile
TECOTHERM NEO is intended for use by healthcare professionals only. Operating a
TECOTHERM NEO requires:
Education as Healthcare Professional
Experience in using life support and life sustaining equipment
Experience in using medical electrical equipment
Personnel must be trained in the use of the TECOTHERM NEO before
operating the device.

Page 5 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Note
:
Operator is requested to carefully check all accepted default or
personally set
parameters for correctness and adequacy before starting treatment.
2. Information for Customers Service & Technical support
For Technical Support in German please contact: TEC COM GmbH
Phone
+49 - 345 - 120 52 04
Fax
+49 - 345 – 120 52 11
E mail info@teccom-halle.de
For Technical Support in English please contact: Inspiration Healthcare Ltd
Phone
+44 - 1455 840555
Fax
+44 - 1455 841464
E mail info@inspiration-healthcare.co.uk
The manufacturer TEC COM or authorized representatives will instruct and introduce the
operation personnel prior putting the equipment into operation
Additional information, technical support, additional manuals may be requested from the
manufacturer and any authorized distribution partner.
Manufacturer
TEC COM GmbH
Gesellschaft für Technik, Technologie und Vermarktung
Am Krümmling 1
D-06184 Kabelsketal
Germany
Supplier Inspiration Healthcare Limited
Gildor House
West Street
Earl Shilton
Leicestershire LE9 7EJ
United Kingdom
Type label
TECOTHERM NEO
Serial Number 2015 / 24 / 01
100-130V / 200-240V
Class I / Schutzklasse I
50-60Hz
max. 350W
IP20
Made in Germany
Fuses / Sicherungen: 5x20mm 250VAC
100-130V: S4AH / T4AH 200-240V: S2,5AH / T2,5AH
Manufacturer / Hersteller: TEC COM GmbH
Am Krümmling 1
D-06184 Kabelsketal 0494

Page 6 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
3. Device Description
The TECOTHERM NEO system is designed for controlled cold & heat treatment procedures
and application of specific cold and heat doses to neonates and babies. The system applies
cold and heat to total body, body parts and particular areas depending on therapy target by
means of mattresses and/or aqua wraps.
One main application is hypothermia treatment of neonate affected by Hypoxic Ischemic
Encephalopathy (HIE).
TECOTHERM NEO consists of a unique cold & heat generating device, applied parts like
mattresses and wrap, interconnecting hoses (tubing set), accessories. Applied parts are
connected to the device via hoses by self- sealing quick- disconnect couplings.
The patient is provided with cold and heat according therapy target in a fully controlled way by a
circulating fluid. This circulating fluid is cooled or warmed in the device and flows through the
mattress or wrap continuously supplying the patient with therapeutically prescribed doses.
Patient temperatures are measured with approved calibrated probes connected to the
TECOTHERM NEO device. Temperature data is permanently communicated to the device
Operational System.
Circulation of thermalizing fluid to provide cold and heat, accurately reaching set points and
operating at set point temperatures accurately with max. deviation of +/-0,3°C, monitoring the
treatment, and alarming when exceeding or falling below temperature limits are performances of
TECOTHERM NEO.
TECOTHERM NEO is a system with built-in physiologic closed loop circuit PCLC.
TECOTHERM NEO is electronically divided into an Operational System and a Controlling
System. Both sub systems are microcomputer (µC) based. Both µC communicate permanently
to ensure safe and proper operation according to therapy needs.
A comfortable user MENU will guide the operator to the treatment modes, advise how to
proceed the treatment and how to manage treatment details.
Menu language may be preselected using Sub–Menu “Language”: English, Deutsch, Espanol …
TECOTHERM NEO offers three separate treatment modes to induce hypothermia and to re-
warm the patients. TECOTHERM NEO uses 2 independent temperature probes:
rectal probe for measuring Body Core Temperature (BCT), mandatory required for
SERVO mode.
skin probe for measuring skin abdominal or forehead temperature etc, optional.
The three treatment modes are
I SERVO CONTROL Programmable Complete Treatment Mode
II SERVO CONTROL Constant Rectal Temperature Mode
III Constant Mattress Temperature Mode
The Operator selects the treatment mode I, II or III and treatment parameters for inducing
hypothermia, normothermia or hyperthermia following the MENU instructions. Attention:
Temperature probes must be properly placed before treatment procedure can start.

Page 7 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Attention Treatment and selection of treatment procedure is fully within the
responsibility of the therapist, physician or trained qualified medical personnel.
Attention For neonate and other patient hypersensitivity or restricted compatibility to
hypothermia and / or hyperthermia is in general not known. Neonate receiving such
treatment must be under careful observation.

Page 8 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
4. Symbols, Indications
Important Information
Attention, Caution, Warning
Electrical Hazard !
Do not touch contacts!
Applied Part Type BF
Consult Instruction for Use
Rectal Temperature Sensor socket
Skin Temperature Sensor socket
Key “Turn On”
System failure
Temperature Alarm
Alarm No or restricted Flow
Alarm Low fluid level
Symbol AUDIO paused
No Mains Power (separate LED indicator)
Internal System Failure (separate LED indicator)
R
SF
SF
S

Page 9 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
5. Warnings, Precaution guidelines
Warnings
Modification of the TECOTHERM NEO not authorized by the manufacturer
is not allowed.
Do not open the device! Risk of electrical shock.
Opening the device is restricted to service
and other authorized personnel.
Do not remove cover part. Risk of electrical shock when touching inner parts and
components.
The TECOTHERM NEO device must be plugged to the mains only to shockproof
sockets. Mains voltage must be 100-130V or 200-240V with 50-60 Hz. Use only
cord supplied with the device or a medical grade approved equivalent cord not
longer than 2,5 m.
Caution During operation and treatment: The operator must
not simultaneously touch the patient and metallic device parts
(plug / connector sockets, grounded connected parts at the device rear,
contacts of fuse compartment).
Both temperature probe sockets on the front of device and the
USB socket on the rear are marked with ESD warning symbols.
They are sensitive against discharge of static electricity, their electrical contacts
should not be touched with the fingers or tooling. When connecting probes or
USB stick to their sockets the following precautionary procedure is required:
Before plugging, touch the fan protective grid at the rear with your other hand.
It is recommended that all staff involved in using TECOTHERM NEO receive an
explanation of the ESD warning symbol and training in ESD precautionary
procedures.
That training should include, in addition to the precautionary procedure
prescribed above, general information on the origin, the possible impact
and the prevention of electrostatic charging.
Repair and maintenance are restricted to authorized personnel only!
For a reliable and safe operation use only original components, applied parts and
spare parts supplied or recommended by the manufacturer.
Substitution of original parts or components of the TECOTHERM NEO system by
parts or components which are not licensed by the manufacturer is likely to put
the system and the patient at risk!
Only use Sterile Water as the circulating fluid. Otherwise it would be
possible to put the system and the patient at risk!

Page 10 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Use only temperature probes in accordance with IfU and
with the technical specification of the manufacturer.
Applying different probes may lead to incorrect and wrong temperature data.
This is likely to put patients at significant risk!
Ensure that probes are properly connected to the TECOTHERM NEO socket
marked "R" (Rectal for Body Core Temperature) and “S” (Skin for Surface
Temperature).
Ensure that Rectal and Skin Temperature Probes are
correctly placed in/on the patient and that probes are
properly secured.
Do not use TECOTHERM NEO with or in presence of flammable agents.
Safety, Reliability
Caution For a reliable and safe operation of the TECOTHERM NEO use only original
components, parts and spare parts and accessories supplied or licensed by the
manufacturer.
Use only such components, parts and accessories for hypothermia treatment with a
TECOTHERM NEO system!
Warning Substitution of original parts or components of the TECOTHERM NEO system
by parts or components which are not licensed by the manufacturer is
likely to put the system and the patient at risk!
Precautions
Consider within Intended Use
Note: Therapeutic Induced Total Body Hypothermia is a systemic treatment method.
Select target temperatures cautiously.
Re- warming: Select low re-warming rates to smoothly reach normal BCT of 37°C.
Patient body mass may severely influence re- warming. The larger the mass the
slower the re- warming.
Further notes
When TECOTHERM NEO is run in the Constant Mattress Temperature Mode to lower
body core temperature an independent temperature measurement is required to monitor
hypothermia.

Page 11 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Note that in this mode applied treatment temperature and duration do not allow a
realistic estimation of the actual degree of lowering of patient’s BCT.
Medical electrical equipment needs special precautions regarding Electromagnetic
Compatibility and needs to be installed and put into service according to the
Electromagnetic Compatibility information provided below in 17. EMC guidance.
Portable and mobile RF communication equipment can affect medical electrical
equipment. Observe the recommended separation distances listed below
in 17. EMC guidance.
The TECOTHERM NEO device should be subject to regular maintenance and
service, see section 13.
Refill with Sterile Water regularly every 2 months, see section 8.5.
Note Circulation may stop, fluid flow stops.
In such cases mattresses may cool neonate or patient slowly down. Especially during
treatment re-warming phase neonate may suffer from extraction of body heat back
into the mattress. Change such condition soon!
The operator or the user should not apply other cleaning, disinfecting and
decontamination procedures than those recommended by the manufacturer. If in
doubt contact your local representative.
Precaution Notes for placement
Location
The TECOTHERM NEO device should not be used adjacent to or stacked with other
equipment. If adjacent or stacked use is necessary, the TECOTHERM NEO should
be observed to verify normal operation in the configuration in which it will be used.
The unit must be placed in such a way that it could be easily disconnected from
mains power. Removing the mains plug must be always possible.
The unit must be placed horizontally onto a plane support
The system is fan cooled. Sufficient space must be allocated so that a free flow of air
from all sides can reach the bottom of the device when it is in operation.
Device should be located so that there is a distance of at least 15cm between rear
side and a wall or another limiting surface to ensure free outflow of the cooling air.
Do not place the device into small cabinet compartment or onto small scale boards.
Do not cover the device!
The unit should be placed avoiding air to be blown towards the patient.
The unit should be placed so that optical blink alarms are clearly seen and acoustic
alarms are clearly audible.

Page 12 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Do not place mattresses and hoses onto hot or warm supports during operation
Do not place the device during operation near intensive heat sources.
Attention Ensure there is enough space around the TECOTHERM NEO not to obstruct
passage of acting personnel. Ensure that hoses, cable cord, temperature probes etc. do
not form obstacles.
Ensure that placement of TECOTHERM NEO is not forming trapping zones for hands
and fingers to avoid contusions and other injuries.
In case of reusable mattresses and wraps, apply a thin disposable interlayer
which must at least fully cover the mattress with some border. Such interlayer must
be coated at lower side with plastic coating to prevent penetration of blood, liquids or
liquid media onto the upper surface of the mattress and to protect the patient.
Note: Place mattress/aqua wrap onto a 10 - 20 mm thick foam material that has
good thermal insulation during operation.
Using an incubator
When using an incubator to perform hypothermia treatment:
Pay attention that there is enough space to properly place mattress or cool wrap.
Otherwise kinking of hose set and / or tubing near mattress and mattress folding may
cause restricted fluid flow, bad circulation or even flow blocking
Place the hoses set in a way that the hoses are in a straight line. Fasten the hoses in
such a way to avoid kinking of the tubing near the mattress
Note: Place mattress onto a 10 - 20 mm thick foam material that has good
thermal insulation during operation.
Note: Do not put mattress directly onto compact silicon inlays used in incubators.
Attention Ensure that incubator heaters are shut off! Ensure that there is no forced
air circulation. It may cool down the neonate in a re- warming phase of the treatment.

Page 13 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Indications for hazardous substances
TECOTHERM NEO does not contain parts or substances stemming from derivatives of
blood or human / animal tissues.
TECOTHERM NEO does not contain parts made of Latex or its derivatives.
TECOTHERM NEO Applied Parts do not contain parts made of PVC with DEHP
softener / plasticizer
Thermalizing Fluid is Sterile Water.
Skin contact with fluid is harmless.
Ambient Conditions
To ensure a proper operation in normal use pay attention to the following conditions
Protection The device should be protected from dampness and wetness
(e.g. splash water.)
Do not operate device in rooms where flammable mixtures of anaesthesia gases
with oxygen, laughing gas N
2
O or air may evolve.
To have full cooling power ambient temperature should not exceed 27°C. Otherwise
the TECOTHERM NEO system may not achieve the lowest possible set temperature
if using a large mattress.
Relative Humidity during treatment within a range of 30 % - 80 %
Ensure that during treatment / operation no installations, systems, devices and the
like are operating or are intended to operate next to TECOTHERM NEO and
producing
ultraviolet radiation
intense infrared radiation
strong electromagnetic radiation
mechanical shocks, vibrations.

Page 14 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
6. TECOTHERM NEO operating functions
Operators should familiarize themselves with the operating instructions. It is of crucial
importance that they are fully familiar with the actions to be taken in the case of alarms
and initial operating errors occurring during use.
Operators should furthermore develop an understanding of the TECOTHERM NEO as a
system, comprising of a physiologic closed-loop circuit (PCLC).
6.1
The physiologic closed-loop circuit (PCLC)
In accordance with specified applications (see 1.1) TECOTHERM NEO is used to
regulate rectal measured body core temperature of patients, which is a physiologic
variable, in a specific way or to maintain it at a constant level. Following an as rapid as
possible cooling down to 33.5°C this temperature is subsequently to be maintained over
a 72 hour period, followed by a gradual steady re-warming up to 37°C within a period of
7 hours or longer.
This is achieved by placing the patient into effective thermal contact with a coolant fluid
perfusion mattress (see 8.7). The temperature of the fluid determines the changes
which will take place in the patient’s core body temperature: if it is lower than that of the
patient, the patient’s temperature will fall – if it is higher the patient’s temperature will
rise. The greater the difference in temperature between patient and the fluid the faster
the change in the patient’s core body temperature, whereby a change of 0.5°C/hour
should already be considered being rather “rapid”. The mattress temperature (i.e. the
average temperature of the fluid) must obviously be maintained within certain limits in
order to prevent possible damage to tissue (frostbite, burns). These limits are set at
12°C and 39°C respectively, whereby temperatures around 12°C are needed only
during the initial treatment phase to allow for an as rapid as possible cooling down
process; subsequently these temperature levels will no longer be required.
In order to achieve the intended progression in the patient’s core body temperature the
mattress must be kept at the correct temperature level, at all times. The right
temperature will depend on a number of factors: what is the current stage of treatment;
what are the (changing) ambient conditions, environmental factors; how effective is the
thermal contact between mattress and the patient; how intensive or reduced is the
patient’s own level of thermal output. First of all, sufficient information concerning the
patient’s current core body temperature must be available in order to determine the
correct mattress temperature required. However, the impact of external factors is often
rather complex and difficult to assess, so that manual setting of the mattress
temperature by the operator will often result in more or less wide fluctuations of the core
temperature around the target level, especially as the actual results of a temperature
adjustment do rarely become apparent within less than a half-hour period. This also
does lead to increased nursing requirements, since repeated adjustments in
temperature will become necessary.

Page 15 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
The operator is spared these complex considerations and corresponding decision-
making process by the automatic temperature control system performing these tasks in
the two automatic operating modes. The operator now merely needs to schedule the
overall intended progression of change in the patient’s core body temperature in
advance, using a limited number of parameters, which can be intuitively understood.
The temperature control system using the rectal temperature measurements
subsequently calculates the exact mattress temperature required, on a continuous
basis, in order to stay within the set schedule. The TECOTHERM NEO temperature
control module ensures that the mattress delivers the temperature required based on
these calculations, as fast as possible.
Like any other temperature control system TECOTHERM NEO comprises a closed-loop
control circuit. Any deviation from target settings is counteracted immediately.
Assuming that the rectal temperature measurement is 0.1° higher than it should be, at
any given point in time, the control system would lower the mattress temperature by 1° if
the internally programmed amplification factor had a value of 10. With a certain delay
this lowering of mattress temperature will result in a corresponding decrease in rectal
temperature and the subsequent gradual convergence back towards the target value.
As a result the decrease in mattress temperature will in turn be reduced. Thus the cycle
is closed and since the regulated rectal temperature is a physiologic variable of
measurement this represents a physiologic closed-loop circuit (PCLC).
During the initial stages of treatment, i.e. rapid cooling down of the patient, an inevitable
overshoot will occur – actual values will in fact fall somewhat short of the target value
of 33.5°C. Standard parameters have been chosen to reduce such overshoot to below
0.5°. A stable status is subsequently reached within a settling time of approx. 1 hour;
there are no remaining deviations from set parameters. During this constant phase,
which usually lasts about 72 hours, potential fluctuations will be less than 0.3°.
Following commencement of the re-warming phase there will initially be a rise in
mattress temperature. Only after a response period of approx. 30 minutes will there
be any noticeable change in rectal temperature. This will subsequently increase only
gradually as well and therefore initially lag marginally behind the intended progression.
This tracking error is gradually reduced and will in any event always remain below 0.5°.
This physiologic closed-loop circuit can obviously operate properly only if all
elements of this functional chain do perform their designated tasks as intended.
Assuming normal operations of the TECOTHERM NEO unit a number of additional
requirements need to be met:
The thermalizing fluid must circulate at a flow rate sufficiently high to ensure an
efficient thermal transfer to or away from the patient. This process is monitored by the
unit and, if required, the operator will be alerted to initiate appropriate corrective
measures.
There must be sufficient thermal contact between the fluid perfusion mattress and the
patient, as any change in mattress temperature may otherwise have no or only
limited effect on rectal temperature. It is of crucial importance for the operator to
position the mattress correctly and in accordance with the operating instructions (see

Page 16 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
8.7). The equipment can detect any fault in this respect only after lapse of the
response period of approx. 30 minutes, at the earliest, if despite constant adjustment
of mattress temperature the expected reaction in rectal temperature does not occur
and the rectal temperature eventually moves outside the permissible range of +/- 0.5°
around the target value. Only at that point in time will a temperature alarm be
activated.
Rectal temperature, as the measurement ultimately to be regulated, must be
recorded accurately. Incorrect measurements taken over an extended period of time,
regardless of cause, would immediately result in an unwanted change in the patient’s
actual core body temperature.
Example: An incorrectly placed rectal probe (e.g. slipped out) will record a temperature lower
than the actual core body temperature, since the rectal probe will now measure the air
temperature in proximity of the rectum. The current temperature measurement is shown on
the display. This temperature will in fact be lower than the rectal target temperature of
33.5°C. Consequently there is now a deviation in temperature (cause). This will immediately
result in an increase in mattress temperature (effect), since the unit’s control system will
work to again increase the core body temperature, which is now perceived being too low.
Upon activation of the alarm, the operator can conclude from the low rectal temperature
recording shown on the display that the rectal probe may have slipped out and will need to
check this immediately.
6.2
Fallback mode
Amongst various possible causes which may lead to a malfunctioning of the physiologic
closed-loop circuit the systematically false recording of rectal temperature would be the
most disadvantageous, especially if it went unnoticed for an extended period of time.
Only this kind of false measurement would lead directly to the wrong core body
temperature for the patient. Such false readings can have a number of different causes:
incorrect placement of the rectal probe, e.g. slipped out
excessively high levels of electromagnetic interference from the environment
deficiencies in contact(s) at plug connections
defective rectal probe.
Unfortunately it is not possible to permanently monitor the rectal probe with the aid of a
second control probe, as the insertion of 2 rectal probes would be impossible in the
case of an infant. The otherwise recommended control by means of an additional skin
probe is not sufficiently accurate and too exposed to potential external impact for such
readings to be used in arriving at an informed decision. For these reasons measuring
results taken from the rectal probe are checked as to plausibility, whereby it will depend
upon the relevant stage of treatment as to what range of values for rectal temperature
recordings will be classified as being plausible and therefore acceptable. In the case of
measurements occurring systematically outside this specified range of acceptance

Page 17 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
TECOTHERM NEO will stop operating as a physiologic closed-loop circuit and
instead switch into fallback mode. The operator will be alerted and informed about the
current status and subsequently needs to decide upon an appropriate course of action.
Depending upon the stage of treatment a choice of suitable options will be given for the
unit to immediately resume operations. The operator can now follow these prompts or
make changes according to his own assessment of what actions may be required.
The key characteristic of the fallback mode is, that the required mattress temperature
will no be longer calculated by the temperature control system, but that it now needs to
be specified by the operator. In order to be able to take an informed decision under
these circumstances the operator immediately needs to arrange for alternative methods
of continuing a reliable recording of the patient’s rectal temperature, completely
independent from the TECOTHERM NEO system.
Although it will generally be possible to continue the current treatment process up to the
end entirely in fallback mode, one should always try to identify and eliminate the actual
cause of any false measurement. If no obvious reasons can be detected a replacement
of the rectal probe is recommended. As soon as acceptable measurements are
available again the unit will switch back automatically into the physiologic closed-loop
circuit operating mode and the operator will be advised accordingly. Only in rare cases
is it possible, that measurements may again be correct but still marginally remain
outside the valid range of acceptance. If the operator can see that the measurements
are indeed correct and there is still no automatic reversal, then this reversal can be
prompted through use of the “Servo” button.
6.2.1
Plausibility limitations in rectal temperature measurement
In accordance with the designated applications for the TECOTHERM NEO system (see
1.1) it would be possible in the extreme case for the rectal temperature of a “patient”
(not necessarily an infant!) to vary between 30°C and 38°C, at the beginning of the
treatment cycle. Initial temperature recordings between 29°C and 39°C are
consequently categorized as plausible readings. In the case of measurements outside
this range the control system cannot be started and activation will be denied, with
corresponding notification.
This comparatively broad range of tolerances, however, does not entail any untenable
elements of risk. On the one hand it can be assumed, that intensive care and monitoring
of the process is safeguarded during the initial stages of treatment, when the rapid
achievement of stable conditions is the primary objective. The range of acceptable
tolerances on the other hand is rapidly reduced following the initial stages of treatment
until a status of stable condition has been reached. From that point forward the
acceptable range of tolerances will be merely 1° above or below the corresponding set
target value. In the event of adjustments being made to the relevant target values the

Page 18 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
corresponding threshold values will change accordingly, e.g. they will rise during the re-
warming phase at the same speed as the rectal temperature target value.
Besides the monitoring of compliance with these absolute tolerance thresholds rectal
temperature measurements are also checked as to their speed of change. The
threshold value in this respect is 0.3°/minute. Any changes faster than this, as in the
case of the probe having slipped-out, will be read and evaluated as non-plausible and
will trigger the switch to fallback mode.
6.2.2
Operations during fallback mode
As soon as the fallback mode has been activated, due to an infringement of tolerance
thresholds, the operator must intervene and determine how the mattress temperature is
to react from that point on forward. In this context it very much depends on the current
stage of treatment in deciding on how best to proceed in an expedient manner.
Correspondingly parameters are set for immediate activation of the fallback mode which
will, at least initially, not result in any additional exposure to risks. The operator will need
to adjust these parameters to prevailing conditions. With the aid of the graphic display
the operator can obtain a good indication from the diagram of the progression of
mattress temperature up to that point. Only once these steps have been taken is it
advisable to commence with any trouble-shooting efforts or even a replacement of the
rectal probe, in order to restore automatic operations as quickly as possible.
Depending on the current stage of treatment two essentially different types of
parameters and optional settings are available for operations in fallback mode.
If the rectal temperature is to be either kept constant or to be adjusted to a specific
value as quickly as possible, then the mattress temperature will be used as an
immediate control parameter which can be reset, if required, at any time.
During the initial phase of rapid cooling down to e.g. 33.5°C the pre-defined setting for
fallback mode will be a mattress temperature of 20°C. This will initially ensure that the
cooling down process, once initiated, will be continued. Depending on how far the
cooling down process has progressed up to this point, the temperature level of 20°C
may still be too high or otherwise already too low. This needs to be assessed by the
operator on the basis of an independent measurement of the patient’s actual rectal
temperature and the temperature will subsequently need to be adjusted accordingly. As
soon as the (independently measured) rectal temperature does approach the target
value of 33.5 °C additional adjustments to mattress temperature will become necessary,
in order to stabilize the temperature at 33.5°C and to prevent any further cooling down
of the patient.
In phases during which the rectal temperature is to be kept constant, e.g. at 33.5°C or
finally at 37°C, the pre-setting for the mattress temperature will be the same as that for
the rectal temperature to be maintained at a constant level. Depending on ambient

Page 19 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Rectal set temperature
Rectal measured temperature
Mattress temperature
conditions as well as the patient this may be marginally too high or somewhat too low.
The progression of mattress temperature up to that point, as shown in the diagram, will
provide useful guidance in this respect for corrective measures to be taken. If the
fallback mode continues to hold any longer, the operator will again have to assess, on
the basis of independently taken readings of the patient’s rectal temperature, whether
the choice of mattress temperature has been correct.
Different criteria do apply during treatment phases when the rectal temperature is to be
gradually adjusted at a pre-determined speed. It is known that in this case the mattress
temperature will gradually change, at the same speed, albeit with a certain delay with
regard to the pre-determined rectal temperature. A correspondingly accurate automatic
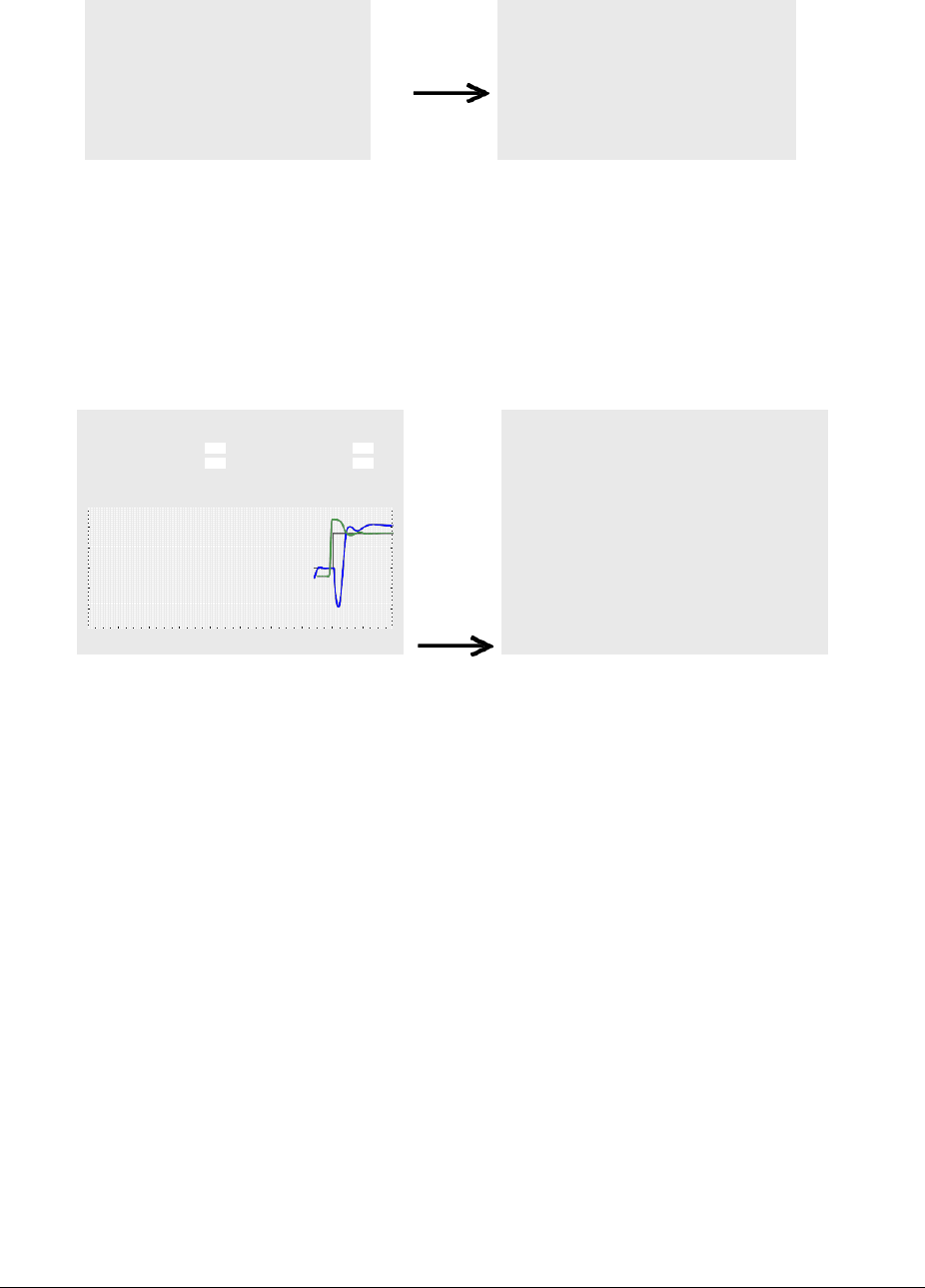
re-warming can schematically be illustrated as follows:
40
35
30
time
25
In practice, however, the mattress temperature will not always need to be exactly 1°
above the rectal temperature and depending on the patient it may be even higher, as
well as lower, than the rectal temperature. Certain fluctuations may also be due to
interference, as indicated in the left segment of the diagram.

Page 20 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
FALLBACK MODE
automatically activated
Future scheduled Rectal set temperature
Rectal measured temperature
Future Mattress set temperature, Shift = 0 (default)
FALLBACK MODE
automatically activated
Future scheduled Rectal set temperature
Rectal measured temperature
Future Mattress set temperature, Shift = +1.0 (good choice)
If the fallback mode is activated following parameters are applied: the target value for
the mattress temperature will be set at the currently applicable target value for the rectal
temperature. In further progression it will gradually rise at the same speed at which the
rectal temperature is set to increase, as schematically shown in the following illustration:
40
35
30
time
25
Comparing this illustration with the preceding one it becomes apparent, that the pre-
determined parameters cannot produce in the originally desired progression in rectal
temperature: Mattress temperatures would systematically fall 1° short of requirements.
In these cases the operator therefore is given an option to offset the future progression
of mattress temperature by an appropriate margin (maximum up to ±3°). The following
graphic illustrates how treatment would progress once the operator had interpreted the
progression of mattress temperature up to that point correctly and adjusted the system
accordingly to the appropriate level of offset:
40
35
30
time
25

Page 21 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
7. TECOTHERM NEO System
TECOTHERM NEO system, components and accessories:
TECOTHERM NEO device
Application parts like mattresses and cooling wraps
Temperature probes with connectors
Extension cables for single use Temperature probes
Hose set, thermally shielded to connect application parts to the device
Fill-up set, includes necessary components for filling/re- filling
Thin protective interlayer for multiple use mattresses and cooling wraps
Electrical Power cord, up to 2,5 m
Warning: The use of accessories other than those specified in this Instruction for Use,
in particular Power cord, Temperature probes and their Extension cables, may result in
increased emissions or decreased immunity of the TECOTHERM NEO Hypothermia
System.
Warning: Accessories specified for use with TECOTHERM NEO, especially Power
cord, Temperature probes and their Extension cables, should not be used with other
medical electrical equipment or systems. This may result in increased emissions or
decreased immunity of the medical electrical equipment or system in question.
Optional accessories:
Repair set for small mattress defects like punctures and flaws
Fluid Emptying Aid for mattress
Storage boxes
Aqua Wrap/Mattress TC-MATT-NEO for total body cooling of neonate and infants for
Multiple Use,
Aqua Wrap/Mattress TC-MATT-DISP for total body cooling of neonate and infants for
Single Use,
Aqua Pad Mattress TC-MATT (L) for total body cooling 50 x 90 cm (medium size), for
Multiple Use,
Interlayer Foils TC-FFL separate mattresses/wraps from patient body preventing
intimate direct contact with Multiple Use Application parts.

Page 22 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
For total body cooling treatment the TECOTHERM NEO device pumps thermalizing fluid
along the hydraulic lines through mattresses or wraps to lower or increase body core
temperature to the set value. Operators may preselect the most appropriate treatment
procedure and target temperatures.
During circulation the microcomputer of the Control Board is permanently comparing /
analyzing the measured and set rectal temperatures. The larger the deviation between
the two temperatures the more cooling or heating power is to be supplied by the
TECOTHERM NEO device.
The circulating liquid extracts heat from or delivers heat to the patient. Circulation is
monitored by the controlling system. Heating and cooling rates also are monitored by the
microcomputer of the controlling system and checked for observing defined limits.
Treatment procedure temperatures for inducing hypothermia in neonate are strictly limited
Rectal Temperature standing for BCT 32°C / 38°C
(lower limit / upper limit) in the treatment modes I and II
Mattress temperature 12°C / 39°C
Device internal temperature alarm limits 10°C lower limit
41°C upper limit

Page 23 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
7.1
TECOTHERM NEO operating modes
TECOTHERM NEO is designed as a physiologic closed- loop control system ( PCLCS)
see section 6.1.
Note All parameters can be changed from set position at ANY TIME should the need
arise. Select menu mode “Options” .Changes will be stored on the TECOTHERM NEO
and can be seen on later analysis.
Three treatment and operation modes of TECOTHERM NEO
I Programmable Complete Treatment Mode (Servo controlled)
II Servo Control Mode (constant rectal temperature)
III Constant Mattress Temperature Mode
Note The operator can permanently follow the Rectal Temperature on the display
screen, see section 7.3.
7.1.1
Programmable Complete Treatment Mode (Servo controlled),
Treatment profiles
System is designed for inducing hypothermia in a patient by total body cooling. Details
for initial operation see section 8.
Note This mode with its target temperatures and
profiles is based upon the TOBY Study protocol
(see reference) for inducing hypothermia in neonate
suffering from Hypoxic Ischemic Encephalopathy.
TOBY protocol recommends a set target rectal
temperature of 33,5°C (default), then in treatment section 2 holding it constant for 72 h
followed by a linear increase to 37°C over at least 7 h in treatment section 3.
Treatment mode I generally allows selecting and setting rectal temperatures within a
range 32°C to 38°C. For treatment sections 1 and 2, however, setting is limited to
32
33,5°C
35°C. Duration of treatment section 2 can generally be set within
1 to 100 h, and in treatment section 3 between 1 and 24 h.
Temperature is accurately adjusted and finely tuned in all treatment sections.
All the pre-defined standard values of temperatures and durations as recommended by
the TOBY protocol may be modified to some extent by the user prior to starting the
treatment as well as later on whilst treatment is already running.
time
temperature

Page 24 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Within “Servo Control Complete Treatment Mode“ the user can set
up to 9 (user-defined) Treatment Profiles.
When changes are made to the default temperatures or times before the start of
treatment, the user is given an option to save this new settings as a treatment profile.
If selecting this option, this new settings will get the next free treatment profile number
(from 1 to 9) for identification. If the starting point for the changes was a previously
generated treatment profile, it can be redefined instead of creating another new
treatment profile.
After a treatment profile has been saved, there is available a further option to declare
this profile as the future default. Accepting this option with "Yes", in the future each
new call of this treatment mode will offer exactly this set of defaults. So treatment can
be started immediately, without first having to make any changes.
Once the user has created at least one own treatment profile, when selecting the “Servo
Control Complete Treatment Mode” in addition to the displayed temperatures and times
corresponding to the profile declared as standard, the option to choose another profile
will be offered. The corresponding specifications are each displayed immediately. So
the user can see the temperature and time defaults the treatment would proceed with.
In the delivery state of TECOTHERM NEO system there exists just the profile № 0 with
the above mentioned standard specifications according TOBY protocol. This profile
cannot be overridden, it always remains unchanged. Any changes can only be saved
into a user-defined profile. If there is no more free number available (9 profiles have
been created already), only profile № 9 can be redefined.
Of course, the process of creating a treatment profile can be canceled at any time. In
any case, the treatment will always be performed using the temperature and time
settings that are shown on the display at the time of pressing the button "Start".
During the treatment, temperature and time settings can be changed if necessary by
pushing the "Options" button. However, these changes cannot be saved in profiles
during the course of treatment. They apply only to the currently running treatment.

Page 25 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Necessary TECOTHERM NEO system equipment to run Programmable Complete
Treatment Mode is
TECOTHERM NEO device
Mattress or wrap for total body cooling, filled, maybe protecting interlayer
Rectal Temperature Probe (Skin temperature probe is optional)
Themally shielded hoses to connect mattress to the device.
All equipment parts should be checked and prepared to start treatment, see section 8 of
this IfU. Patient – neonate – should be placed onto the aqua wrap/mattress.
Rectal probe should be inserted and secured.
Operation Mode I has four (4) treatment sections / phases 1 … 4.
To start treatment follow and observe the instructions of the MENU.
Treatment section 1 Rapid Cooling- Down Phase
TECOTHERM NEO is operating to cool down as fast as possible. The system is
operating with maximum cooling power. Body heat from the patient/ neonate is
extracted by the cooling mattress and transferred to the circulating fluid. The central
device cooling unit extracts the heat and cools the fluid on demand.
The default target set temperature in this automatic mode is 33,5°C as recommended
by the TOBY protocol.
When rectally measured BCT is reaching the target temperature 33,5°C the system will
reduce the cooling power automatically.
Control board microcomputer determined a sufficiently small deviation of measured and
set temperature to start power reduction. Control system approaches set temperature
without remarkably overshooting or undershooting.
Treatment section 2 Cooling Phase
Reaching the set temperature 33,5°C the system continues treatment to hold BCT of
the patient constant. Treatment time is preselected by the operator (default 72 h). The
Rectal (BCT) temperature is pre-selected (default 33,5 °C). Typically, deviations of BCT
from set temperature will be 0,2°C.
The control board permanently compares measured rectal temperature with set BCT
33,5°C. The central cooling unit is permanently adjusting the temperature of the
circulating fluid to hold BCT at 33,5°C 0,5 °C. If during treatment section 2 BCT of the
patient is increasing / decreasing caused by disturbances the difference between
measured and set rectal temperature increases.
The system initially will respond with more cooling power or increased warming.
If deviation exceeds 0,5 °C in any direction, for some longer time the internal monitoring
system will detect this discrepancy. TECOTHERM NEO starts alarming.

Page 26 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Treatment section 3 Re- Warming Phase
After cooling phase 2 the system automatically starts the re- warming phase. If needed,
this phase can be started from menu “Options” at any time before cooling phase 2
automatically ends. Re-warm target Rectal (BCT) Temperature and time to achieve this
are pre-selected (Default BCT 37°C and 7 hours).
The TECOTHERM NEO system will linearly increase the BCT until selected target
temperature 37°C is reached.
System then stops re- warming. BCT remains at the selected value.
In case of disturbances the system is responding as described in section 2.
Treatment section 4 (optional) Holding BCT at Normal Temperature
The operator can continue the treatment. TECOTHERM NEO will automatically
continue to hold neonate at the selected BCT 37°C or at a modified temperature.
Operator can finish such treatment at any time using menu “Options”.
To continue the treatment with further warming or cooling you must leave
treatment mode I and select treatment mode II or mode III.
NOTE: All parameters can be changed from set position at ANY TIME should the need
arise. Changes will be stored on the TECOTHERM NEO and can be seen on later
analysis.
In treatment mode I all temperatures / time dates are recorded/ logged and can be read
out/ transferred to an USB stick, see section 7.3 for the USB port.
Fallback Mode
Note: Consulting section 6.2.2 is strongly recommended
TECOTHERM NEO will perform treatment of the patient very safely as long as rectal
temperature of the patient is accurately measured. If measured temperature
deviates more than 1°C from the expected value TECOTHERM NEO will switch from
automatic to the Fallback Mode being a manual mode. Now the operator has to
assess BCT by choosing appropriate mattress temperature. Details see section
6.2.2.
As long as fallback mode is active, BCT monitoring requires installation of another
independent accurate calibrated temperature probe (rectal probe or bladder
catheter sensor) with a separate display showing the rectal temperature.
Substitution of the used rectal probe by a new one of the same type is a First Aid
Measure. Install it into the rectum and connect it to the device front panel socket “R”.
If failure cause is eliminated TECOTHERM NEO will automatically switch back to
servo treatment mode I.

Page 27 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
7.1.2
Servo Control Mode (constant rectal temperature)
In this mode system is designed for inducing hypothermia in a patient by total body
cooling. Details for initial operation see section 8.
Note In treatment mode II you can select treatment temperatures in a wider range
than in mode I: Target rectal temperatures or BCT 30° ….. 38°C.
Treatment temperatures and durations can be defined more freely in the treatment
sections. But after ending a treatment section, the reached final temperature will be
maintained constant for unlimited time. Operator is requested to either continue, or to
introduce a new section by changing treatment parameters (temperatures, duration),
or to stop the treatment.
If a time of 0 h (default) is selected to reach target temperature, the TECOTHERM
NEO will try to reach that temperature as fast as possible. By selecting a reasonable
time interval operator can define an appropriate cooling or warming rate.
Necessary TECOTHERM NEO system equipment to run treatment is
TECOTHERM NEO device
Mattress or wrap for total body cooling, filled, maybe protecting interlayer
Rectal Temperature Probe
Thermally shielded hoses to connect mattress to the device.
All equipment parts should be checked and prepared to start treatment, see section 8 of
this IfU. Patient – neonate- should be placed onto the mattress/ cool wrap.
Rectal probe should be inserted and secured.
To start treatment follow the instructions of the MENU.
The TECOTHERM NEO device pumps thermalizing fluid through mattresses or wraps
and back to the device. During circulation the microcomputer of the Control Board is
permanently comparing/analyzing the measured and set rectal temperatures.
The larger the deviation between the two temperatures the more cooling or heating
power is to be supplied by the TECOTHERM NEO device.
After having reached set rectal temperature the system automatically retains this
temperature.
Treatment section 1 Rapid Cooling- Down Phase
Note that depending on initial BCT and target BCT this section may be a warming up as
well. TECOTHERM NEO is operating to cool down as fast as possible. The system is
operating with maximum cooling power. Body heat from the patient / neonate is
extracted by the cooling mattress and transferred to the circulating fluid. The central
device cooling unit extracts the heat and cools the fluid on demand.
The operator can preselect target rectal temperature within 30°C 38°C, say 33,5°C
e.g. When rectally measured BCT is reaching the target temperature system will
reduce the cooling power automatically. Control board microcomputer determined a
sufficiently small deviation of measured and set temperature to start power reduction.

Page 28 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
.docx
Control system approaches set temperature without remarkably overshooting or
undershooting.
Alternatively, a time interval could have been selected to reach the final temperature.
Treatment section 2 Cooling Phase
This is a constant temperature phase.
Reaching set temperature of section 1 system continues treatment to automatically hold
BCT of the patient constant. It permanently compares measured rectal temperature with
set BCT. The central cooling unit is permanently adjusting temperature of circulating
fluid to hold BCT at selected value.
Operator may either finish treatment or change treatment parameters during treatment
operation (MENU entry “Modifying Treatment”.) Then Control board attempts to reach
the new target temperatures as fast as possible or within the pre-defined time interval.
That is, a new section 1 has been started. For example, this might be a slow re-
warming within 7 hours.
Elapsed treatment times are shown in the display feature DIAGRAM.
Observing the temperature profiles the operator is able to check the temperature
constancy over the treatment time. Rectal temperature is held within a temperature
range 0,5 °C , say 33,5 °C 0,5 °C. Typical deviations are < 0,2°C.
NOTE TECOTHERM NEO will not alarm the end of any section. The operator must
observe whether the intended time of treatment elapsed!
If during treatment section 2, BCT of the patient is increasing/decreasing caused by
disturbances the difference between measured and set rectal temperature increases.
The system initially will respond with more cooling power or increasing warming.
If deviation exceeds 0,5 °C in any direction, for some longer time the internal monitoring
system will detect this discrepancy. TECOTHERM NEO starts alarming.
NOTE All parameters can be changed from set position at ANY TIME should the need
arise. Changes will be stored on the TECOTHERM NEO and can be seen on later
analysis. All temperatures / time dates are recorded / logged and can be read out /
transferred to an USB stick, see section 7.3 for the USB port.

Page 29 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Fallback Mode
Note: Consulting section 6.2.2 is strongly recommended
TECOTHERM NEO will perform treatment of the patient very safely as long as rectal
temperature of the patient is accurately measured. If measured temperature
deviates more than 1°C from the expected value TECOTHERM NEO will switch from
automatic to the Fallback Mode being a manual mode. Now the operator has to
assess BCT by choosing appropriate mattress temperature. Details section 6.2.2.
As long as fallback mode is active BCT monitoring requires installation of another
independent accurate calibrated temperature probe (rectal probe or bladder
catheter sensor) with a separate display showing the rectal temperature.
Substitution of the used rectal probe by a new one of the same type is a First Aid
Measure. Install it into the rectum and connect it to the device front panel socket “R”.
If failure cause is eliminated TECOTHERM NEO will automatically switch back to
servo treatment mode II.
7.1.3
Constant Mattress Temperature Mode
This Treatment mode is designed for inducing hypothermia in a patient by total body
cooling. Details for initial operation see section 8.
Note Treatment Mode III is a non-servo controlled mode. Rectal Temperature
is no more a reference variable for automatically running the treatment.
Selectable mattress temperature range is 12°C …. 39°C. In Treatment Mode III the
operator is fully responsible for performing and selecting a treatment procedure. He has
to select appropriate mattress temperatures and treatment times for inducing
hypothermia and re warming.
NOTE During Initial treatment phase operator should observe treatment very
carefully and when needed correct / adapt treatment parameters.
Under normal conditions of heat transfer it takes about 15 minutes to warm up the
mattress from 20°C to 37°C. Naturally, the core temperature of a patient being placed
onto the mattress cannot follow such a high speed of temperature change.
Attention Only the Mattress temperature is permanently displayed on the screen
in the display feature LARGE SIZE NUMBERS.
If using the TECOTHERM Rectal or Skin probes, temperatures are
displayed only in the display feature DIAGRAM (as profile and in the
parameter boxes). One cannot rely on these temperatures, however,
if Mode III is used as Fallback Mode for a Servo Mode in case the
rectal temperature measurement was in doubt.
Necessary TECOTHERM NEO system equipment to run treatment mode III is
TECOTHERM NEO device
Mattress or wrap for total body cooling, filled, maybe protecting interlayer
Thermally shielded hoses to connect mattress to the device.

Page 30 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Attention External Temperature Probe !
If the internal rectal temperature measurement is in doubt, BCT monitoring requires
installation of an independent accurate calibrated temperature probe (rectal probe or
bladder catheter sensor) with a separate display screen showing the rectal temperature.
The screen should indicate rectal BCT clearly and visible from distance.
All equipment parts should be checked and prepared to start treatment, see section 8 of
this IfU.
Treatment Procedure
Section Cool Down Phase Start treatment following the MENU instructions.
Select mattress temperature and treatment time 0 h to cool down the patient as fast as
possible, or different time to cool in a definite rate.
Measured BCT being 1,0°C higher before approaching the target rectal temperature
operator should enhance the mattress temperature up to 34 - 34,5°C. Depending on the
rate of approaching operator may decide to select slightly higher or lower mattress
temperatures to smoothly reach target value. Keep in mind that changes in mattress
temperature as a rule are seen in the BCT only after 30 minutes.
In the Cooling Phase to hold BCT at the Target Temperature Value the operator
should observe the BCT regularly. Deviations should be corrected by slightly changing
mattress temperature if need arises.
After termination of Cooling Phase (say 72 hours) the operator is requested to perform
the transition into Re- warming Phase. Following the MENU Options the operator is
required to select new treatment parameters. Start re-warming cautiously rising
mattress temperatures to 34- 35°C. Observing the rewarming rate, the operator must
reduce / enhance mattress temperature on demand. Maximal mattress temperature is
39°C. It is possible and useful to initiate a linear rise of the mattress temperature with an
appropriate rate of temperature rise.
For neonates, rewarming to normothermia should take at least 7 hours.
Keep in mind: Patient body mass may severely influence the re-warming. The larger
the mass the slower the re-warming.
All temperatures / time dates are recorded / logged and can be read out / transferred to
an USB stick, see section 7.3, USB port.

Page 31 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
7.2
TECOTHERM NEO Hypothermia system Information
For the TECOTHERM NEO Hypothermia system no ESSENTIAL PERFORMANCES have
been determined.
TECOTHERM NEO is a light- weight, efficient and powerful hypothermia system
Options
Cooling/Warming, Normothermia
Dimensions
375 x 190/ 215 x 310 mm (W x L x H)
Mass / Weight
7,2 kg
Operation modes
automatic
I Servo Control Complete treatment mode
automatic
II Servo Control Constant Rectal Temperature
manual
III Constant Mattress Temperature
Mattress temperatures for Total Body cooling
Neonate and babies,
children up to 50 kg body mass +12 °C to + 39 °C
Temperature constancy 0,3 °C
Temperature accuracy 0,1 °C
Body Core Temperature control range Mode I 32°C … 33,5°C … 38°C
Mode II 30°C …. 38°C
Hydraulic circulation system
Fluid Sterile Water
Reservoir capacity approx. 250 ml
Fluid flow rate in operation up to 300ml/ min, with mattress
up to 500 ml/ min short circuited
Circulation System pressure max. 0,5 bar
Electrical power consumption < 350 W (mains 100-130V / 200-240V 50-60Hz)
Applied Parts
Rectal Probes TC-FMT400/AOR-D/10 single use
TC-FMT400/POR reusable
TCM1837A, single use
Skin Probes TC-FMT400/AS-D10 single use
TC-FMT400/AS-THT reusable
Adaptor cable for disposable rectal probe TC-FMT400/AEC-P reusable
Adaptor cable for disposable skin probe TC-FMT-400/AEC-THT reusable
Adaptor cable for disposable rectal probe TC989803162601, reusable
Aqua Pad TC-MATT (L) Reusable, Manufacturer Inspiration Healthcare
Material PUR polyurethane, transparent
Dimensions 50 x 90 cm
Volume 500 ml fluid
Mass (empty) 750 g
Cool Wrap TC-MATT-NEO Reusable, Manufacturer Inspiration Healthcare
Material PUR polyurethane, transparent
Dimensions 620 x 420 mm
Volume 300 - 350 ml fluid
Mass (empty) 155 g
Cool Wrap TC-MATT-DISP Single Use, Manufacturer Inspiration Healthcare
Material PUR polyurethane, coated
Dimensions 620 x 420 mm
Volume 300 - 350 ml fluid
Mass (empty) 220 g

Page 32 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Modules and Main Components are
Central Cooling / Warming Module
Hydraulic Module for controlled circulation of thermalizing fluid
Micro Computer controlled Operating and Control Board,
MENU, user interface
Display for visualization of MENU operations and treatment / therapy scenario.
Alarm and monitoring system
Temperature probes
Detailed software is implemented.
Indicators and operation key elements / buttons are clearly arranged at the front panel.
Mains socket and sockets for USB are positioned at the rear side.
Figure TECOTHERM NEO Hypothermia Device
More details, see section 7.3
Central Cooling / Warming module
The Central Cooling / Warming module is a thermoelectric based unit which cools or
warms the circulating fluid. This module is controlled and monitored by means of a
microcomputer in the Control Board and supplied by a modern efficient switching power
supply. It is fan cooled to remove heat produced by the Peltier elements.
It is operating exactly to reach the target temperatures adjusted by the operator, and
hold them constant according treatment protocol.

Page 33 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Hydraulic Module and Circulation System
The Hydraulic Module is made of a pump, pressure valve, inner fluid container, quick
disconnect couplings to connect hoses and mattresses, aqua wraps, and an internal
flow meter to detect circulation flow volume. Pump is driving the thermalizing fluid to
circulate through the mattresses/aqua wraps. Operation pump pressure is limited to 0.5
bar.
Micro Computer controlled Operating Board and Control Board, MENU
TECOTHERM NEO is furnished with an Operating Board and a Control Board to
control its internal operation features. Both boards are based on their separate powerful
microcomputers which are in permanent communication.
TECOTHERM NEO has three main operation and treatment modes. The operator can
select operation modes using the MENU, see section 7.1.
A large display serves as user interface for the operator. MENU operations and
treatment modes are visualized on the display screen.
The operator either selects, confirms or modifies treatment modes, treatment options,
operations and settings using MENU operation Arrow Keys to move to MENU entries.
Pushbuttons below display screen enable performing instructions like Select, Confirm,
Cancel, Apply, Start etc.
Currently selectable instructions and entries are highlighted turquoise.
Display with MENU
Arrow Cursor
Arrow keys
Page 34 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
SF
Alarm System and Monitoring Features
Alarm symbols shown on the display are indicating system troubles and failures
System failure.
Temperature Alarm.
Alarm No or restricted flow
Low fluid level
Audible alarm, paused
TECOTHERM NEO is equipped with detailed alarm and monitoring elements. Main
purpose is monitoring and detection of temperatures and flow of the circulating fluid, of
internal temperatures in the Central Cooling/Warming module, of the temperature limits,
mains power failure.
When detecting deviations from the limits and/or failures the alarm system initiates
optical and audible alarms, and the above shown indicators appear on the screen.
Details on indicators see section 4.
Mains Power failure and a certain internal system failure are indicated
by LED indicators in the lower front panel part just below the display screen. Details see
section 11 Alarm System.
Mains Cable Cord
TECOTHERM NEO is plugged via a medical grade cable cord to a shock-proofed mains
socket with 100-130V or 200-240V and 50-60Hz. Cable should be 2,5 m long and
approved for shock- proofed sockets only.
SF

Page 35 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
7.3
Indicators and Operation Key elements, Display screen
Figure TECOTHERM NEO front panel view
(1) Pushbutton to power the device on, marked ”I“
(S) Temperature Probe Socket Skin Probe
(R) Temperature Probe Socket Rectal Probe
(4)
LED Indicator Mains Failure
(5)
LED Indicator System Failure “SF”
(T 1) Pushbutton for MENU operations, meaning indicated on display
(T 2) Pushbutton for MENU operations, meaning indicated on display
(T 3) Pushbutton for MENU operations, meaning indicated on display
(T 4) Pushbutton Arrow Key: menu upwards or increase value
(T 5) Pushbutton Pausing Audible Alarm
(T 6) Pushbutton Arrow Key: menu downwards or decrease value
(6)
Female Coupling / socket for connecting hoses or mattresses
(7)
Female Coupling / socket for connecting fill- up set for filling

Page 36 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Figure TECOTHERM NEO Rear side
(8)
Mains Socket
(9)
USB socket
Further details see section 7.9
Indicating Temperatures at the Display screen
Running the 3 main treatment modes, see section 7.2, treatment temperature is shown
on the screen in a large size (display feature LARGE SIZE NUMBERS).
Rectal temperature is displayed when selecting treatment mode I or II. Mattress
temperature is shown when selecting treatment mode III.
Treatment modes I and IIAs long as measured Rectal Temperature deviates more
than 0,5 °C from the Set Point the rectal temperature is appearing RED .So for example
if Set Point is 33,5°C a measured rectal temperature of 36,4 °C is displayed RED. Only
when deviation is < 0,5°C colour changes to GREEN.
Servo controlled complete treatment mode
36.4
Set Point 33.5
Details
Servo controlled complete treatment mode
33.4
Set Point 33.5
Details
Page 37 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Treatment mode III As long as measured Mattress Temperature deviates more
than 0,5 °C from the Set Point the mattress temperature is appearing RED .So for
example if Set Point is 30,0°C a measured temperature of 34,1 °C is displayed RED.
Only when deviation is < 0,5°C colour changes from RED to BLUE.
Constant Mattress Temperature Mode
34.1
Set Point 30.0
Details
Constant Mattress Temperature Mode
30.2
Set Point 30.0
Details
60 seconds after starting treatment in each of the main treatment modes the display
feature DIAGRAM is changed to the feature LARGE SIZE NUMBERS.
Here shown for treatment mode II Servo Control Constant rectal temperature.
Servo control mode (constant rectal temperature)
33.6
Set Point 33.5
Details
Servo control mode (constant rectal temperature)
Set Temperature
Rectal Temperature
33.5 Mattress Temperature 35.4
33.6 Skin Temperature
-----
Constant rectal temperature for 01:34 hours
23.03.2010 08:55:42
35
35
30
30
25
25
20
20
15
15
9
8
7
6
5
4
3
2
1 hour
Options

Page 38 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
R
S
7.4
External Temperature Probes
Patient Temperatures should be measured using approved calibrated Temperature
probes recommended by the manufacturer, only.
TECOTHERM NEO applies Rectal Temperature probes and a Skin temperature probe:
Rectal Probes TC-FMT400/AOR-D/10 single use
TC-FMT400/POR reusable
TCM1837A, single use
Skin Probes TC-FMT400/AS-D10 single use
TC-FMT400/AS-THT reusable
Adaptor cable for disposable rectal probe TC-FMT400/AEC-P reusable
Adaptor cable for disposable skin probe TC-FMT-400/AEC-THT reusable
Adaptor cable for disposable rectal probe TC989803162601, reusable
NOTE Rectal probe and Skin probe connectors have
their individually mating sockets R and S!
Ensure correct connections!
Body core temperature BCT is measured with the rectal probe. Ensure that the probe is
correctly inserted in the patient and that it is properly secured. Also ensure that the
probe is properly connected to the TECOTHERM NEO socket marked !
The second temperature probe (reference probe) is directly plugged to the
TECOTHERM NEO socket . It serves as a means independently monitoring a
second patient temperature.
Figure TECOTHERM NEO with Temperature Probes

Page 39 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
7.5
Hoses, Hydraulic lines
The hose set is composed of fluid tubing made of transparent polyurethane material,
and a thermally shielding envelope made of silicone foam material. One of the two
lines is marked blue at both ends.
Mattresses or wraps are connected to TECOTHERM NEO by means of the hoses.
Hoses are connected to the device with male connectors, to the mattresses with
female connectors. No need to take account of the blue marking.
Male connectors are plugged to the device ports (6), see section 7.3. and 8.3.
Mattresses and other applied parts are connected to the female connectors of the
hoses.
The blue marking facilitates identification of flow direction within a non-transparent
mattress bearing in mind that liquid leaves the device at the left port (6).
All connectors are self- sealing quick disconnect couplings.
Figure Hoses
Standard size of hoses is 2 m. 1m or 3 m upon request.
Special medical diagnostic checkups and applications like MRI with need of patient
cooling require lack of metallic parts. In such cases mattresses are directly connected
to hoses of various size and length up to 5m without using any couplings avoiding
disturbing metallic springs.
NOTE Prior to order such special application contact
your service partner or the manufacturer.

Page 40 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
7.6
Fill- up set for Filling / Refilling thermalizing fluid.
Fill- up set is a 500 ml fluid container made of HD polyethylene or of polypropylene
material, marked every 50 ml.
The cap has two connecting adapters made of polyurethane tubing equipped with male
QDC couplings. To fill – up / refill TECOTHERM NEO device with sterile water, male
connectors are plugged to ports (7) in the device front panel, section 7.3.
Figure Fill- up set
7.7
Thermalizing Fluid
TECOTHERM NEO uses Sterilized Water as a circulating fluid to bring cold and heat to
the patient. Sterilized Water can be sourced locally or supplied in 5 l containers made of
HD polyethylene or of polypropylene.

Page 41 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
7.8
Mattresses, Cool Wraps and protective layers
For transfer of cold and heat to the neonate or generally to a patient within total body
treatment cool wraps and mattresses are used. All have connector parts connecting
them to the hoses, see Figures.
Both types are limited to working pressure 0,5 bar.
Figure Mattress Therm Aqua Pad, 50 x 90 cm, with male QDC connectors
Mattresses of Therm Aqua Pad type are multi- use applied parts for total body cooling,
see section 7.2 for sizes and details.
Figure Cool wrap TC-MATT-NEO/TC-MATT-DISP 42 x 62 cm with male QDC
connectors
Eyelets

Page 42 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Figure
Cool Wrap TC-MATT-NEO/TC-MATT-DISP with its typical pattern structure to ensure
proper flow and fluid distribution
The shown wrap is specified for Multiple Use. Details see section 7.2 for size and
details. Wraps intended for Multiple Use are made of transparent PUR material.
The working pressure is limited to 0,5 bar.
For positioning TNEO cool wraps we recommend small felt fixation tapes. Tapes are fed
through eyelets at right and left sides of the wrap then forming a loop. Both tape ends
are knotted. The operator may select, by choosing the appropriate loop length, to what
extent the neonate or patient is wrapped. For details see section 8.7.
Protecting Foil layer
Use thin disposable interlayer which must at least fully prevent direct contact of
neonate skin with mattress or wrap intended for multiple use. Such interlayer must be
coated at lower side with thin plastic coating to prevent penetration of blood, body
liquids or other liquid media onto the upper surface of the mattress and to protect the
patient against liquid escaping the mattress.
420 mm
365 mm
620 mm

Page 43 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
7.9
MENU and the User Interface
The operator is working within the framework of the Operator System (Operation
Board).
TECOTHERM NEO provides a comfortable Men- Machine Interface: MENU as the
User Interface, display screen as Visual Interface and Pushbuttons / Keys to move
along the MENU instruction entries.
Display visualizes MENU operations and treatment procedures
The operator either selects, confirms or modifies treatment modes, treatment options,
operations and parameter settings using the Arrow Keys to move to MENU entries.
Such active entry is coloured turquoise. Direction of movement corresponds to the
arrow keys, see section 7.3.
Pushbuttons below display screen enable performing instructions like Select, Confirm,
Cancel, Apply, Start etc.
TECOTHERM NEO Main MENU always serves as a Starting point for navigation. Using
Main MENU the operator selects a Menu language: entry Language, see section 8.3.
Entries at the MENU left side are accessible for the operator except entry SERVICE.
Access to entry SERVICE is only for service personnel using a password.
Alarms and troubles are indicated optically by ICON symbols
TECOTHERM NEO Main Menu
Highlight and Select Function Required:
Servo control mode (constant rectal temperature)
Constant Mattress Temperature Mode
Alarm check
Display and export of treatment data
Service
Power Off
Select
Language
Servo controlled complete treatment mode
Ser.-Nr. 2013/38/01
Rev. 060/02.15
SF
TECOTHERM NEO
A check of the internal functions has been performed and did not
detect any malfunction.
Now the alarm functions will be checked. Can you see the various
Symbols and clearly hear the two alarm sounds?
No
Yes

Page 44 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
No Yes
ERROR
It is not allowed to run the device without working alarm
functions. It will shut off in 30 seconds (press "Confirm" to shut
off immediately).
Confirm
Important entries, instructions, notes and failures are displayed as turquoise- highlighted
Pop- Up Windows
Important conditions and preconditions to perform treatments are displayed on the
screen. The operator has to Confirm or Cancel.
TECOTHERM NEO
A check of the internal functions has been performed and did not
Now the alarm functions will be checked. Can you see the various
Symbols and clearly hear the two alarm sounds?
detect any malfunction.
No Yes
ERROR
It is not allowed to run the device without working alarm
Confirm
functions. It will shut off in 30 seconds (press "Confirm" to shut
off immediately).
Servo controlled complete treatment mode
NOTE: A rectal probe is required to be inserted prior to treatment.
Please ensure its correct placement prior to starting treatment.
For reference, an additional skin temperature probe is strongly
recommended.
Please confirm the requirement for a rectal probe by pressing the
"Confirm" button!
Cancel
Confirm

Page 45 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
7.10
Display and export of treatment data
To read out and export treatment data and data log files select Entry
Review and export of treatment data in the Main MENU.
This submenu offers 3 options:
Export new treatment data
Export all treatment data
Select, review and export particular treatment data.
Here, "new treatment data" means all those treatments which never have been
exported so far, including in any case a possibly just only finished treatment.
Option "Select, review and export particular treatment data" provides a comfortable
possibility to graphically represent the complete progress of an earlier treatment
identified by date and time at which it has been started. If necessary, this data
can then be copied to a USB stick.
To find a specific patient record, the user must first select the year of the treatment, then
the month, the day and finally the specific treatment itself. Should there be more entries
than available lines, the list automatically scrolls when the last (or the first) line is
reached. The function can also be used to simply display the graphics, without
actually exporting the data at the end.
To perform export plug an USB stick into the USB port (9) at the rear side of the device.
Then follow MENU instructions. The operator gets a message on the screen notifying
the user whether or not data export was successful. After successful export unplug USB
stick.
Display and export of treatment data
Highlight and select the desired procedure.
Export new treatment data
Export all treatment data
Select, display and export particular treatment data
Back
Select

Page 46 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
8. TECOTHERM NEO Hypothermia System Putting into Operation
8.1
Initial Set Up / Initial Operation
The manufacturer or authorized service personnel should initially set up TECOTHERM
NEO system. The operators should be trained how to run the system.
8.2
Pre- operation Check up
Caution Ensure that power cord matches the shockproof
socket at site (100-130V or 200-240V, 50-60Hz).
Caution Ensure that right proper temperature probes
have been prepared and prepositioned.
Caution Ensure that proper mattress / wrap and maybe
interlayer foils have been prepared and prepositioned.
Pre- operation Check up
Prior to putting the system into operation, check the conditions of section 5 to ensure
safe and proper operation:
Warning: For a reliable and safe operation use only original components and
applied parts (mattresses) supplied or recommended by the
manufacturer. Otherwise proper and safe operation cannot be
guaranteed.
Check that only sterile water is used as circulating fluid to avoid a risk to
the neonate. Otherwise damages of device components may occur.
Check type of mattress / wrap and maybe prescribed interlayer. Use only thin
interlayers with plastic coating.
Check whether the right temperature probe is prepared. Its connector must
match the socket “R” at the device front panel
Without rectal temperature probe plugged to port “R” it is not possible to
start treatment modes I and II.
If all this preconditions are fulfilled device can be put into operation.

Page 47 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
8.3
Initial Operation by the customer
If mattress is positioned into an incubator read notes in section 5.
Plug the cable cord into rear socket (8). Then plug the system to the mains into shock-
proofed socket.
Fuse data see section 14, Technical Data, and type label.
Note Immediately after plugging to mains TECOTHERM NEO is in its Stand-
by mode. Key (1) is lit light green.
Next Step Connect mattress / cool wrap to the TECOTHERM NEO
Prior to connecting check whether
mattress / wrap is filled completely. If empty or only partially filled, see section 8.5
for filling instructions.
defects like punctures and flaws are existing. If fluid escapes replace or repair
defect mattress / wrap.
tubing of hoses and mattress / wrap is kinked or situation may appear to kink.
Connect QDC couplings of hoses to the QDC counterparts of the mattress. Then plug to
the ports (6) at the device right lower front part, see figure. If they do not engage
properly push metallic unlocking keys at the QDC, then repeat plug procedure.
Figure Connection to ports (6)
Unlocking Keys
Note To unlock push metallic unlocking keys downwards.
Place completely filled mattress/ cool wrap onto a 10 - 20 mm thick plastic- foam
material that has good thermal insulation, e.g. in a prepared incubator.
Note In case of Incubators see section 5.
Place a thin protective interlayer with plastics coating at the bottom downwards onto the
mattress if it is of multiple use type. Protective interlayer should be somewhat larger
than the mattress and fully cover the mattress with a border for wrapping the mattress.

Page 48 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Connecting Temperature Probes to TECOTHERM NEO device
NOTE: For treatment modes I and II a rectal probe is required.
NOTE Ensure that only calibrated probes are used!
Patient temperature measurement must be accomplished only with probes approved
by the manufacturer of the TECOTHERM NEO as listed in section 7.4. Only these
have been tested as required and ensure a sufficiently accurate and reliable
temperature measurement even in an unfavorable electromagnetic environment.
Using other temperature probes is likely to put the patient at risk!
Rectal Probes TC-FMT400/AOR-D/10 single use
TC-FMT400/POR reusable
TCM1837A, single use
Skin Probes TC-FMT400/AS-D10 single use
TC-FMT400/AS-THT reusable
Adaptor cable for disposable rectal probe TC-FMT400/AEC-P reusable
Adaptor cable for disposable skin probe TC-FMT-400/AEC-THT reusable
Adaptor cable for disposable rectal probe TC989803162601, reusable
NOTE Rectal probe and Skin probe connectors have
their individually mating sockets R and S
If patient is already prepared for treatment:
Ensure that the rectal probe is correctly plugged to its socket “R”. Probe must
be correctly inserted in the patient and properly secured.
The reusable rectal probe D- RB2A must be plugged to socket “R” directly.
Rectal probes for single use must be connected to their respective extension
cables, and these in turn plugged to socket “R”.
Always insure to keep dry the connection between probe and extension cable!
If a skin probe is used plug it correctly to its socket “S”. Place it correctly abdominal or
at the forehead and secure it.
Putting TECOTHERM NEO into operation
After passing the above mentioned preparations you may put system into operation:
Press Pushbutton (1). The lit key changes to intensive Green.
Note TECOTHERM NEO is running a self- test to check internal functions
followed by a test of the alarm functions.
Display screen must be illuminated, LED indicators (4) and (5) must flash. Device is
emanating an intensive BEEP, followed by a blue flash of the T5 button and intensive
Page 49 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
double BEEP. The operator is asked to confirm YES by pressing key T3, that one can
see the various symbols and clearly hear the two alarm sounds. Should anything be
wrong, answer NO pressing key T1 or simply do nothing. Device will shut down.
After confirmation YES the TECOTHERM NEO Main MENU is displayed.
Using Main MENU and pushing arrow keys the operator can select entry Language
and is led to a list of available languages (English, Deutsch, Español, ….. ). After
selection display screen promptly shows the Main Menu in the selected language.
Pushing arrow keys T4 and T6 you may select the operation / treatment mode, see
section 7.1. Selected entry is highlighted turquoise.
After selection of a treatment mode: Follow the Instructions in the MENU.
TECOTHERM NEO Main Menu
Highlight and Select Function Required:
Servo control mode (constant rectal temperature)
Constant Mattress Temperature Mode
Alarm check
Display and export of treatment data
Service
Power Off
Select
Language
Servo controlled complete treatment mode
Ser.-Nr. 2013/38/01
Rev. 060/02.15
SF
TECOTHERM NEO
A check of the internal functions has been performed and did not
detect any malfunction.
Now the alarm functions will be checked. Can you see the various
Symbols and clearly hear the two alarm sounds?
No
Yes

Page 50 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Adjustment of treatment parameters, treatment start
In main menu, select one of the treatment modes, details see section 7.1:
I Programmable Complete Treatment Mode (Servo controlled mode)
II Servo Control Mode (constant rectal temperature)
III Constant Mattress Temperature Mode
All treatment modes are specified by their respective menus.
The menu approach to induce hypothermia in a patient is described in detail in an
example on page 53.
Note Pay attention to the entries highlighted turquoise. Entry instructions are
requesting adjustment to treatment temperatures and times / durations pushing the
arrow keys
once or repeatedly.
Treatment options are subdivided into different phases. All details for the 3 treatment
modes and their phases can be found in section 7.1.
Having selected treatment mode, having accepted default values or modified the
relevant treatment parameters, and having positioned the neonate on the mattress,
press button Start. Treatment begins. In treatment modes I and II, the measured rectal
temperature must be within 29°C to 39°C. Otherwise, Start will be denied because
rectal temperatures outside this range are regarded to be unacceptable (measured
incorrectly).
Treatment is started
Normally TECOTHERM NEO reaches the set rectal temperature 33,5°C after 25 – 35
min (small mattress 30x 45 cm) and 30 – 35 min (cool wrap). Have in mind that cooling
down rate is depending on weight of the patient and on the ambient temperature: the
higher, the more time is required.
TECOTHERM NEO is programmed to reach target rectal temperature 33,5°C or any
other selected temperature as soon as possible if no other rate input is chosen by the
operator (time to reach final temperature > 0 h ).
The operator may observe the temperature – time profile in the display feature
DIAGRAM and actual temperature values in the small parameter boxes in the upper
part.
Independent on treatment mode, feature DIAGRAM changes after 60 seconds
automatically to feature LARGE SIZE NUMBERS.

Page 51 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Treatment is running
Treatment is executing according to and depending on selected treatment mode and
applied treatment parameters. All treatment data like temperatures / time dates are
automatically recorded/ logged and can be read out / transferred to an USB stick.
We recommend that during treatment operator should from time to time monitor patient
temperatures. This is especially important during transition from cooling section 3 to
re warming section 4.
NOTE: All parameters can be changed from set positions pushing key T3 Options and
selecting entry Change the current settings, see below. You may go back to the original
settings pushing key T1 Back .
End treatment
When treatment approaches the end the operator may continue with a treatment section
4, see section 7.1. Otherwise following the MENU instructions he is requested to select
the instruction End Treatment and save data using the arrow keys.
Doing so you return to the main menu. From there, you can start another treatment,
export stored treatment data or turn off the device.
Options Available
Final temperature is currently unchanged
Highlight and select the desired option:
End treatment and save data
Back
Select
Options during operation
The current operation continues to run.
Highlight and select the desired option:
Changes the current settings
Immediately start the re-warming phase
End treatment and save data
Alarm check
Back
Select

Page 52 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Export treatment data/ Data storage
To read out and export treatment data and data log files select entry Display and export
of treatment data in the Main MENU. To perform export plug an USB stick into the USB
port (9) at the device rear side. Then follow MENU instructions, push Select.
The operator gets an information on the screen whether data export was successful.
After successful export unplug USB stick.
After successful export push key T3 Confirm to return to Main MENU.
It is not necessary to export new data immediately after the end of a treatment. All data
are stored by the TECOTHERM NEO on a memory card with large capacity.
8.4
Stop operation / Turn off device
To stop or to interrupt operation turn back to the TECOTHERM NEO Main MENU.
Then push Arrow Key ▼ to move to entry Power off and finally push button “Select”.
After few seconds device is shut off.
NOTE Push button (1) is lit (but dimmed) as long as system is plugged to mains.
Only unplugging mains cause green push button light to turn off.
Disconnection from mains: Unplug the cable cord from the mains shock-proofed
socket or from the rear socket (8). Only after this the device is completely disconnected
from mains!
TECOTHERM NEO Main Menu
Highlight and Select Function Required:
Servo controlled complete treatment mode
Servo control mode (constant rectal temperature)
Constant Mattress Temperature Mode
Alarm check
Display and export of treatment data
Service
Select
Language
Power Off
Ser.-Nr. 2013/38/01
Rev. 060/02.15
Display and export of treatment data
Highlight and select the desired procedure.
Export new treatment data
Export all treatment data
Select, display and export particular treatment data
Back
Select

Page 53 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Example How to perform hypothermia treatment in Treatment Mode III
Exemplary approach: Treatment Mode III Constant Mattress Temperature
Treatment Mode III Constant Mattress
Temperature
NOTE Selected Instruction entry is
highlighted turquoise
1
st
Step Set Mattress temperature
Use key T3 Select
Standard set 33,5°C displayed
Moving to a different temperature
say 28°C pushing arrow key
28,0 °C
Then Push Key T3 Apply set
temperature and store it
2
nd
Step
Select Time to reach set final
temperature
Use key T3 Select
Standard time 0 h is displayed
Keep this value: change as fast as
possible to reach 28°C
Or adjust another time
pushing arrow key to adjust
new time, say 2 h
2 h is displayed
Then Push Key T3 Apply time and
store it.
3
rd
Step
Instruction Start
Push key T2 Start
Display feature DIAGRAM with
treatment parameter boxes is displayed.
Treatment starts. 60 seconds later
display feature LARGE SIZE NUMBERS
appears displaying current mattress
temperature.
Constant Mattress Temperature Mode
Apply standard settings or highlight, select and change parameter
settings as required:
Set point for constant mattress temperature
Time to reach final temperature
(Change as fast as possible)
33.5 °C
0 h
Cancel
Start
Select
Constant Mattress Temperature Mode
Apply standard settings or highlight, select and change parameter
settings as required:
Set point for constant mattress temperature
Time to reach final temperature
(Change as fast as possible)
28.0 °C
0 h
Apply
Constant Mattress Temperature Mode
Set Temperature
Rectal Temperature
28.0 Mattress Temperature 28.2
34.2 Skin Temperature -----
Constant mattress temperature for 00:43 hours
23.03.2010 08:55:42
35
35
30
30
25
25
20
20
15
15
9
8
7
6
5
4
3
2
1 hour
Options

Page 54 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
8.5
TECOTHERM NEO system: Filling / Refilling Procedures
TECOTHERM NEO’s hydraulic module is equipped with an internal fluid tank of 250 ml
volume containing circulating fluid. This container is prepared and prefilled by the
manufacturer ready for operation.
To ensure a safe operation and a proper fluid circulation prior to each treatment
fill/refill device and mattresses properly and correctly.
Attention Do not use partially filled mattress in hypothermia treatment.
Procedures:
Preparation of fresh or empty / drained mattress:
Check mattress for defects and damages like punctures and flaws.
Place mattress horizontally spread onto a plane support (table).
Connect QDC couplings of hoses to the mattress QDC counterparts. Then plug to
the ports (6) at the device right lower front part, see section 8.3 of this IfU.
Preparation of fill- up set, see also section 7.6 of this IfU.
Check fill- up set to see whether defects, damages, leaks are existing.
Fill- up fluid bottle with sterile water up to the mark 450 ml.
After filling close bottle cap tightly.
Filling / Refilling
Connect QDCs of the fill-up bottle to the refill port QDC counterparts (7) at the
device front face
Put TECOTHERM NEO device into operation, see section 8 of this IfU.
In Main MENU, select desired Treatment Mode and start operation.
Now lift fill- up bottle and turn it until cap is directed downwards.
Attention If Flow rate alarm is appearing ignore it or push key T5 to silence audible
alarm (AUDIO paused, is appearing at the display.)
Fill up until air bubbles inside the fill- up bottle disappear.
Then disconnect bottle QDC from ports (7) ).
To remove residual air bubbles in the mattress via mattress tubing outlet, move
and swing mattress slightly after filling operation. Air must be given the possibility
to move upwards. The blue marking of the hoses helps to identify the flow
direction within a non-transparent mattress.
Re- fill the fluid bottle with sterile water and connect it again as described above.
Continue filling up until air bubbles inside the fill- up bottle disappear again.
If audible alarm is active, silence it by pushing blue button T5. Close an open Pop-
Up window if activated.

Page 55 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Continue TECOTHERM NEO operation for 1 min. If within this time period alarm “No
Flow” is appearing or reappearing follow the instructions in the display Pop- Up window.
End procedure as described above. Now TECOTHERM NEO system is ready to
perform the intended treatment.
Note A large mattress like Therm Aqua Pad 50 x 90 may require more than 450ml of
liquid during filling / refilling process, and the fill-up bottle becomes empty. In that case
fill up the bottle again and proceed as described above.
Refilling TECOTHERM NEO device during treatment
TECOTHERM NEO device is put into operation, mattress is correctly connected and
treatment is properly running. During treatment symbol Low liquid level is appearing.
Low liquid level may be caused by loss of fluid, see section 11.4.
Lack of sterile water/ low liquid level is indicated by the symbol appearing at the
screen accompanied by an audible alarm. Button T5 is lit blue.
You have to refill sterile water.
Proceed as follows
1.
Push key T5 to silence audible alarm. AUDIO paused, is appearing at the
display.
2.
If not prepared fill the fill- up fluid bottle with sterile water up to the upper mark
450 ml. After filling close bottle cap tightly.
3.
Turn bottle until cap is directed downwards and keep in this orientation until the
whole fill-up procedure will be finished.
4.
Connect QDC of the fill-up bottle to the refill port QDC counterparts (7) at the
device front face.
Attention If alarm No Flow appears ignore it or push button T5 again.
5.
Refill until symbol disappears, continue filling until rising air bubbles in the
bottle disappear.

Page 56 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
6.
Observe liquid level in the bottle, keep it in mind.
7.
Now disconnect the refill bottle from the device.
If alarm Low liquid level is reappearing repeat refilling procedure as described.
Attention Frequent or permanent appearance of the alarm indicates a malfunction
or system failure. Please, follow sections 11.4 and 11.6.
Substitution of sterile water see section 13.3
8.6
Draining a used mattress
First drain liquid from the inner container:
Disconnect hoses
Connect the empty Re-fill bottle instead, with cap upwards
Start mattress mode and wait until all liquid is in the bottle
Ignore (or mute) any flow alarm or fluid level alarm
Disconnect bottle, discard fluid and connect again as described.
Now drain liquid from the mattress:
Connect mattress to the refill port couplings (7) at the device front face, turn
mattress upwards
Wait for about 1 minute until all the liquid is drained from the right half of the
mattress
Disconnect mattress and re-connect with connectors swapped, turn mattress
upwards
Wait for about 1 minute until all the liquid is drained from the mattress
Disconnect mattress
Disconnect Re-fill bottle, discard fluid.

Page 57 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
8.7.
Application of mattresses to patients
Hypothermia in neonate and infants is induced by whole body cooling. For that the
following mattresses are available, see also section 7.8:
Dimensions
Cool wraps TC-MATT-NEO and TC-MATT-DISP 420 x 620 mm
Reusable
Aqua Pad TC-MATT (L) 500 x 900 mm
Attention: Do not kink hose set and tubing! Do not fold mattress!
Kinking and folding will stop fluid circulation and hence cooling
or warming operation of the TECOTHERM NEO system.
Alarm No Flow may appear.
For further details see section 11.3, too.
Before applying to human body pay attention to the following items
Use only mattresses specified in accordance with the intended application to neonate
and babies.
Place protective interlayer between multiple use mattress surface and neonate skin;
see below
Mattresses must not be touched with sharp or tipped objects due to risk of puncture
damage. Liquid may escape!
Attention: Avoid direct contact of mattress or cool wrap with fresh
or non- closed wounds, infectious areas, areas with
ulceration and abscesses, rash and burns.
Application
Place mattress horizontally spread onto a plain support. Ensure that mattress and ports
(6) are on the same level, approximately.
Use only mattresses specified or recommended by TEC COM as the manufacturer of
the TECOTHERM NEO systems, or authorized distributors.
In case of multiple use mattresses, use a thin disposable protective interlayer which
must at least fully cover the mattress with 5 cm larger border area all around. Such
protective interlayer must be coated at lower side with plastics coating to prevent
penetration of blood, liquids or liquid media onto the surface of the mattress. It also
provides protection against fluid escaping from the mattress and coming in contact
with the patient’s skin. After use, discard protective interlayer.
Place mattress onto a 10 - 20 mm thick thermally insulating layer (elastic foamy
plastics) for ensuring good thermal insulation during hypothermia operation.

Page 58 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Application of mattress type Cool Wrap
TC-MATT-NEO and TC-MATT-DISP Cool wraps are designed and performed for
neonate in a way to induce hypothermia more appropriately, see figure in section 7.8
and below. The size of the lower part is abt. 420x360mm. Neonate can be wrapped to
an extent not disturbing other treatments or actions with the patient, diagnostics e.g.
Place cool wrap horizontally spread onto a plain support. Ensure that mattress and
ports (6) are on the same level, approximately.
Place mattress onto a 10 - 20 mm thick thermally insulating layer (elastic foamy
plastics) for ensuring good thermal insulation during hypothermia operation.
Maybe place protective interlayer onto the cool wrap mattress.
Place neonate onto A - B - C region. Place head onto A. Feet should be located at part
C and body / chest along part B.
Place neonate in a way to allow partial wrapping around the body below axilla.
NOTE Do not touch axilla. Avoid chafing!
For positioning T NEO cool wraps use small felt fixation tapes. Tapes are fed through
eyelets at right and left sides of the wrap then forming a loop. Both tape ends are
knotted. The operator may select by choosing the appropriate loop length to what extent
the neonate or patient is wrapped.
Felt fixation tape
Wrap neonate only to an extent that free access
to the patient is ensured.
Wrap creates a cool ambient around and
near neonate.
A
B
C

Page 59 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
9. Hygienic Requirements
We draw your attention to the following minimal hygienic requirements for the
application of TECOTHERM NEO hypothermia system, procedures and components.
To meet these minimal hygienic requirements we recommend a careful disinfecting
cleaning of applied parts (mattresses etc., hoses, QDC and temperature probes.)
This disinfecting cleaning may be performed by wiping with a mild liquid disinfectant
appropriate for medical purposes.
9.1. Cleaning and Disinfecting TECOTHERM NEO
Attention Prior to cleaning and disinfecting switch off system
and unplug mains!
The external cleaning and disinfecting of the device including hoses should be
performed using a damp sponge or cloth both soaked with a liquid disinfectant, or a
spray disinfectant in combination with a dry cloth.
Clean and disinfect device bottom side (ventilation holes) generally once every 1-2
months and just before next treatment as a part of the system preparation.
Inspect once every three months that air ventilation holes in the device bottom side are
not fluff covered and free from dust. Remove fluff and dust using a small vacuum
cleaner.
9.2
Mattresses, thermally insulated hoses, tubing
Some mattresses can be used multiple times and must be cleaned / disinfected. Usually
such mattresses will not directly come into contact with normal skin surface due to the
protective interlayer. After treatment, disinfectant cleaning of the mattress should be
performed by wiping a mild liquid disinfectant appropriate for medical purposes.
Attention Do not try to sterilise mattress!
Attention: Application of multiple use mattresses is only allowed
in combination with thin fabric protective interlayer
coated with plastics coating at bottom side. Such interlayer components
are part of the TECOTHERM NEO accessories and can be supplied by the
distributors or the manufacturer. Such protective interlayer is disposable
single-use product.
Attention: When the mattress has been in contact with blood or
human secretion during treatment, it must not be used
more than once! Dispose of mattress in accordance with
local standards for single use items.
The same holds for hoses and couplings after such contact.
Note Drain and replace fluid in the mattresses at least every
2
months
after last filling.

Page 60 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
9.3
Temperature Probes
Reusable Temperature Probes
Rectal and Skin Temperature Probes are delivered as autoclavable applied parts.
Probes have to be disinfected and sterilised after treatment as usual in clinical practice
of I C U.
For details read the Instructions for Use of the manufacturer of the temperature probes.
Disposable Temperarature Probes, Single Use
Dispose of such temperature probes after use in accordance with local standards for
single use items.
The corresponding Adapter and Extension Cables, however, are reusable. After use
disinfecting cleaning should be performed, see section 9.2.
Caution Keep cable connectors dry to ensure proper probe and device operation!
Do not dip them into liquids!

Page 61 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
10. Storage and Transport
10.1
Storage of the TECOTHERM NEO Device
The TECOTHERM NEO Hypothermia Equipment includes the Basic TECOTHERM
NEO device unit, electrical mains cable, fill- up set and hoses. Keep all parts together.
Store equipment in a closed cabinet.
Basic Unit The TECOTHERM NEO basic unit should be stored in a closed cabinet
to protect it against mechanical damage and dust. Put it onto a solid
board horizontally standing on feet.
Store in a dry ambient.
Mains cable Keep cable near by the basic unit. Put it into a separate plastic storage
bag. Close bag.
Fill- up Set The empty set is supplied in a box or bag.
Put filled or partially filled set into the set box beside the basic unit.
Keep cap tightly closed to avoid leakage of coolant fluid. Protect QDC
couplings against mechanical damage. Disinfect it prior putting back.
Store in a dry ambient.
Hoses Hoses are supplied in a closed airtight plastic envelope or a closed box.
Keep it closed until preparing TECOTHERM NEO for operation.
After use disinfect hoses and put them back into the box or the plastic
envelope.
Keep hoses near by the basic unit.

Page 62 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
10.2
Storage of mattresses
Fresh or unused empty mattresses have to be stored in the original box or package.
Storage in a dry and dark environment.
Filled or partially filled used or unused mattresses containing fluid must be stored in a
closed storage box in a cool and dark environment *).
Drain and replace fluid after a 2 month storage period. For replacement procedure,
see section 13.4. After replacing fluid, store mattress as described above.
Storage time for empty fresh mattresses in the closed storage box or envelope should
be no more than 3 years.
*) The mattress material is polyurethane plastic. It is slightly permeable for water
and ethanol. Fluid evaporates from the mattress surface if storage box is not closed.
Fluid volume is reduced.
10.3
Transport
TECOTHERM NEO is a light- weight hypothermia system with weight 7,2 kg when the
fluid reservoir is full.
Carry it to move the device over short distances or use an appropriate trolley.
When carrying do not touch or damage display screen.

Page 63 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
SF
11. Alarm system, malfunctions, incident management
Five (5) alarm functions are activated during operations to indicate any malfunction of
the system. Alarms during operations are indicated by an acoustic signal and a
corresponding symbol on the display. The push-button T5 will light up at the same time
and may be used to switch the acoustic alarm to mute, for a period of eight (8) minutes.
Alarm functions are all of medium priority.
No patient- assigned alarms exist.
The alarm “No mains power / No system voltage” has a sound level of dB(A) 63
approximately, the other alarms of dB(A) 57, approximately.
Interruption of power supply does not influence or alter the alarm settings. They are
automatically restored when power is on and alarm cause is persisting.
Assignment of alarm functions to failures and conditions:
System alarm Internal system failure.
Temperature alarm System operation temperature deviation of
more than 0,5°C from set temperature.
No flow No or very restricted circulation of the fluid.
Fluid level low Fluid deficit in the internal fluid container.
AUDIO paused.
No mains power No system voltage due to mains power failure.
Alarm No Mains Power appears when device operation is stopped due to lack of
electrical power. Hence this kind of alarm cannot be displayed at the display screen.
This failure is indicated optically by a separate LED lit indicator (4) at the device lower
front side part, see section 7.3, and by a separate intensive audible alarm. This alarm
can be silenced finally pushing key T5 AUDIO paused.

Page 64 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
No Yes
ERROR
It is not allowed to run the device without working alarm
functions. It will shut off in 30 seconds (press "Confirm" to shut
off immediately).
Confirm
NOTE Whenever an alarm is appearing, first push key T5
to silence audible alarm (AUDIO paused, is
appearing at the display.)
After Switch On of the TECOTHERM NEO the operation readiness of the alarm system
is automatically checked through self- testing.
Display screen is showing the Information
Confirm YES when you see the various symbols and clearly hear the two alarm sounds.
If not press NO. The display then shows a Pop Up window ERROR.
It is not allowed to run the device without working alarm functions. TECOTHERM NEO
will shut off at after 30 seconds.
Push key T3 Confirm to immediately shut off device !
TECOTHERM NEO
A check of the internal functions has been performed and did not
Now the alarm functions will be checked. Can you see the various
Symbols and clearly hear the two alarm sounds?
detect any malfunction.
No Yes
ERROR
It is not allowed to run the device without working alarm
Confirm
functions. It will shut off in 30 seconds (press "Confirm" to shut
off immediately).
SF
TECOTHERM NEO
A check of the internal functions has been performed and did not
detect any malfunction.
Now the alarm functions will be checked. Can you see the various
Symbols and clearly hear the two alarm sounds?
No
Yes

Page 65 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Operator can select at any time during treatment operation an Alarm Check using
MENU. First push key T3 Options and than use arrow keys to highlight this function.
In some critical cases, for instance a potential disruption in normal circulation of the
coolant fluid, there will be not only just the warning symbol shown on the display, but a
detailed text display with instructions will be appearing first. This text field will give
information concerning the nature of the fault, as well as providing instructions as to
what measures need to be taken to eliminate the problem. This text field will not
disappear automatically, even though the AUDIO alarm might have been silenced. It
must be separately closed, using push button T3, thus allowing sufficient time for the
information displayed to be read and understood by the operator.
Note: Prior to closing a Pop Up window the AUDIO alarm must be silenced,
otherwise it will re-appear again and again.
In the same way, detailed information and advice is given, if the system switched back
from automatic to FALLBACK MODE caused by problems with rectal temperature
measurement.
Options during operation
The current operation continues to run.
Highlight and select the desired option:
Changes the current settings
Immediately start the re-warming phase
End treatment and save data
Alarm check
Back
Select
Page 66 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
SF
SF
SF
SF
SF
SF
11.1
System Alarm, System failure
System Alarm is caused by a serious internal failure.
Two (2) SF alarm features are used:
1
Symbol
is displayed at the screen , display feature DIAGRAM,
accompanied by acoustic alarm.
2
System alarm LED (5) in the lower part of the front panel. The
LED pictogram is activated accompanied by acoustical alarm.
Caution System failure will not be reset automatically,
symbol clearly visible remains on display!
Device cannot longer be used in this error state.
System alarm can be released accidently. Symbol is appearing at the screen.
1. Step Unplug mains connector from the device socket at rear side
TECOTHERM NEO is switched OFF.
Wait 5 seconds. Plug mains connector back to the device socket
TECOTHERM NEO is switched ON.
If System alarm disappeared continue treatment.
Note: Previous treatment normally is continued after confirming Yes
at the screen appearing when power returned:
If System alarm remains or is appearing again after some short time
Shut off device by unplugging Mains cable
TECOTHERM NEO
Power failure during treatment - do you wish to continue with
previous treatment?
Confirm to continue with "Yes"
or
Start a new treatment with "No".
No
Yes
We draw your attention to a very rare event
SF
Page 67 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Symbol System Alarm is displayed at the screen.
Caused by Failure in the TECOTHERM NEO system
-
Defective Central Cooling Module
-
Defective pump
-
Temperature exceeding internal limits
-
Communication problems between Operational System and Control system
Measures Step1 has been carried out, but did not solve the problem:
Shut off device by unplugging Mains cable
Or
Push key T3 Options, end treatment and return to the Main MENU.
Pushing key T6 select Power Off and turn off device.
NOTE It is not allowed to Turn On the device again.
Device cannot further be used. Contact your local service provider, for assistance.
If possible Replace TECOTHERM NEO device by a replacement unit or a spare unit.
Put it into operation according IfU.
System Alarm Alarm feature 2
LED (5) in the lower part of the front panel is activated.
In case a serious communication error between Operational Board and subordinated
Control Board emerged, after 10 seconds the Control Board creates an acoustic alarm,
and SF LED (5) is lit. System continues operating with the set parameters unless
operator is silencing audible alarm pushing key T5 AUDIO paused. Then, another 10
seconds later the Control Board will switch off the device.
The operator can now analyse the situation and make an attempt to restore device
operation by turning it ON again.
In any case subsequently contact your service partner.
TECOTHERM NEO Main Menu
Highlight and Select Function Required:
Servo controlled complete treatment mode
Servo control mode (constant rectal temperature)
Constant Mattress Temperature Mode
Alarm check
Display and export of treatment data
Service
Select
Language
Power Off
Ser.-Nr. 2013/38/01
Rev. 060/02.15
SF
SF
SF

Page 68 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
25
15
11.2
Temperature alarm
Temperature alarm
The display changes from Temperature indication LARGE NUMBER to DIAGRAM
feature indicating the alarm symbol. Audible alarm also appears.
Servo controlled complete treatment mode
32.7
Set Point 33.5
Details
Current Rectal Temperature Temperature alarm, alarm paused
Caused by Rectal Temperature probe disconnected from the device or sensor break
Rectal Temperature probe is detached from patient
System operation temperature deviates more than 0,5°C from set
rectal temperature
Mattress temperature incorrectly measured
Fan cooling insufficient
Power of Central Cooling Module insufficient.
NOTE When alarm is appearing push key T5 to silence audible
alarm (AUDIO paused, is appearing at the display.)
There are now 8 minutes to resolve the problem.
Elimination/ Management
Attention Check immediately whether Rectal Temperature probe is correctly
connected to the device and correctly positioned and secured in the
patient.
The operator should check and analyse the temperature profiles in the DIAGRAMM
feature. Look at first the temperatures in the upper part of the display and check
whether indicated temperatures are corresponding to the treatment section temperature
settings and are plausible and make sense.
In all following cases 1 - 5 If possible replace TECOTHERM NEO device with a
replacement unit or a spare unit. Put new system into operation following IfU.
Servo controlled complete treatment mode
Set Temperature
Rectal Temperature
33.5 Mattress Temperature
36.8
32.7 Skin Temperature
-----
Elapsed time of cooling phase 02:34 of 72 hours
23.03.2010 08:55:42
35
35
30
30
25
20
20
15
9
8
7
6
5
4
3
2
1 hour
Options

Page 69 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Case 1 There is no indication of patient rectal temperature, in the
DIAGRAM feature
Device immediately switches to FALLBACK MODE, and first a decision on the future
progression of the mattress temperature is required, see section 6.2.
Measure Check whether Rectal Temperature Probe is plugged correctly to the
socket R. If not plug connector tightly.
If cause is eliminated, rectal temperature reappears in the information box. Observe
display until measured rectal temperature approaches the set rectal temperature.
If no success probe may have a sensor break or is electrically short circuited.
In this event, replace rectal probe with a prescribed rectal temperature probe correctly in
the rectum of the patient, secure it. Plug the probe into socket R . Observe temperature
indicator at the display.
If still no temperature indication appears repeat procedure with another new rectal
probe. If this still fails, turn off the device, see section 8.4.
Contact and inform your local service.
Case 2 All temperatures are indicated. Rectal temperature deviates more than
0,5°C from the set rectal temperature.
Note In treatment sections 2 Cooling phase and 3 Re- warming phase
a maximum deviation of +/- 0,5°C is allowed (alarm limit).
Example Indicated rectal temperature is lower than
set rectal temperature 33,5°C.
Measure Check whether rectal probe is placed in its correct position or slipped out
completely or partially. The more it is slipped out the lower is the detected
and indicated temperature (approaching ambient temperature).
If slipped out place probe into correct position and secure it.
Example Indicated rectal temperature is higher than
set rectal temperature 33,5°C.
Measure Cooling capacity of the device may be reduced.
System is fan cooled. May be free flow of air is restricted.
Check whether device is placed onto a soft layer or pillow or the like,
which restricts free flow of air from the bottom side.
If so place device onto a plane solid support.
Check whether distances to surrounding walls are at least 15 cm!
------
30,5
34,5

Page 70 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Case 3 Check Mattress temperature indication
If mattress temperature is not indicated in the DIAGRAM feature the Control board
cannot regulate to hold rectal temperature constant.
Measure Push key T3 Options, stop treatment. In Main MENU select entry
Power Off using arrow keys and then push T3 Select. Device is shut down.
Do not switch on the device again! Contact your local service.
Case 4 Check ambient conditions
Check whether ambient temperature is too high exceeding 27°C. This can be caused by
an external heat or infra- red radiation source, within or near the incubator.
Remove causes as appropriate.
Observe rectal temperature. It should reach the set temperature after some time.
If measured rectal temperature more and more deviates from the set rectal temperature
the device is defective. Push key T3 Options, stop treatment. In Main MENU select
entry Power Off using arrow keys and then push T3 Select. Device is shut down.
Do not switch on the device again! Contact your local service.
If possible replace TECOTHERM NEO device by a replacement unit or a spare unit. Put
new system into operation following IfU.
Case 5 External heat sources below mattress
Check whether a warming mattress like electrically heated mattresses in incubators is
placed below the mattress.
Remove causes as appropriate.
Observe rectal temperature. It should reach the set temperature after some time.
If measured rectal temperature more and more deviates from the set rectal temperature
the device is defective. Push key T3 Options, stop treatment. In Main MENU select
entry Power Off using arrow keys and then push T3 Select. Device is shut down.
Do not switch on the device again! Contact your local service.
If possible replace TECOTHERM NEO device by a replacement unit or a spare unit. Put
new system into operation following IfU.

Page 71 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
15
0 74 58 42 26 10 6 4 2
1 hour
Options
15
11.3
Flow rate alarm
Flow rate alarm Insufficient circulation of fluid.
Maintenance of the treatment temperature is no longer guaranteed. An acoustic alarm
will sound and a Pop-Up window will inform the operator what actions need to be taken
in order to restore normal circulation.
Example:
Caution: We recommend to first pause the acoustic alarm
by pressing the lit up push button T5.
Please study the instructions for the correction of errors carefully and then close the text
display window. The diagram will reappear and the icons will remind you that although
the acoustic alarm has been switched to mute the error still remains:
You now have a period of 8 minutes to deal with the problem, following which the
acoustic alarm will be activated again. Although the acoustic alarm can be switched
again to mute, the problem nevertheless remains and needs to be resolved. As long as
this state of alarm does exist the unit is no longer able to regulate the patient’s
temperature in the required manner!
Constant Mattress Temperature Mode
Set Temperature
Rectal Temperature
28.0 Mattress Temperature 28.2
34.2 Skin Temperature
-----
Constant mattress temperature for 00:43 hours
23.08.2011 08:55:42
35
35
30
30
25
25
20
20
15
90
74
58
42
26
10
6
4
2
1 hour
Options
34.2
-----
Constant Mattress Temperature Mode
Set Temperature
Rectal Temperature
Mattress Temperature
Skin Temperature
Constant mattress temperature for 00:43 hours
23.08.2011 08:55:42
35
30
25
20
9
ERROR
The total flow is very low. Check connectors. Check hoses, tubing
and mattress for kinking. If nothing is found top up the system with
fluid. Connect the green marked fill-up set connector to the right
refill port. Fill up with 100 ml fluid, then disconnect.
Close
35
30
25
20
15
28.0
28.2

Page 72 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
SF
No or very limited circulation / flow of the fluid
Alarm indicates that for more than 10 seconds mattress gets too little fluid, or flow is
very low due to some blocking, or there is somewhat too little fluid in the mattress.
Possible causes may be
1
Pump is not working or with insufficient power
2
Kinking of hose set and / or tubing near mattress; mattress folded;
couplings disconnected.
3
Flow blocked, small blocking obstacles in the couplings or tubing.
4
Lack of circulating fluid (slight deficit)
There are now 8 minutes to resolve the problem.
Elimination/ Management
Case 1 Pump is defective / not working
System alarm is additionally appearing at the screen. Proceed as described in
section 11.1: Stop treatment, shut off the device.
Push key T3 Options, stop treatment. In Main MENU select entry Power Off using arrow
keys and then push T3 Select. Device is shut down.
Do not switch on the device again!
If possible replace TECOTHERM NEO device by a replacement unit or a spare unit. Put
new system into operation following IfU.
Contact your local service provider for inspection of the defective device.
Case 2 and 3 Inspection for kinking or blocking.
If you observe any kinking of tubing or folding of mattress, eliminate it.
Disconnect all couplings and re-connect them.
Reverse flow direction in hoses and mattress by interchanging the 2 male couplings of
hoses in ports (6).
Replace hoses if indications of flow blocking are found.
If problems remain contact and inform your local service provider.

Page 73 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Case 4 Volume of circulating liquid slightly too little
Lack of fluid is rather small, Low Fluid Level alarm will not yet be activated. Adding
sterile water will restore circulation.
Proceed as follows (see for details section 8.5):
Plug connectors of the fill-up bottle to the refill port QDC counterparts (7) at the device
front face. Lift fill- up bottle and turn it until cap is directed downwards. Observe liquid
level in the refill container. After adding approximately 100 ml disconnect connectors.
Observe whether symbol No Flow disappears.
Should the problem persist, stop treatment and contact your local service provider for
assistance.
If possible: Replace TECOTHERM NEO device by a replacement unit
or a spare unit. Start treatment according IfU.

Page 74 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
11.4
Alarm: Fluid level low see section 11.6, too
Alarm fluid level too low: Little or no fluid in the container.
NOTE When alarm is appearing push key T5 to silence audible
alarm (AUDIO paused, is appearing at the display.)
This alarm indicates a lack of fluid inside the inner container. There are now 8 minutes
to resolve the problem, until AUDIO alarm will re-appear.
Attention Check immediately whether liquid is escaping
or was escaping from the device.
Check immediately whether a leakage is
in the mattress or hoses.
If a big leak or fluid volume is seen below or near the device immediately shut off the
device. Contact your local service provider.
Possible causes for this alarm are
1
Leakage in the TECOTHERM NEO device / Hydraulic Module.
2
Lack of fluid caused by leakage in hoses, at couplings or in mattress.
3
Slow loss of fluid caused by evaporation through mattress surface.
Elimination / Management
If leakage or / and indication of escaped liquid / traces of liquid in your immediate
inspection were not found refill the system. For refilling sterile water follow the
procedure of section 8.5.
Continue treatment following Main MENU instructions.
If alarm persists and symbol is not disappearing a more systematic troubleshooting is
required.
Case 1 Check / inspection of the device
Inspect the support area below the device, device bottom and lower casing parts for
traces and indications of escaping liquid / moisture.
If you find a substantial or medium leak: Immediately Shut Off the device following the
instructions in the Main MENU. Unplug mains. Contact your local service.
If you find only a small leak contact your local service provider for assistance how to
proceed.

Page 75 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Case 2 Check / inspection of mattresses and hoses
Inspect the mattress, hoses, couplings for traces and indications of liquid / moisture.
If you do not find a leakage that could explain the loss of fluid proceed as follows:
Refill Sterile water liquid in accordance with section 8.5.
Symbol
must vanish. Observe the display screen.
Once alarm disappeared, start a systematic inspection and search for leakage. If
leakage is small and no spare components are at hand, try to provisionally seal the leak
as to finish patient treatment:
Close the small leak by means of an adhesive tape or appropriate plasters
(non-permeable). After finishing the treatment, replace the defective part or repair
the mattress using the repair kit. Mattress must be empty and dry.
You can either stop treatment or temporarily continue until the problem is finally
resolved. Contact your local service.
If there is a big leakage, replace this defective component immediately.
Note: If the alarm repeatedly appears, check mattress, hoses, connectors and
tubing
once again to detect if and where fluid escapes permanently. In such cases, see
instructions here in this section 11.4 and 11.6.
After finishing the treatment, replace the defective mattress or repair it using the repair
kit. For this, mattress must be empty and dry.
Attention After refilling, the TECOTHERM NEO is ready for use.
Usually the added fluid has a different temperature than
the system fluid.
Temperature alarm as described in section 11.2 may appear.
We recommend to push key T5 to silence audible
alarm (AUDIO paused, is appearing at the
display.)
Within a few minutes the system will reach the set operation temperature, alarm
symbols will disappear.

Page 76 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
11.5
Alarm: No Mains Power
No system voltage, mains power failure
Accompanying audible alarm with higher sound intensity.
Device operation is stopped, display is dark.
This failure is indicated optically by a separate LED lit indicator (4) at the device lower
front side part, see section 7.3, and by a separate intensive continuous audible alarm.
The alarm can be silenced pushing key T5 AUDIO paused.
Caused by No supply from the mains.
Accidental shut off of the system.
Disconnected mains cable.
Blown fuses.
Internal defect in the TECOTHERM NEO device.
Elimination/ Management
Check whether key (1) is lit slightly green. If not lit TECOTHERM NEO is disconnected
from mains grid.
If only the TECOTHERM NEO device is shut off (and no other equipment in the
room) check that cable cord is tightly plugged to the mains socket and to the device
rear socket. If unplugged, plug it tightly.
Fuses
Check fuses. Fuses are located in a small compartment of the device socket. Unplug
mains cable and pull- out the small fuse compartment. Replace the defective fuses.
Fuse types and ratings are indicated on the device rear plate / type label and in the
Technical Specification at the end of these Instructions for Use.
Note If fuse blowing again appears stop operation. Contact your local service.
Internal defect in the TECOTHERM NEO device
If the Switching Power Supply SPS fails or the SPS is not supplying the internal
operating voltages 5 VDC and 24 VDC, TECOTHERM NEO will stop operation. Display
is not operating (dark). LED Indicator (4) is activated and lit up brightly green, audible
alarm of higher sound intensity is generated.
The alarm can be silenced pushing key T5 AUDIO paused.
To continue treatment is impossible. Contact your local service provider!
If possible: Replace TECOTHERM NEO device by a replacement unit or a spare unit.
Put new system into operation / start operation following individual Instruction for Use.

Page 77 of 88
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Loss of mains power.
For the moment there is nothing to do. Device will continue the interrupted treatment as
soon as power returns.
TECOTHERM NEO is designed to continue operation in the previous treatment mode
when mains voltage reappears. If the interruption period was shorter than 60 minutes
the previous system configuration will be restored and treatment continued.
The operator is asked wheter to continue the interrupted treatment or not, see MENU
screen picture below. If yes, the log files created before the power loss will be
continued, too.
If you wish to continue the interrupted treatment, confirm YES . Otherwise, to start a
new treatment push key T1 NO . With NO you enter the Main MENU, where you can
shut down the device as well.
TECOTHERM NEO
Power failure during treatment - do you wish to continue with
previous treatment?
Confirm to continue with "Yes"
or
Start a new treatment with "No".
No
Yes

Page 78 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
11.5
Fluid escapes from the TECOTHERM NEO System
Also see section 11.4 of this IfU.
Attention Large amount of fluid is observed escaping from
the system.
Possibly alarm Low Fluid Level is appearing because of leakage.
Attention Skin contact with the fluid is harmless.
But do not swallow or ingest fluid!
Case 1 Large amount of fluid escapes from the TECOTHERM NEO device
Management Stop operation by unplugging mains. Do not touch device before!
Caused by Suddenly appearing internal leak in the circulation system.
Fluid is escaping from inside the unit due to defect or leaking parts or components.
Splashes and wetness at, near or below the device.
If possible: Replace TECOTHERM NEO device by a replacement unit or a spare unit.
Put new system into operation following this IfU
Contact your local service provider.
Case 2 Fluid escapes from the mattress or hoses
Management: Stop operation by unplugging mains.
Caused by Suddenly appearing defect or leak in the mattress, along hoses
or in coupling connections.
Wetness in the close vicinity of the patient is possible.
Try to localize the defect or leak.
If you find the defect: Replace defective component. Small leaks may be provisionally
seald as to finish treatment, by means of an adhesive tape or appropriate plasters, see
also section 11.4.
NOTE Possibly you have to refill fluid, see section 8.5.
After replacement or repair, re-connect TECOTHERM NEO device to the mains. It will
automatically resume the treatment with the same settings as before unplugging, see
section 11.5.
Contact your local service provider.

Page 79 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
12. Training & Qualification of personnel
Hypothermia treatment of patients requires skilled personnel.
Operators must ensure they have been adequately trained on treatment operations with
the TECOTHERM NEO system for inducing systemic hypothermia within Intended Use.
They should know how to manage unusual situations, and deal with problems and
malfunctions in treatment operations for inducing systemic hypothermia with the
TECOTHERM NEO system.
Sales partners offer training courses and provide information on necessary software
updates. They also inform on modernizations and technical improvements.
For more information contact your authorized service provider, see section 2 and 15.
13. Service, preventive maintenance, Software Update
13.1
Service & Maintenance
To ensure and maintain a safe and proper long-term operation of the TECOTHERM
NEO equipment regular system inspection by an authorized service provider is
necessary. The inspection has to be done in compliance with current local legal rules
and regulations.
The manufacturer recommends system check and calibration at least every 12 months.
A check of the basic electrical safety must be carried out and documented annually.
Preventive maintenance measures
TECOTHERM NEO hypothermia system requires only few regular service and
maintenance measures.
To sustain its operating readiness and reliable use we recommend some preventive
activities as follows:
13.2
Cleaning the ventilation hole structure (device bottom)
NOTE Inspection of the bottom ventilation holes should be made every
1-2 months. Dust and flues may cover the holes and reduce
cooling capacity. To remove dust and flues, use a small
vacuum cleaner if necessary.
We recommend hygienic cleaning of the bottom every 1-2 months. This
disinfecting cleaning may be performed by wiping with a mild liquid
disinfectant appropriate for medical purposes.

Page 80 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
13.3
Substitution of Sterile Water in the device
The sterile water fluid circulating in the TECOTHERM NEO system should be
substituted, and the system cleansed every 2 months. It is recommended to perform the
cleansing procedure using a Chlorine Dioxide tablet, which is available from Inspiration
Healthcare Limited.
The cleansing procedure is as follows:
Wear disposable gloves and discard them after completing this procedure.
Note: A Chlorine Dioxide tablet 1.5 – 4ppm (1.5 – 4mg/L) is required for this process.
This tablet can be sourced locally or from Inspiration Healthcare.
First drain the liquid from the inner container:
Disconnect hoses
Connect the empty Re-fill bottle instead, with cap upwards
Start mattress mode and wait until all liquid is in the bottle
Ignore (or mute) any flow alarm or fluid level alarm
Stop mattress mode
Disconnect bottle, discard fluid into a sink
Secondly, prepare the cleansing solution:
Pour 450 ml of sterile water into the Re-fill bottle
Add one Chlorine Dioxide tablet 1.5 – 4ppm (1.5 – 4mg/L)
Close bottle cap tightly
Wait until tablet is dissolved completely
This may take half an hour, shaking the bottle will speed up the process
Thirdly, fill up the cleansing solution: (Immediately after the tablet is dissolved)
Connect hoses with mattress to the device
Connect the fill-up bottle to the refill ports at the device front face
Turn bottle until cap is directed downwards and keep in this orientation
Start mattress mode and wait until most of the liquid is transferred into the device
and rising air bubbles in the fill-up bottle disappear completely
Disconnect bottle, discard rest of fluid into a sink
Let the cleansing solution circulate for 10 minutes
Stop mattress mode
After that, drain the cleansing solution from the inner container just as described above.
Finally, fill up the system with fresh sterile water:
Connect hoses with mattress to the device
Pour in 450 ml of sterile water into the Re-fill bottle
Connect the fill-up bottle to the refill ports at the front of the device
Turn bottle until cap is directed downwards and keep in this orientation
Start mattress mode and wait until most of the liquid is transferred into the device
and rising air bubbles in the fill-up bottle disappear completely
Disconnect bottle
Let the sterile water circulate for 5 minutes
Stop mattress mode
Now, the device can be used for another patient or stored up to 2 months.

Page 81 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
13.4
Substitution of Sterile Water in mattresses and cool wraps
Mattresses and Cool Wraps completely or partially filled and stored for a longer time
should be subject to sterile water substitution every 2 months.
Details see sections 8.5 and 8.6.
13.5
Check / calibration of temperature probes
We recommend checking and calibration of the reusable temperature probes within
every 2 years, in accordance with Technical Specification / Manual of the temperature
probe manufacturer.
To ensure proper operation and intended use of the TECOTHERM NEO system,
use only probes authorized by the manufacturer TEC COM GmbH or Inspiration
Healthcare Limited.
If other temperature probes are used, a correct temperature measurement is not
guaranteed. Likely this would put the patient at significant risk!

Page 82 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Export all log data
Back Select
Export all log data
Back Select
13.6
Software Update
Ensure that the TECOTHERM NEO applies latest software versions.
Customers will be informed on software updates by the manufacturer and / or
authorised service provider.
Normally software Update is done by service personnel.
Error in the up date process is indicated at the display screen of the MENU in
a Pop Up window highlighted turquoise, see below.
If possible, additional instructions will be displayed how to further proceed.
Service
Highlight and select service option
Update
Change password
ERROR
Software could not be updated (error 3). The system is in an
undefined state. Call Service.
Confirm
Sometimes there may be an only partial update. TECOTHERM NEO system then will
reboot. This is not an error, but only an information that it was intended to update only
some components of the software, not all.
Service
Highlight and select service option
Update
Change password
SUCCESS
WARNING: Software was updated only partially. The system
will reboot now.
Confirm
If necessary customer should consult the TECOTHERM NEO manufacturer TEC COM
GmbH or Authorized Service Representative for assistance and support.

Page 83 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
automatically
automatically
manually
Temperature Constancy
Hydraulic Circulation System
System pressure max.
Flow rate without / with mattress
Internal Fluid reservoir capacity
Circulating Fluid
Connectors / Couplings
Fill Up / Refill
Electrical Parameters
Supply Voltage / Mains
Power consumption
Fuses (2 pieces)
1. Microcomputer: hardware control
2. Microcomputer: MENU system
Servo Control Complete Treatment
Servo Control Constant Rectal Temperature
Constant Mattress Temperature
0,3 °C
< 400 µA
2,5 m with hospital grade plug
+ 10°C
+ 41°C
+ 12°C
+ 39°C
optical and audible alarms
Sound pressure level approx. 63 dB(A)
Sound pressure level approx. 57 dB(A)
Sound pressure level approx. 57 dB(A)
Sound pressure level approx. 57 dB(A)
Sound pressure level approx. 57 dB(A)
DIN EN 60601-1, DIN EN 60601-1-2, DIN EN
60601-1-6, DIN EN 60601-1-8, DIN EN 60601-1-10
DIN EN 60601-2-35 E/F
0494
14. Technical Data / TECOTHERM NEO Specification
Device of Class I for use with shockproof mains sockets
Class of risk IIb acc. ann. IX of Medical Device Directive 93/42/EEC.
Type BF applied parts
IP 20
Class 1, Risk Class II b, Type BF
System safety
Protection class
Standards
Ambient conditions
Operation / Treatment A m b i e n t Temperatures + 5 °C to + 27 °C
Operation / Treatment Relative Humidity 10% to 75%, not condensating
Alarm No Mains
Alarm System Failure
Alarm No or restricted flow
Alarm Low fluid level
Alarm Temperature deviation
0,5 bar
500 ml / min (shorted) / up to 300 in use
approx. 250 ml
Sterile Water
Quick Disconnect Couplings
Fill Up Set
100-130V and 200-240V, 50-60 Hz
max. 350 W
5x20mm, 250VAC, slow, high breaking capacity
at 100-130V: S 4A H, at 200-240V: S 2,5A H
Earth Leakage Current
Mains Power Cord
Patient Safety / Alarms
Lower Temperature alarm limit
Upper Temperature alarm limit
Set Temperatures, lower limit
Set Temperatures, upper limit
Alarm System, 5 Channels, Blink LED
Rectal BCT + 30°C to + 38°C
Patient weight max.
50 kg
Control Systems
Control System
Operating System
MainTreatment Modes
Mattress + 12°C to + 39°C
Treatment temperature control ranges
Cooling & Warming
375 x 190 / 215 x 310 mm (W x H x D)
approx. 7,2 kg
Thermoelectrically based module
Options
Dimensions
Weight without accessories
Central Cooling Module
Certificate

Page 84 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
15. Declaration of Conformity
Manufacturer: TEC COM GmbH
Address: Am Krümmling 1
D-06184 Kabelsketal, Germany
The manufacturer, using a Production Quality Management System certified according
EN ISO 13485:2012 + AC:2012 (ISO 13485:2003 + Cor 1:2009) herewith declares that
each actual device TECOTHERM NEO complies to the requirements of
Medical Device Directive 93/42/EEC
Safety, general EN 60601-1: 2007
Electromagnetic Compatibility EN 60601-1-2: 2007
Usability EN 60601-1-6: 06.2008
Alarm systems EN 60601-1-8: 09.2008
Physiologic closed loop systems PCLS EN 60601-1-10:2008
Safety of mattresses etc EN 60601-2-35: 2009:10 E/F
Restriction of Hazardous Substances Directive 2011/65/EU
The general requirements of Medical Device Directive 93/42/ EEC are fulfilled acc.
ann. I Essential Requirements
ann. III EC type examination.
Device Code UMDNS: 12-068. In accordance with MDD 93/42/EEC (as amended),
Annex lX, Rule 9, the device is Class llb.
Each device is produced in full compliance with the technical documentation underlying
the EC type examination procedures.
Place, date Kabelsketal, November 2015
Signature
Manufacturer
TEC COM GmbH
Gesellschaft für Technik,
Technologie und Vermarktung
Am Krümmling 1
06184 Kabelsketal, Germany
Tel.: +49 (0)345 / 120 52 04
Fax: +49 (0)345 / 120 52 11
E Mail inf[email protected]e
16. Disposal
This device must not be disposed of as common industrial or household
waste. It must be delivered to a local regular collecting point, to a waste
disposal company or returned to the distributor or the manufacturer.
Inspiration Healthcare Ltd.
Gildor House
West Street
Earl Shilton
Leicester
Leicestershire LE9 7EJ
UK
Tel: + 44(0)1455 840555
Fax: + 44(0)1455 841464
E Mail: inf[email protected]

Page 85 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
17. EMC guidance for TECOTHERM NEO
Guidance and manufacturer’s declaration – electromagnetic emissions
The TECOTHERM NEO is intended for use in the electromagnetic environment specified below. The customer or
the user of the TECOTHERM NEO should assure that it is used in such an environment.
Emissions test
Compliance
Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The TECOTHERM NEO uses RF energy only for its
internal function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby
electronic equipment.
RF emissions
CISPR 11
Class A
The TECOTHERM NEO is suitable for use in all
establishments other than domestic and those directly
connected to the public low-voltage power supply network
that supplies buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations /
flicker emissions
IEC 61000-3-3
Complies
Guidance and manufacturer’s declaration – electromagnetic immunity
The TECOTHERM NEO is intended for use in the electromagnetic environment specified below. The customer or
the user of the TECOTHERM NEO should assure that it is used in such an environment.
Immunity test
IEC 60601 test
level
Compliance level
Electromagnetic environment
- guidance -
Electrostatic
± 6 kV contact
± 6 kV contact
Floors should be wood, concrete or
discharge
discharge
discharge
ceramic tile. If floors are covered with
(ESD)
synthetic material, the relative humidity
IEC 61000-4-2
± 8 kV air discharge
± 8 kV air discharge
should be at least 30 %.
Electrical fast
± 2 kV for power
± 2 kV for power
transient / burst
supply
supply
Mains power quality should be that of a
IEC 61000-4-4
lines
line
typical commercial or hospital
± 1 kV for input/output
Not applicable,
environment.
lines
because not present.
Surge
± 1 kV
± 1 kV
IEC 61000-4-5
differential mode
differential mode
Mains power quality should be that of a
± 2 kV
± 2 kV
typical commercial or hospital
common mode
common mode
environment.
Voltage dips,
< 5 % U
T
< 5 % U
T
Mains power quality should be that of a
short interruptions
(> 95 % dip in U
T
)
(> 95 % dip in U
T
)
typical commercial or hospital
and voltage variations
for ½ cycle
for ½ cycle
environment. If the user of the
on power supply
TECOTHERM NEO requires continued
input lines
40 % U
T
40 % U
T
operation during power mains
IEC 61000-4-11
(60 % dip in U
T
)
(60 % dip in U
T
)
interruptions, it is recommended that the
for 5 cycles
for 5 cycles
TECOTHERM NEO be powered from a
non- interruptible power supply or a
70 % U
T
70 % U
T
battery.
(30 % dip in U
T
)
(30 % dip in U
T
)
for 25 cycles
for 25 cycles
< 5 % U
T
< 5 % U
T
(> 95 % dip in U
T
)
(> 95 % dip in U
T
)
for 5 sec
for 5 sec
Power frequency
3 A/m
3 A/m
Power frequency magnetic fields should
(50 Hz)
be at levels characteristic of a typical
magnetic field
location in a typical commercial or
IEC 61000-4-8
hospital environment.
NOTE: U
T
is the a.c. mains voltage prior to application of the test level.

Page 86 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Guidance and manufacturer’s declaration – electromagnetic immunity
The TECOTHERM NEO is intended for use in the electromagnetic environment specified below. The customer or
the user of the TECOTHERM NEO should assure that it is used in such an environment.
Immunity test
IEC 60601 test
level
Compliance level
Electromagnetic environment
- guidance -
Portable and mobile RF communications
equipment should be used no closer to any
part of the TECOTHERM NEO, including
cables, than the recommended separation
distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance:
Conducted RF
3 Vrms
3 V
d = 1,2 √ P
IEC 61000-4-6
150 kHz to 80 MHz
d = 1,2 √ P 80 MHz to 800 MHz
Radiated RF
3 V/m
3 V/m
IEC 61000-4-3
80 MHz to 2,5 GHz
d = 2,3 √ P 800 MHz to 2,5 GHz
where P is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in metres
(m).
Field strength from fixed RF transmitters as
determined by an electromagnetic site
survey,
a
should be less than the compliance
level in each frequency range.
b
Interference may occur in the vicinity of
equipment marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site
survey should be considered. If the measured field strength in the location the TECOTHERM NEO is used
exceeds the applicable RF compliance level above, the TECOTHERM NEO should be observed to verify normal
operation. If abnormal performance is observed additional measures may be necessary, such as re-orienting or
relocating TECOTHERM NEO.
b Over the frequency range 150 kHz to 80 MHz field strength should be less than 3 V/m..
Note on Radiated RF: Interference field strength more than 3 V/m may affect the “Rectal Temperature
control” by causing erroneous Rectal Temperature measurements. However, TECOTHERM NEO is safe
up to 10 V/m.

Page 87 of 87
Ref No. TN300 EN-18
IfU TECOTHERM NEO TN300 EN-18.docx
Recommended separation distances between portable and mobile RF communications
equipment and the TECOTHERM NEO
The TECOTHERM NEO is intended for use in an electromagnetic environment in which radiated RF disturbances
are controlled. Customer or user of the TECOTHERM NEO can help to prevent electromagnetic interference by
maintaining at least minimum distance between portable and mobile RF communications equipment (transmitters)
and TECOTHERM NEO as recommended below, according to the maximum output power of the communications
equipment.
Rated maximum
output power of
transmitter
(W)
Separation distance according to frequency of transmitter
( m )
150 kHz to 80 MHz
d = 1,2 √ P
80 MHz to 800 MHz
d = 1,2 √ P
800 MHz to 2,5 GHz
d = 2,3 √ P
0,01
0,12
0,12
0,23
0,1
0,38
0,38
0,73
1
1,2
1,2
2,3
10
3,8
3,8
7,3
100
12
12
23
For transmitters rated at a maximum output power not listed above the recommended separation distance d in
metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
