
1
Electrolytes: Enteral and Intravenous – Adult – Inpatient
Clinical Practice Guideline
Note: Active Table of Contents – Click each header below to jump to the section of interest
Table of Contents
INTRODUCTION .................................................................................................................................. 6
DEFINITIONS ....................................................................................................................................... 6
RECOMMENDATIONS ........................................................................................................................ 7
1. POTASSIUM (K
+
) ............................................................................................................................. 7
2. PHOSPHATE (PO
4
3-
) ........................................................................................................................ 9
3. MAGNESIUM (MG
2+
) ...................................................................................................................... 10
4. CALCIUM (CA
2+
) ............................................................................................................................ 12
5. SLIDING SCALE ELECTROLYTES ............................................................................................... 14
METHODOLOGY ............................................................................................................................... 15
COLLATERAL TOOLS & RESOURCES ........................................................................................... 17
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

2
Content Expert:
Name: Philip Trapskin, PharmD, BCPS – Department of Pharmacy
Phone Number: (608) 263-1328
Email Address: ptrapskin@uwhealth.org
Contact for Changes:
Name: Philip Trapskin, PharmD, BCPS – Department of Pharmacy
Phone Number: (608) 263-1328
Email Address: ptrapskin@uwhealth.org
Guideline Authors:
Emily Jackson, PharmD
Sara Shull, PharmD, BCPS
Joshua Vanderloo, PharmD, BCPS
Original authors: Lindsey Goldsmith, PharmD; Gordon Sacks, PharmD
Reviewers:
Pierre Kory, MD – Critical Care
Kenneth Kudsk, MD – General Surgery
Edward Lalik, MD – Hospitalist
Joshua Medow, MD – Neurological Surgery
Anne O’Connor, MD – Cardiovascular Medicine
David Yang, MD – Medical Director of UW Health Clinical Laboratories
Theodore Berei, PharmD, BCPS – Cardiovascular Medicine
Caitlin Curtis, PharmD, BCNSP – Nutrition Support
Jeff Fish, PharmD, BCPS – Critical Care
Marie Pietruszka, PharmD, BCPS – General Medicine
Committee Approvals:
Pharmacy and Therapeutics Committee – December 2017
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017
3
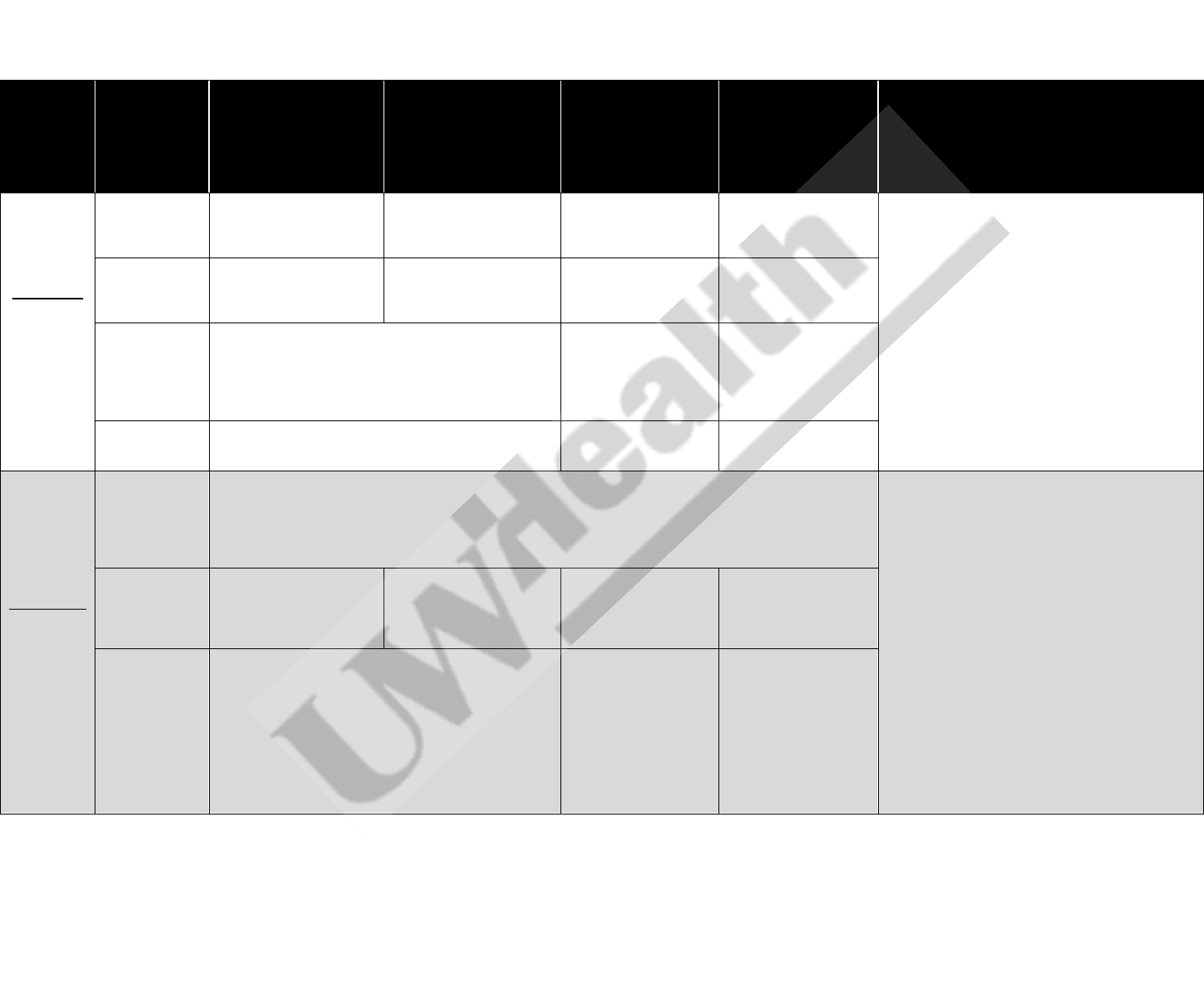
Table 1. UW Health Guidelines for the Use of Oral, Enteral, and Intravenous Electrolytes in Adults
1-34
Electrolyte
Concentration
Oral
Gastric (NG/OG/PEG)
For patients with
enteral access in small
bowel, IV preferred due
to adverse GI effects
Standard Adult IV
dose
High-risk
A
Adult IV
dose
Notes
Potassium
Normal
reference:
3.5-5.1
mmol/L
3.6-3.9 mmol/L
20 mEq potassium
chloride
caps/tabs/powder
20 mEq potassium
chloride powder packets
(dilute in ~100 mL per
packet, see note 3)
Use oral/enteral
supplementation
20 mEq (see note 4)
1. If CrCl < 30 mL/min, reduce dose by 50%
2. For oral doses >20 mEq, divide into
increments of 20 mEq given every 2 hours
3. If patient is fluid restricted, dilute oral powder
in 50 mL
4. Intravenous infusion rate:
Peripheral line: max 10 mEq/hour
Central line: max 20 mEq/hour
3.1-3.5 mmol/L
40 mEq potassium
chloride
caps/tabs/powder
(see note 2)
40 mEq potassium
chloride powder packets
(dilute in ~100 mL per
packet, see note 3)
Consider oral/enteral
repletion; 40 mEq
(see note 4)
40 mEq (see note 4)
2.5-3.0 mmol/L
If asymptomatic: may consider combination of enteral
and IV repletion with 20 mEq oral potassium chloride
caps/tabs/powder and 40 mEq IV (see note 4)
If symptomatic: IV repletion recommended (see IV
dose columns)
60 mEq (see note 4)
60 mEq (see note 4)
< 2.5 mmol/L
IV repletion recommended (see IV dose columns)
80 mEq (see note 4)
80 mEq (see note 4)
Magnesium
Normal
reference:
1.6-2.6
mg/dL
1.5-1.8 mg/dL
Consider no replacement, except in patients admitted on cardiac units, who have had recent cardiac
surgery, or who have cardiac disorders, including arrhythmias, prolonged QTc, and digitalis toxicity, or in
patients with eclampsia or pre-eclampsia
In these patient populations, consider 0.05 g/kg IV
(see note 8, 9, 10)
5. If CrCl < 30 mL/min, reduce dose by 50%
6. If CrCl < 30 mL/min and using magnesium
sulfate solution:
Magnesium 1.5-1.8 mg/dL: Magnesium
sulfate solution 2000 mg (dilute in ~50
mL) x 1 dose
Magnesium 1.1-1.4 mg/dL: Magnesium
sulfate solution 2000 mg (dilute in ~50
mL) every 4 hours x 3 doses
7. See Table 2 for alternative product contents
8. For IV dosing, use actual body weight
unless actual is >130% ideal body weight; in
these cases, use ideal body weight
9. Maximum IV dose is 8g/day
10. Intravenous infusion rate
Infuse doses of ≤ 0.05 g/kg over 12
hours or over 24 hours for supplements
>0.05 g/kg
Maximum infusion rate is 0.5 to 1 g/hr
1.0-1.4 mg/dL
Magnesium oxide
elemental tablets 500 mg
BID x 2 doses
Magnesium sulfate
solution 2000 mg (dilute
in ~50 mL) every 4 hours
x 5 doses
0.05 g/kg
(see note 8, 9, 10)
0.1 g/kg
(see note 8, 9, 10)
< 1.0 mg/dL
IV repletion recommended (see IV dose columns)
0.1 g/kg
(see note 8, 9, 10)
0.15 g/kg
(see note 8, 9, 10)
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

4
Electrolyte
Concentration
Oral
Gastric (NG/OG/PEG)
For patients with
enteral access in small
bowel, IV preferred due
to adverse GI effects
Standard Adult IV
dose
High-risk
A
Adult IV
dose
Notes
Phosphate
Normal
reference:
2.3-4.7
mg/dL
2.4-3.0 mg/dL
Phosphate-potassium
packet (PHOS-NAK
powder) 1 packet every
4 hours while awake x 3
doses
B,C
Phosphate-potassium
packet (PHOS-NAK
powder) 1 packet every
4 hours while awake x 3
doses (dilute in ~75
mL)
B,C
Consider no
replacement
C
or use
oral/enteral
supplementation
0.16 mmol/kg (see
notes 15 to 18),
consider oral/enteral
supplementation
11. Consider oral/enteral supplementation in
any asymptomatic patient, or combination of
oral/enteral and IV.
12. If CrCl <30 mL/min, reduce IV dose by 50%
13. If CrCl <30 mL/min, use of phosphorus
tablet (K-PHOS Neutral) preferred due to
lower potassium content:
Phosphate 2.4-3.0 mg/dL: 1 tablet
every 4 hours while awake x 2 doses
Phosphate 1.6-2.3 mg/dL: 1 tablet
every 4 hours while awake x 3 doses
Phosphate 1.0-1.5 mg/dL: 1 tablet
every 4 hours while awake x 4 doses
14. See Table 2 for alternative product content
15. For IV dosing, use actual body weight
unless actual is >130% ideal body weight; in
these cases, use ideal body weight
16. Maximum IV dose is 45 mmol; recheck
phosphate level 6 to 12 hours after dose to
determine if further supplementation is
necessary
17. Administer IV dose over 2 to 3 hours for mild
or moderate hypophosphatemia and over 6
to 8 hours for severe hypophosphatemia
18. Round IV supplementation to the nearest
7.5 or 15 mmol increment
1.6-2.3 mg/dL
Phosphate-potassium
packet (PHOS-NAK
powder) 2 (two) packets
every 4 hours while
awake x 3 doses
B
Phosphate-potassium
packet (PHOS-NAK
powder) 2 (two) packets
every 4 hours while
awake x 3 doses (dilute
in ~75 mL)
B
0.16 mmol/kg (see
notes 15 to 18),
consider oral/enteral
supplementation
0.32 mmol/kg (see
notes 15 to 18),
consider oral/enteral
supplementation
1.0-1.5 mg/dL
Phosphorus TABLET (K-
PHOS Neutral) 2 (two)
tablets every 4 hours
while awake x 4 doses
B
Phosphorus TABLET (K-
PHOS Neutral) 2 (two)
tablets every 4 hours
(crush & dilute in ~75
mL)
B
0.32 mmol/kg (see
notes 15 to 18),
consider oral/enteral
supplementation
15 mmol IV once over
2 hours, then 0.32
mmol/kg (see notes
15 to 18)
< 1.0 mg/dL
IV repletion recommended (see IV dose columns)
0.64 mmol/kg (see
notes 15 to 18)
15 mmol IV once over
2 hours, then 0.64
mmol/kg (see notes
15 to 18)
Ionized
Calcium
Normal
reference:
Serum: 4.6-
5.2 mg/dL
Whole
blood: 4.9-
5.6 mg/dL
Serum:≤4.59
mg/dL
Whole blood:
≤4.89 mg/dL
Calcium carbonate chew
tabs 1000 mg every 4
hours x 4 doses
Calcium carbonate
suspension 1250 mg
every 4 hours x 4 doses
Consider oral/enteral replacement if
asymptomatic
D
2 g calcium gluconate or 1 g calcium
chloride (optimally infused over 2 hours)
19. Ionized (unbound) calcium concentrations
are preferred over total serum calcium
concentrations for hypocalcemia monitoring
20. Use oral/enteral calcium for asymptomatic
hypocalcemia ONLY
21. Maximal oral calcium absorption occurs at
elemental doses ≤500 mg and when given
with a meal
22. For IV administration, calcium gluconate
preferred due to extravasation risk with
calcium chloride
23. See Table 2 for alternative product contents
A
High-risk: clinically malnourished, active alcoholism, recent surgery, admitted for cardiac disease, graft-versus-host disease, diabetic ketoacidosis, or undergoing diuresis
B
Patients with hyperkalemia, impaired renal function, or that require larger doses of phosphate replacement should receive replacement using the phosphorus tablet (K-PHOS Neutral) as it
contains less potassium than the phosphate-potassium packet (PHOS-NAK powder)
C
Replete if: active alcoholism, malnourished, liver cirrhosis, critical status, hepatectomy, parenteral nutrition, or burn injury if benefit outweighs risk
D
Symptoms of hypocalcemia: tetany, numbness in extremities, stridor, mental status changes, hypotension
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

5
Table 2. Available oral, enteral, and intravenous electrolyte products at UW Health
Oral
Intravenous
Notes
Product
Elemental Content
Magnesium
Magnesium oxide elemental 250 mg tablets
250 mg
Magnesium Sulfate:
1 g /100 mL
2 g /50 mL
4 g /100 mL
Magnesium sulfate 1 g = 8 mEq = 98.6 mg
elemental magnesium
Magnesium sulfate 1 gram
98 mg
Calcium
Calcium carbonate chew tabs 500 mg
200 mg per tab
Calcium gluconate (preferred):
1 g/100 mL normal saline
2 g/100 mL normal saline
Calcium gluconate 1 g = 93 mg of
elemental calcium
Preferred due to lower extravasation risk
Calcium carbonate suspension 1250 mg/5
mL
500 mg per 5 mL
Calcium Chloride:
1 g/100 mL normal saline
2 g/100 mL normal saline
Calcium chloride 1g = 273 mg elemental
calcium
Phosphate
Phosphorus TABLET (K-PHOS Neutral)
8 mmol
phosphorus
1.1 mEq
potassium
13 mEq
sodium
Phosphate Sodium:
15 mM PO
4
/20 mEq Na/100 mL
Phosphate-potassium packet (PHOS-NAK
powder)
8 mmol
phosphorus
7.1 mEq
potassium
7.1 mEq
sodium
Phosphate Potassium:
7.5 mM PO4 /11 mEq K/100 mL
15 mM PO4 /22 mEq K/100 mL
(central line recommended)
Peripheral access may be used for IV
administration in high-risk patients when
benefit outweighs the risk
Potassium
Oral packet
20 mEq
Potassium Chloride
10 mEq/100 mL
20 mEq/100 mL
(central line recommended)
Peripheral access may be used for IV
administration in high-risk patients when
benefit outweighs the risk
Oral liquid (contains sorbitol)
40 mEq/15 mL (20%)
Oral solution
13.3 mEq/5 mL (20%)
Phosphate (Potassium)
7.5mM PO
4
/11 mEq K/100 mL
15 mM PO
4
/22 mEq K/100 mL
(central line recommended)
Extended-release oral tablet
10 mEq; 20 mEq
Potassium Acetate
10 mEq/100 mL
20 mEq/100 mL (central line
recommended)
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

6
Introduction
Significant electrolyte depletion can result in serious complications for patients. Intravenous electrolyte
replacement can produce life-threatening complications, serious arrhythmias and phlebitis; therefore
supplementation must be carefully monitored.
2
There are multiple underlying factors for electrolyte disorders in adult inpatients. Alterations in absorption,
distribution, hormonal, and/or homeostatic mechanisms can all cause disturbances. Therefore, treating the
underlying cause and prescribing adequate therapy is essential for repletion. In addition, the intracellular vs.
extracellular electrolyte concentrations must be considered. Due to distribution variances, labs may not
directly correlate with true electrolyte level. Therefore, continuous monitoring is essential to properly replete
patients.
18
Scope
Intended Users: Physicians, advanced practice providers, nurses, pharmacists
Objectives: To identify the appropriate patients requiring intravenous, oral, or enteral electrolyte replacement,
to recommend appropriate treatment, and to minimize inappropriate use of electrolyte replacement.
Target Population: Adult inpatients with a clinical condition warranting oral, enteral, or intravenous electrolyte
replacement therapy. This document does not pertain to parenteral nutrition (PN).
Clinical Questions Considered:
How to provide clinically appropriate oral, enteral, and IV electrolyte supplementation (potassium,
phosphate, magnesium, calcium) in adult inpatients?
What patient populations are appropriate for oral and enteral electrolyte supplementation?
What patient populations are appropriate for intravenous electrolyte supplementation?
Definitions
Ideal Body Weight (>60 inches) = 50* kg + [2.3 x (height (inches) – 60)]
Ideal Body Weight (<60 inches) = 50* kg + [- 2.3 x (60 – height (inches))]
o *Use 45.5 kg for females
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

7
Recommendations
1. Potassium (K
+
)
Potassium is mainly found in the intracellular space (98%) versus extracellular space (2%). Some physiologic
functions of this cation include: cellular metabolism, protein synthesis and regulation of action potentials across
cell membranes. Entry of potassium into cells is dependent on the sodium-potassium ATPase pump. This
pump is regulated by many factors including insulin, glucagon, catecholamines, and acid-base balance.
18,35
Mild to-moderate hypokalemia can increase the likelihood of cardiac arrhythmias in patients with cardiac
ischemia, heart failure, or left ventricular hypertrophy.
1,3
Hypokalemia can have profound effects on the
excitability of electrical tissues in cardiac, skeletal, and smooth muscle which in some patients can lead to
cardiac arrhythmias, muscular paralysis, or respiratory failure.
3,35
1.1. Dosing recommendations for patients with normal renal function (>30 mL/min) are listed in Table 1. (UW
Health GRADE High quality evidence, strong recommendation)
1.2. Current literature does not support supplementing normal electrolyte levels in adult patients who are not
classified as high risk. (UW Health GRADE Low quality evidence, strong recommendation)
1.3. Oral and Enteral Potassium Replacement, Administration, and Monitoring
1.3.1. If creatinine clearance is <30 mL/min, administer approximately 50% of normally recommended
doses. Use caution in patients with anuria and patients with ESRD receiving dialysis.
4
(UW
Health GRADE Moderate quality evidence, strong recommendation)
1.3.2. Oral doses greater than 20 mEq should be given in divided doses every two to twelve hours to
increase tolerability and decrease gastrointestinal discomfort.
36,37
(UW Health GRADE Moderate
quality evidence, strong recommendation)
1.3.3. Higher doses may be required for patients with ongoing or chronic causes of potassium loss.
1-4
(UW Health GRADE Moderate quality evidence, strong recommendation)
1.3.4. Very low serum potassium concentrations can reflect a large total body deficit of both intracellular
and extracellular potassium so higher doses may be required.
1
(UW Health GRADE Low quality
evidence, strong recommendation)
1.3.5. Higher serum potassium concentrations are targeted in patients with cardiac disease.
2
(UW
Health GRADE Moderate quality evidence, strong recommendation)
1.3.6. Potassium requirements can be much greater in patients on loop or thiazide diuretic therapy (e.g.,
furosemide, bumetanide, hydrochlorothiazide, chlorothiazide).
38
(UW Health GRADE Moderate
quality evidence, strong recommendation)
1.3.7. Maintain appropriate magnesium serum concentrations with potassium supplementation since
magnesium is an important cofactor for potassium uptake.
2
(UW Health GRADE Moderate quality
evidence, strong recommendation)
1.3.8. Potassium replacement utilizing potassium elixirs should be avoided, and should instead be
achieved by utilizing potassium powder packets or tablets swallowed whole or dissolved in
approximately 100 mL of water per tablet or packet prior to oral administration, especially when
delivered to the small bowel due to the risk of hyperosmolar-induced diarrhea and/or abdominal
pain.
36,37
(UW Health GRADE Moderate quality evidence, strong recommendation)
1.3.8.1. Patients on fluid restrictions should receive dissolved potassium supplementation in
approximately 50 mL of water per tablet or packet.
36,37
(UW Health GRADE Moderate
quality evidence, strong recommendation)
1.3.9. The appropriate route of administration should be chosen based on availability and tolerability:
(UW Health GRADE Low quality evidence, strong recommendation)
Oral - Use capsules or tablet formulation. Consider powder packet if patient is unable to
swallow tablets.
Gastric (Nasogastric tube, Orogastric tube, or Percutaneous Endoscopic Gastric tube) - Use
powder packet.
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

8
Small Bowel (Nasoduodenal tube, Nasojejunal tube, or jejunostomy tube) – IV or diluted oral
powder recommended.
1.3.10. In cases of severe hypokalemia, serum potassium levels may be evaluated every two to six hours
to ascertain response to therapy.
4,39
(UW Health GRADE Moderate quality evidence,
weak/conditional recommendation)
1.4. Intravenous Potassium Replacement, Administration, and Monitoring
1.4.1. Very low potassium concentrations can reflect a large total body deficit of both intracellular and
extracellular potassium so higher doses may be required.
1
(UW Health GRADE Low quality
evidence, strong recommendation)
1.4.2. Decrease supplemental potassium doses by 50% in patients with a creatinine clearances <30
mL/min.
3,4,11,40,41
(UW Health GRADE Moderate quality evidence, strong recommendation)
1.4.3. Modest bolus doses of potassium given over 1 hour, 10-20 mEq for potassium levels 2.5-3.4
mmol/L should be considered a starting point in anephric or hemodialysis-dependent
patients.
3,4,11,40,41
(UW Health GRADE Moderate quality evidence, strong recommendation)
1.4.4. Potassium concentrations greater than 3.5 mmol/L are targeted in patients with cardiac
disease.
2,41
(UW Health GRADE Moderate quality evidence, strong recommendation)
1.4.4.1. In a prospective, observational, case-control, multi-center study, serum potassium levels
<3.5 mmol/L was a predictor for serious perioperative (odds ratio [OR] 2.2, 95%
confidence interval [CI] 1.2-4.0) intraoperative (OR 2.0; 95% CI 1.0-3.6), and
postoperative arrhythmias (OR 1.7; 95% CI, 1.0-2.7). In total, 2402 patients were
included in the two-year study period and were undergoing elective coronary artery
bypass graft surgery. Some limitations of this study were small sample size (10000
needed to show statistical significance between hypokalemia and death), as well as not
accounting for other electrolyte therapies (including magnesium). Authors concluded
that potassium repletion for those undergoing a cardiac procedure was a low-risk, low-
cost, and beneficial option. Therefore, UW Health Guidelines supports targeting levels >
3.5 mmol/L for patients with cardiac disease.
1.4.5. Potassium requirements can be much greater in patients on loop or thiazide diuretic therapy (e.g.,
furosemide, bumetanide, hydrochlorothiazide, chlorothiazide).
9
(UW Health GRADE Low quality
evidence, strong recommendation)
1.4.6. Maintain normal magnesium concentrations with potassium supplementation since magnesium is
an important cofactor for potassium uptake.
2
(UW Health GRADE Moderate quality evidence,
strong recommendation)
1.4.7. Never administer potassium IV push.
3
(UW Health GRADE Moderate quality evidence, strong
recommendation)
1.4.8. Administer parenteral potassium either slowly by adding it to maintenance fluids or over a shorter
period via intermittent infusion depending on the degree of hypokalemia.
4
(UW Health GRADE
Low quality evidence, weak/conditional recommendation)
1.4.9. An infusion pump is required for all one liter continuous infusions bags containing potassium 40
mEq or more.
4
(UW Health GRADE Low quality evidence, strong recommendation)
1.4.10. Infusion rates faster than 10 mEq/hour require a smart infusion pump.
3
(UW Health GRADE Low
quality evidence, strong recommendation)
1.4.11. The amount of potassium in any liter of fluid for either peripheral or central administration should
not exceed 88 mEq (except in parenteral nutrition formulations).
2,3
(UW Health GRADE Moderate
quality evidence, strong recommendation)
1.4.12. Peripheral Administration
1.4.12.1. Rate of peripheral administration should rarely exceed 10 mEq/hour. In extreme
circumstances, potassium may be peripherally administered at 20 mEq/hour.
3
(UW
Health GRADE Moderate quality evidence, weak/conditional recommendation)
1.4.12.2. The addition of lidocaine to potassium chloride infusions to decrease pain from
peripheral vein irritation is not recommended due to limited evidence of safety or
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

9
efficacy.
42-45
(UW Health GRADE Moderate quality evidence, weak/conditional
recommendation)
1.4.12.2.1. Potential adverse drug events with the use of lidocaine include, but are not
limited to: numbness and tingling in the fingers and toes, unusual sensations
around the mouth, metallic taste, tinnitus, lightheadedness, and/or
dizziness.
42-44
1.4.12.2.2. The addition of lidocaine to potassium chloride may also mask pain reducing
the ability to detect an extravasation event.
1.4.12.3. To minimize irritation associated with potassium chloride, it is recommended to
administer via Y-site with intravenous maintenance fluids whenever feasible. (UW Health
GRADE Low quality evidence, strong recommendation)
1.4.13. Central Administration
1.4.13.1. Administer IV supplemental bolus potassium via the central route at concentrations less
than 0.2 mEq/mL to minimize concentrated potassium boluses into the heart.
3
(UW
Health GRADE Moderate quality evidence, strong recommendation)
1.4.13.1.1. Administering potassium boluses at higher concentrations may lead to
serious, life-threatening arrhythmias.
2,3
(UW Health GRADE Moderate
quality evidence, strong recommendation)
1.4.13.2. The maximum central line infusion rate should not exceed 20 mEq/hour unless the
patient is experiencing a life-threatening condition due to hypokalemia. Faster infusions
can result in cardiac arrhythmias and death.
3
(UW Health GRADE Moderate quality
evidence, strong recommendation)
1.4.14. Infusion rates ≥20 mEq/hour require telemetry.
3
(UW Health GRADE Low quality evidence, strong
recommendation)
1.4.15. Potassium levels are required following supplementation, especially if correcting severe
hypokalemia (serum potassium ≤2.5 mmol/L) or in patients with multiple medical problems.
2
(UW
Health GRADE Moderate quality evidence, strong recommendation)
1.4.16. In cases of severe hypokalemia, potassium levels are recommended every two to six hours
initially to ascertain response to therapy.
4,39
(UW Health GRADE Low quality evidence,
weak/conditional recommendation)
2. Phosphate (PO
4
3-
)
Phosphate is the primary anion within the intracellular space. Functions of this electrolyte include: nerve and
muscle conduction, ATP production, glucose utilization, and glycolysis. Since critically ill patients are often
hypermetabolic, phosphate requirements and therefore supplementation can be higher than adult patients
admitted to general care.
18,46
Clinical manifestations of moderate-to-severe hypophosphatemia affect many organ systems and include
rhabdomyolysis, glucose intolerance, respiratory distress, and arrhythmias.
47
Hypophosphatemia is a common
electrolyte abnormality in patients who are malnourished, have an alcohol dependency, or are receiving
nutrition support.
14
2.1. Dosing recommendations for patients with normal renal function (>30 mL/min) are listed in Table 1 (UW
Health GRADE Moderate quality evidence, strong recommendation)
2.1.1. Order intravenous phosphate (sodium) or phosphate (potassium) boluses in millimole (mmol)
amounts of phosphate rounded to the nearest 7.5 mmol or 15 mmol increments. (UW Health
GRADE Low quality evidence, strong recommendation)
2.2. Supplementation of phosphate concentrations 2.5 to 3.0 mg/dL may be beneficial for patients at high risk
for developing hypophosphatemia (e.g. critical care, active alcohol abuse, malnourished patients).
Supplement with one phosphate-potassium packet or tablet every 4 hours while awake for 3 doses. (UW
Health GRADE Low quality evidence, strong recommendation)
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

10
2.3. Oral and Enteral Replacement, Administration, and Monitoring
2.3.1. If creatinine clearance is <30 mL/min, administer approximately 50% of normally recommended
doses. See potassium dosing information. Use caution in patients with anuria and patients with
ESRD receiving dialysis.
3,4
(UW Health GRADE Moderate quality evidence, strong
recommendation)
2.3.2. Patients with hyperkalemia, impaired renal function, or patients that require large doses of
phosphate replacement should receive replacement using the phosphorus tablet (K-PHOS
Neutral) as it contains less potassium than the phosphate-potassium packet (PHOS-NAK
powder). (UW Health GRADE Moderate quality evidence, strong recommendation)
2.3.3. Phosphate is poorly absorbed via the gastrointestinal tract. If oral/enteral phosphate repletion
does not increase serum phosphate, consider using intravenous repletion.
18
(UW Health GRADE
Low quality evidence, strong recommendation)
2.3.4. Oral/enteral phosphate supplementation can cause diarrhea. If this becomes problematic,
consider phosphate administration via the intravenous route.
18
(UW Health GRADE Low quality
evidence, strong recommendation)
2.3.5. Phosphate tablets or powder packets should be diluted in approximately 75 mL of water prior to
enteral tube administration (Nasogastric tube, Jejunostomy tube, Orogastric tube, or
Percutaneous Endoscopic Gastric tube).
36,37
(UW Health GRADE Moderate quality evidence,
strong recommendation)
2.4. Intravenous Replacement, Administration, and Monitoring
2.4.1. If creatinine clearance is <30 mL/min, administer 50% of the above dose. See potassium dosing
information. Use caution in patients with anuria and patients with ESRD receiving dialysis.
3,4
(UW
Health GRADE Moderate quality evidence, strong recommendation)
2.4.2. Use actual body weight unless patient’s actual weight is >130% ideal body weight (IBW), in which
case use ideal body weight for dose calculations.
14,47
(UW Health GRADE Moderate quality
evidence, strong recommendation)
2.4.3. The maximum intravenous dose of phosphate is 45 mmol based on weight-based dosing in Table
1. Recommend to re-check levels as listed in 2.4.6.
24
(UW Health GRADE Moderate quality
evidence, strong recommendation)
2.4.4. Administer total calculated dose over two to three hours for mild or moderate hypophosphatemia
and over four to six hours for severe hypophosphatemia.
14
(UW Health GRADE Moderate quality
evidence, strong recommendation)
2.4.5. For severe hypophosphatemia (phosphate concentrations less than 1 mg/dL), the first dose of
phosphate can be administered at a more aggressive rate of 7.5 mmol of phosphate/hour.
47
(UW
Health GRADE Moderate quality evidence, strong recommendation)
2.4.6. Since response to phosphate supplementation is not predictable, re-check levels six to twelve
hours post-supplementation.
28,46,48
(UW Health GRADE Moderate quality evidence, strong
recommendation)
3. Magnesium (Mg
2+
)
In comparison to potassium, magnesium is the second most abundant intracellular cation. Magnesium serves
as a cofactor for biochemical and adenosine triphosphatase reactions.
49,50
Magnesium homeostasis can be
affected by multiple factors which include: renal function, gastrointestinal function, and medication therapy (e.g.
diuretics).
18
Moderate to severe hypomagnesemia can result in numerous complications such as cardiac arrhythmias,
muscle weakness, and metabolic disturbances, including hypokalemia and hypocalcemia.
16
3.1. Symptomatic hypomagnesaemia should be promptly treated as a medication emergency with intravenous
magnesium supplementation.
16,18
(UW Health GRADE Moderate quality evidence, strong
recommendation)
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

11
3.2. Dosing recommendations for patients with normal renal function (CrCl >30 mL/min) are listed in Table 1.
(UW Health GRADE Moderate quality evidence, strong recommendation)
3.3. Current literature does not support supplementing normal electrolyte levels in adult patients who are not
classified as high risk, as defined in 3.5.9. (UW Health GRADE Low quality evidence, strong
recommendation)
3.4. Oral Magnesium Replacement, Administration, and Monitoring
3.4.1. In patients with creatinine clearance <30 mL/min, administer approximately 50% of normally
recommended doses.
3,4
(UW Health GRADE Moderate quality evidence, strong recommendation)
3.4.2. Take into consideration that serum concentrations may not accurately reflect total body stores
since magnesium is primarily an intracellular ion.
16
(UW Health GRADE High quality evidence,
strong recommendation)
3.4.3. Oral preparations are considered first-line for minor and asymptomatic hypomagnesemia.
31,32
(UW
Health GRADE Moderate quality evidence, strong recommendation)
3.4.4. The majority of oral magnesium-containing preparations can cause diarrhea, which is most
frequently associated with single doses greater than 250 mg of elemental magnesium. If diarrhea
is problematic, consider intravenous magnesium sulfate.
31,32
(UW Health GRADE High quality
evidence, strong recommendation)
3.4.5. Magnesium sulfate solution doses of 2000 mg should be diluted in approximately 50 mL of liquid
prior to enteral administration to increase tolerability.
36,37
(UW Health GRADE Moderate quality
evidence, strong recommendation)
3.4.6. Reassess magnesium concentration 36 to 48 hours after the final dose to allow for tissue
distribution.
16
(UW Health GRADE Moderate quality evidence, strong recommendation)
3.5. Intravenous Magnesium Replacement, Administration, and Monitoring
3.5.1. In patients with creatinine clearance <30 mL/min, administer approximately 50% of normally
recommended doses.
50
(UW Health GRADE Moderate quality evidence, strong recommendation)
3.5.2. Use actual body weight for calculations unless patient’s actual weight is >130% of ideal body
weight. In these patients, use ideal body weight for dose calculations.
50
(UW Health GRADE
Moderate quality evidence, strong recommendation)
3.5.3. Take into consideration that blood concentrations may not accurately reflect total body stores
since magnesium is primarily an intracellular ion.
16
(UW Health GRADE Moderate quality
evidence, strong recommendation)
3.5.4. For treatment of hypomagnesemia without symptoms the optimal rate of administration is over 12
hours for supplements of ≤0.05 g/kg or 24 hours for supplements >0.05 g/kg. If medically
necessary supplemental doses can be administered over four to six hours.
16,50
(UW Health
GRADE Moderate quality evidence, strong recommendation)
3.5.5. For a severely low magnesium concentration (<1 mg/dL) and/or symptomatic patient (tetany or
seizures), administer a bolus (1 to 2 g) over 10 minutes, then administer the remaining calculated
dose as separate infusion(s) over 12 to 24 hours.
16,50
(UW Health GRADE Moderate quality
evidence, strong recommendation)
3.5.6. If medically necessary, the infusion rate can be increased to a maximum of 0.5 (preferred) to 1
g/hr. Infusing magnesium over a shorter time period is less effective as faster rates may result in
much of the dose being excreted.
49,51
(UW Health GRADE Moderate quality evidence, strong
recommendation)
3.5.7. Administer the total magnesium dose as multiple of 1 or 2 g doses.
16,49
(UW Health GRADE
moderate quality evidence, strong recommendation)
3.5.8. Consider a maximum daily dose of 8 g of magnesium.
52,53
(UW Health GRADE Low quality
evidence, strong recommendation)
3.5.9. Consider not repleting magnesium levels of ≥1.5 mg/dL except in patients admitted on cardiac
units, in patients with recent cardiac surgery, in patients with cardiac disorders (including
arrhythmias, prolonged QTc, digitalis toxicity) or in patients with eclampsia or pre-
eclampsia.
51,52,54-57
(UW Health GRADE Moderate quality evidence, strong recommendation)
3.5.10. Administration: Life-threatening Emergencies
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

12
3.5.10.1. When ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest is associated
with torsades de pointes, administer IV bolus magnesium 1 to 2 g over one to two
minutes (central line preferred).
58
(UW Health GRADE Low quality evidence,
weak/conditional recommendation)
3.5.10.2. For the treatment of polymorphic ventricular tachycardia with a prolonged QT interval,
administer IV magnesium 1 to 2 g over 15 minutes.
58
(UW Health GRADE Low quality
evidence, weak/conditional recommendation)
3.5.10.3. For the treatment of adult status asthmaticus administer single 2 g dose over 20
minutes.
59
(UW Health GRADE Low quality evidence, strong recommendation)
3.5.11. Reassess magnesium concentration 36 to 48 hours after the final dose to allow for tissue
distribution.
16
(UW Health GRADE Moderate quality evidence, strong recommendation)
3.5.12. Even if level is checked during next lab draw, a follow-up lab should be ordered (36 to 48 hours)
post-infusion to allow for complete magnesium distribution and avoid “falsely” high concentrations.
(UW Health GRADE Very low quality evidence, strong recommendation)
4. Calcium (Ca
2+
)
Calcium is primarily found within the bones (99%) versus serum (1%). Within the serum, calcium (~40%) is
primarily bound to albumin or other proteins and is not active. Therefore, in patients with low albumin levels,
total calcium levels do not reflect active calcium.
19
Ionized, unbound calcium is the active form of calcium within the blood. Direct measurement of ionized
calcium is preferred since its levels are not affected by albumin.
18
Untreated hypocalcemia can lead to serious neurologic and cardiovascular complications.
20,38
In addition to
supplementing calcium, it is also important to identify and correct any underlying causes of hypocalcemia (e.g.,
hypomagnesemia, decreased vitamin D concentrations, hypoparathyroidism, hyperphosphatemia).
20,38,60,61
4.1. Dosing recommendations for patients with normal renal function (>30 mL/min) are listed in Table 1. (UW
Health GRADE Moderate quality evidence, strong recommendation)
4.2. Indications for IV calcium supplementation for hypocalcemia:
20,38,60,61
(UW Health GRADE Moderate
quality evidence, strong recommendation)
Patient is symptomatic (muscle tetany, paresthesias)
Clinically relevant ionized calcium concentration (whole blood Ca ≤4.89 mg/dL or serum blood Ca
≤4.59 mg/dL)
Calcium channel blocker overdose
Massive blood transfusion (≥5 units of packed red blood cells)
Patient is receiving inotropic/vasopressor support
Cardiac protection when treating hyperkalemia
Prevention of worsening hypocalcemia
4.3. Oral Calcium Replacement, Administration, and Monitoring
4.3.1. Oral calcium supplementation may be safely used in patients with impaired renal function,
hyperphosphatemia, and underlying cardiac dysrhythmias including patients receiving CVVH.
Serum calcium levels should be closely monitored in these patients due to their underlying
conditions.
19,38,60
(UW Health GRADE Moderate quality evidence, strong recommendation)
4.3.2. Ionized (unbound) calcium concentrations, which measure the unbound, active form of calcium,
are preferred over total serum calcium concentrations for monitoring.
19
(UW Health GRADE
Moderate quality evidence, strong recommendation)
4.3.3. “Corrected” total serum calcium concentrations for serum albumin <4 mg/dL (“corrected” total
calcium = measured serum calcium + (4 – measured serum albumin)*(0.8)) often overestimate
total serum calcium concentrations and should not be used to estimate the severity of
hypocalcemia.
20,60
(UW Health GRADE Moderate quality evidence, strong recommendation)
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

13
4.3.4. In order to maximize absorption, oral calcium carbonate should be administered in doses of 500
mg or fewer and with food.
62,63
(UW Health GRADE Moderate quality evidence, strong
recommendation)
4.3.5. Monitor ionized calcium concentration in patients that are hypoalbuminemic. Approximately 40%
of calcium is bound to protein (primarily albumin) and hypoalbuminemia can cause low total
calcium concentrations, while ionized calcium concentrations remain within normal limits.
20
(UW
Health GRADE Moderate quality evidence, strong recommendation)
4.3.6. Monitor ionized calcium concentration in patients with increases in extracellular fluid
concentrations of phosphate, citrate, or bicarbonate. These changes increase the proportion of
bound calcium and decrease ionized calcium. Similarly, alkalosis increases bound calcium and
decreases concentrations of ionized calcium.
19,57,62-64
(UW Health GRADE Moderate quality
evidence, strong recommendation)
4.4. Intravenous Calcium Replacement, Administration, and Monitoring
4.4.1. Intravenous calcium may be safely used in patients with impaired renal function (including
patients receiving CVVH), hyperphosphatemia, and underlying cardiac dysrhythmias. Blood
calcium levels should be closely monitored in these patients due to their underlying conditions.
60,61
(UW Health GRADE Moderate quality evidence, strong recommendation)
4.4.2. During cardiac resuscitation efforts (including severe hypocalcemia, hyperkalemia, and/or calcium
channel blocker and beta blocker overdose), ACLS guidelines recommend calcium chloride as the
preferred agent due to the higher concentration of elemental calcium and faster calcium
repletion.
60,61
(UW Health GRADE Moderate quality evidence, strong recommendation)
4.4.3. Due to less venous irritation and less risk of metabolic acidosis, calcium gluconate is the preferred
intravenous salt for replacement.
61
(UW Health GRADE Moderate quality evidence, strong
recommendation)
4.4.3.1. Since calcium gluconate is the preferred intravenous agent, calcium chloride should only
be used if a shortage of calcium gluconate arises.
4.4.3.2. Due to the risk for extravasation with IV calcium chloride administration, central line
administration is preferred; however peripheral lines may be used when benefit
outweighs the risk. (UW Health GRADE Moderate quality evidence, strong
recommendation)
4.4.4. In patients with severe, symptomatic hypocalcemia, a 1 to 2 g bolus of intravenous calcium
gluconate may be given over 10 to 20 minutes followed by a longer infusion over 2 to 4 hours.
61
(UW Health GRADE Moderate quality evidence, strong recommendation)
4.4.5. Administration over a shorter period of time (fewer than 10 minutes), can increase the risk of
cardiac toxicities.
61
(UW Health GRADE Moderate quality evidence, strong recommendation)
4.4.6. Due to the risk of serious arrhythmias or cardiovascular collapse in digitalized patients,
administration of intravenous calcium should be given slowly over several hours and monitored
with telemetry.
38,61
(UW Health GRADE Moderate quality evidence, weak/conditional
recommendation)
4.4.7. Administering intravenous calcium concurrently with a calcium channel blocker can inhibit the
pharmacologic effect of the blocker.
60,61
(UW Health GRADE Moderate quality evidence, strong
recommendation)
4.4.8. Ionized (unbound) calcium concentrations, which measure the unbound, active form of calcium,
are preferred over total calcium concentrations for monitoring.
61
(UW Health GRADE Moderate
quality evidence, strong recommendation)
4.4.9. Check ionized calcium concentrations at least 10 hours after the completion of a calcium infusion
to assess efficacy of therapy.
60
(UW Health GRADE Moderate quality evidence, strong
recommendation)
4.4.10. Since approximately 40% of calcium is bound to protein (primarily albumin), hypoalbuminemia can
cause total calcium to appear lower than it actually is.
20
(UW Health GRADE Moderate quality
evidence, strong recommendation)
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

14
4.4.10.1. Ionized (unbound) calcium concentrations, which measure the unbound, active form of
calcium, are preferred over total serum calcium concentrations for monitoring.
19
(UW
Health GRADE Moderate quality evidence, strong recommendation)
4.4.10.1.1. Monitor ionized calcium levels in patients with increases in extracellular fluid
concentrations of phosphate, citrate, or bicarbonate. These changes
increase the proportion of bound calcium and decrease ionized calcium.
Similarly, alkalosis increases bound calcium and decreases concentrations
of ionized calcium.
61
(UW Health GRADE Moderate quality evidence, strong
recommendation)
4.4.10.2. “Corrected” total serum calcium concentrations for serum albumin < 4 mg/dL (“corrected”
total calcium = measured serum calcium + (4 – measured serum albumin)*(0.8)) often
overestimate total serum calcium concentrations and should not be used to estimate the
severity of hypocalcemia.
20,60
(UW Health GRADE Moderate quality evidence, strong
recommendation)
5. Sliding Scale Electrolytes
5.1 Due to staffing, timing of lab draws, and diverse patient populations, sliding scale electrolyte
supplementation is not safe in all patients (UW Health GRADE Low quality evidence, strong
recommendation)
5.2 Both intravenous and oral sliding scale electrolyte replacement are only acceptable in ICU and IMC
status patients on B4/3, B4/5, B6N3, B6S3, D4/5, D6/5, F4/4, F4M5, and F8/4. Additionally, general care
status patients on cardiology units (B4/5, D4/5, F4M5, F4/5) may receive sliding scale electrolytes. (UW
Health GRADE Very low quality evidence, strong recommendation)
5.3 Electrolyte replacement may not be applicable for patients on renal replacement therapy. (UW Health
GRADE Very low quality evidence, strong recommendation)
Disclaimer
Clinical practice guidelines assist clinicians by providing a framework for the evaluation and treatment of
patients. This guideline outlines the preferred approach for most patients. It is not intended to replace a
clinician’s judgment or to establish a protocol for all patients. It is understood that some patients will not fit the
clinical condition contemplated by a guideline and that a guideline will rarely establish the only appropriate
approach to a problem.
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

15
Methodology
Development Process
Each guideline is reviewed and updated a minimum of every 3 years. All guidelines are developed using the
guiding principles, standard processes, and styling outlined in the UW Health Clinical Practice Guideline
Resource Guide. This includes expectations for workgroup composition and recruitment strategies, disclosure
and management of conflict of interest for participating workgroup members, literature review techniques,
evidence grading resources, required approval bodies, and suggestions for communication and
implementation.
Methods Used to Collect the Evidence:
The following criteria were used by the guideline author(s) and workgroup members to conduct electronic
database searches in the collection of evidence for review.
Literature Sources:
PubMed
Cochrane Review
International Pharmaceutical Abstracts
Time Period: Through 2017
Search Terms:
“Intravenous electrolyte administration”
“Intravenous electrolyte replacement”
“Potassium repletion”
“Magnesium intravenous supplementation”
“Calcium intravenous repletion”
“Intravenous phosphate replacement”
“Intravenous electrolyte supplementation for high risk patients”
“Electrolyte repletion cardiac”
“Electrolyte repletion burn”
“Electrolyte repletion”
“Supplementation hepatectomy”
“Oral or enteral electrolyte administration”
“Oral or enteral electrolyte replacement”
Methods to Select the Evidence:
In addition to electronic database searches, literature searches were extended to reviews and studies
conducted in humans and published in English. Reference lists of relevant studies were also reviewed.
Methods Used to Formulate the Recommendations:
The workgroup members agreed to adopt recommendations developed by external organizations and/or
created recommendations internally via a consensus process using discussion of the literature and expert
experience/opinion. If issues or controversies arose where consensus could not be reached, the topic was
escalated appropriately per the guiding principles outlined in the UW Health Clinical Practice Guideline
Resource Guide.
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

16
Methods Used to Assess the Quality of the Evidence/Strength of the Recommendations:
Recommendations developed by external organizations maintained the evidence grade assigned within the
original source document and were adopted for use at UW Health.
Internally developed recommendations, or those adopted from external sources without an assigned evidence
grade, were evaluated by the guideline workgroup using an algorithm adapted from the Grading of
Recommendations Assessment, Development and Evaluation (GRADE) methodology (see Figure 1).
Figure 1. GRADE Methodology adapted by UW Health
Rating Scheme for the Strength of the Evidence/Recommendations:
GRADE Ranking of Evidence
High
We are confident that the effect in the study reflects the actual effect.
Moderate
We are quite confident that the effect in the study is close to the true effect, but it
is also possible it is substantially different.
Low
The true effect may differ significantly from the estimate.
Very Low
The true effect is likely to be substantially different from the estimated effect.
GRADE Ratings for Recommendations For or Against Practice
Strong
The net benefit of the treatment is clear, patient values and circumstances
are unlikely to affect the decision.
Weak/conditional
Recommendation may be conditional upon patient values and
preferences, the resources available, or the setting in which the
intervention will be implemented.
Recognition of Potential Health Care Disparities: None identified
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

17
Collateral Tools & Resources
The following collateral tools and resources support staff execution and performance of the evidence-based
guideline recommendations in everyday clinical practice.
Metrics
Assess appropriateness of oral, enteral, and IV supplementation based on patient lab results and
appropriate documentation
Associated Guidelines
UW Health – Intravenous Administration of Formulary Medications – Adult – Inpatient/Ambulatory
UW Health Management Extravasation of Non-Chemotherapeutic Agents – Adult/Pediatric –
Inpatient/Ambulatory
Order Sets & Smart Sets
IP – Cardiac Surgery – Electrolyte Supplementation – Adult – Supplemental [3534]
IP – Electrolyte Supplementation – Adult – ICU/IMC Supplemental [3439]
Protocols
Nutrition Support Care – Adult/Pediatric/Neonatal – Inpatient/Ambulatory [6]
References
1. Freedman BI, Burkart JM. Endocrine crises. Hypokalemia. Crit Care Clin. Jan 1991;7(1):143-153.
2. Kruse JA, Clark VL, Carlson RW, Geheb MA. Concentrated potassium chloride infusions in critically ill patients
with hypokalemia. J Clin Pharmacol. Nov 1994;34(11):1077-1082.
3. Kruse JA, Carlson RW. Rapid correction of hypokalemia using concentrated intravenous potassium chloride
infusions. Arch Intern Med. Mar 1990;150(3):613-617.
4. Hamill RJ, Robinson LM, Wexler HR, Moote C. Efficacy and safety of potassium infusion therapy in hypokalemic
critically ill patients. Crit Care Med. May 1991;19(5):694-699.
5. Couture J, Letourneau A, Dubuc A, Williamson D. Evaluation of an electrolyte repletion protocol for cardiac
surgery intensive care patients. The Canadian journal of hospital pharmacy. Mar 2013;66(2):96-103.
6. Rhoda KM, Porter MJ, Quintini C. Fluid and electrolyte management: putting a plan in motion. JPEN J Parenter
Enteral Nutr. Nov 2011;35(6):675-685.
7. Choi SH, Kwon TG, Kim TH. Active potassium supplementation might be mandatory during laparoscopic
adrenalectomy for primary hyperaldosteronism. J Endourol. Jun 2012;26(6):666-669.
8. Polderman KH, Girbes AR. Severe electrolyte disorders following cardiac surgery: a prospective controlled
observational study. Crit Care. Dec 2004;8(6):R459-466.
9. Hollifield JW, Slaton PE. Thiazide diuretics, hypokalemia and cardiac arrhythmias. Acta Med Scand Suppl.
1981;647:67-73.
10. Scotto CJ, Fridline M, Menhart CJ, Klions HA. Preventing hypokalemia in critically ill patients. Am J Crit Care. Mar
2014;23(2):145-149.
11. Sanghavi S, Whiting S, Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013 Sep-Oct
2013;26(5):597-603.
12. Bielecka-Dabrowa A, Mikhailidis DP, Jones L, Rysz J, Aronow WS, Banach M. The meaning of hypokalemia in
heart failure. Int J Cardiol. Jun 2012;158(1):12-17.
13. Khow KS, Lau SY, Li JY, Yong TY. Diuretic-associated electrolyte disorders in the elderly: risk factors, impact,
management and prevention. Curr Drug Saf. Mar 2014;9(1):2-15.
14. Clark CL, Sacks GS, Dickerson RN, Kudsk KA, Brown RO. Treatment of hypophosphatemia in patients receiving
specialized nutrition support using a graduated dosing scheme: results from a prospective clinical trial. Crit Care
Med. Sep 1995;23(9):1504-1511.
15. Brown KA, Dickerson RN, Morgan LM, Alexander KH, Minard G, Brown RO. A new graduated dosing regimen for
phosphorus replacement in patients receiving nutrition support. JPEN J Parenter Enteral Nutr. 2006 May-Jun
2006;30(3):209-214.
16. Sacks GS, Brown RO, Dickerson RN, et al. Mononuclear blood cell magnesium content and serum magnesium
concentration in critically ill hypomagnesemic patients after replacement therapy. Nutrition. Apr 1997;13(4):303-
308.
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

17. al-Ghamdi SM, Cameron EC, Sutton RA. Magnesium deficiency: pathophysiologic and clinical overview. Am J
Kidney Dis. Nov 1994;24(5):737-752.
18. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive
care unit. Am J Health Syst Pharm. Aug 2005;62(16):1663-1682.
19. Dickerson RN, Morgan LG, Cauthen AD, et al. Treatment of acute hypocalcemia in critically ill multiple-trauma
patients. JPEN J Parenter Enteral Nutr. 2005 Nov-Dec 2005;29(6):436-441.
20. Dickerson RN, Alexander KH, Minard G, Croce MA, Brown RO. Accuracy of methods to estimate ionized and
"corrected" serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition
support. JPEN J Parenter Enteral Nutr. 2004 May-Jun 2004;28(3):133-141.
21. Dickerson RN. Treatment of hypocalcemia in critical illness--part 2. Nutrition. May 2007;23(5):436-437.
22. Felsenfeld AJ, Levine BS. Approach to treatment of hypophosphatemia. Am J Kidney Dis. Oct 2012;60(4):655-
661.
23. Amanzadeh J, Reilly RF. Hypophosphatemia: an evidence-based approach to its clinical consequences and
management. Nat Clin Pract Nephrol. Mar 2006;2(3):136-148.
24. Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. Treatment of hypophosphatemia in the
intensive care unit: a review. Crit Care. 2010;14(4):R147.
25. Yoshimatsu S, Chisti MJ, Hossain MI, et al. Hypophosphataemia among severely-malnourished children: case
series. J Health Popul Nutr. Dec 2012;30(4):491-494.
26. Kim YJ, Kim MG, Jeon HJ, et al. Clinical manifestations of hypercalcemia and hypophosphatemia after kidney
transplantation. Transplant Proc. Apr 2012;44(3):651-656.
27. Yang HT, Yim H, Cho YS, et al. Change of serum phosphate level and clinical outcome of hypophosphatemia in
massive burn patient. J Trauma Acute Care Surg. Nov 2012;73(5):1298-1302.
28. Liamis G, Milionis HJ, Elisaf M. Medication-induced hypophosphatemia: a review. QJM. Jul 2010;103(7):449-459.
29. Schwartz A, Brotfain E, Koyfman L, et al. Association between Hypophosphatemia and Cardiac Arrhythmias in
the Early Stage of Sepsis: Could Phosphorus Replacement Treatment Reduce the Incidence of Arrhythmias?
Electrolyte Blood Press. Jun 2014;12(1):19-25.
30. Buell JF, Berger AC, Plotkin JS, Kuo PC, Johnson LB. The clinical implications of hypophosphatemia following
major hepatic resection or cryosurgery. Arch Surg. Jul 1998;133(7):757-761.
31. Agus ZS. Hypomagnesemia. J Am Soc Nephrol. Jul 1999;10(7):1616-1622.
32. Weisinger JR, Bellorín-Font E. Magnesium and phosphorus. Lancet. Aug 1998;352(9125):391-396.
33. Dickerson RN, Morgan LM, Croce MA, Minard G, Brown RO. Dose-dependent characteristics of intravenous
calcium therapy for hypocalcemic critically ill trauma patients receiving specialized nutritional support. Nutrition.
Jan 2007;23(1):9-15.
34. Fong J, Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician. Feb
2012;58(2):158-162.
35. Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. May 2014;32(2):329-347.
36. Niemiec PW, Vanderveen TW, Morrison JI, Hohenwarter MW. Gastrointestinal disorders caused by medication
and electrolyte solution osmolality during enteral nutrition. JPEN J Parenter Enteral Nutr. 1983 Jul-Aug
1983;7(4):387-389.
37. Dickerson RN, Melnik G. Osmolality of oral drug solutions and suspensions. Am J Hosp Pharm. Apr
1988;45(4):832-834.
38. Dickerson RN. Treatment of hypocalcemia in critical illness--part 2. Nutrition. Vol 23. United States2007:436-437.
39. Sterns RH, Cox M, Feig PU, Singer I. Internal potassium balance and the control of the plasma potassium
concentration. Medicine (Baltimore). Sep 1981;60(5):339-354.
40. Gervasio JM, Garmon WP, Holowatyj MR. Nutrition Support in Acute Kidney Injury. Nutrition in Clinical Practice.
2011;26(4):374-381.
41. Wahr JA, Parks R, Boisvert D, et al. Preoperative serum potassium levels and perioperative outcomes in cardiac
surgery patients. Multicenter Study of Perioperative Ischemia Research Group. JAMA. Vol 281. United
States1999:2203-2210.
42. Pucino F, Danielson BD, Carlson JD, et al. Patient tolerance to intravenous potassium chloride with and without
lidocaine. Drug Intell Clin Pharm. Sep 1988;22(9):676-679.
43. Morrill GB, Katz MD. The use of lidocaine to reduce the pain induced by potassium chloride infusion. J Intraven
Nurs. 1988 Mar-Apr 1988;11(2):105-108.
44. Lim ET, Khoo ST, Tweed WA, Kloo ST. Efficacy of lignocaine in alleviating potassium chloride infusion pain.
Anaesth Intensive Care. May 1992;20(2):196-198.
45. Safety issues with adding lidocaine to IV potassium infusions ISMP Medication Safety Alert. 2004.
46. Lentz RD, Brown DM, Kjellstrand CM. Treatment of severe hypophosphatemia. Ann Intern Med. Dec
1978;89(6):941-944.
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017

47. Brown KA, Dickerson RN, Morgan LM, Alexander KH, Minard G, Brown RO. A new graduated dosing regimen for
phosphorus replacement in patients receiving nutrition support. JPEN J Parenter Enteral Nutr. Vol 30. United
States2006:209-214.
48. Halevy J, Bulvik S. Severe hypophosphatemia in hospitalized patients. Arch Intern Med. Jan 1988;148(1):153-
155.
49. Rude RK, Bethune JE, Singer FR. Renal tubular maximum for magnesium in normal, hyperparathyroid, and
hypoparathyroid man. J Clin Endocrinol Metab. Dec 1980;51(6):1425-1431.
50. al-Ghamdi SM, Cameron EC, Sutton RA. Magnesium deficiency: pathophysiologic and clinical overview. Am J
Kidney Dis. Vol 24. United States1994:737-752.
51. Rude RK, Ryzen E. TmMg and renal Mg threshold in normal man and in certain pathophysiologic conditions.
Magnesium. 1986;5(5-6):273-281.
52. Efstratiadis G, Sarigianni M, Gougourelas I. Hypomagnesemia and cardiovascular system. Hippokratia. Oct
2006;10(4):147-152.
53. Magnesium sulfate. Lexi-Comp Online TM , Hudson, Ohio: Lexi-Comp, Inc.; November 1, 2017.
54. Geiger H, Wanner C. Magnesium in disease. Clin Kidney J. Feb 2012;5(Suppl 1):i25-i38.
55. Gröber U, Schmidt J, Kisters K. Magnesium in Prevention and Therapy. Nutrients. Sep 2015;7(9):8199-8226.
56. Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. Feb 2012;5(Suppl 1):i3-i14.
57. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with
ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of
Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for
Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular
Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. Sep 2006;48(5):e247-346.
58. Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart.
Circulation. Nov 2 2010;122(18 Suppl 3):S729-767.
59. Janson S. National Asthma Education and Prevention Program, Expert Panel Report. II: Overview and application
to primary care. Lippincotts Prim Care Pract. 1998 Nov-Dec 1998;2(6):578-588.
60. Dickerson RN, Morgan LM, Croce MA, Minard G, Brown RO. Dose-dependent characteristics of intravenous
calcium therapy for hypocalcemic critically ill trauma patients receiving specialized nutritional support. Nutrition.
Vol 23. United States2007:9-15.
61. Dickerson RN, Morgan LG, Cauthen AD, et al. Treatment of acute hypocalcemia in critically ill multiple-trauma
patients. JPEN J Parenter Enteral Nutr. Vol 29. United States2005:436-441.
62. Harvey JA, Zobitz MM, Pak CY. Dose dependency of calcium absorption: a comparison of calcium carbonate and
calcium citrate. J Bone Miner Res. Jun 1988;3(3):253-258.
63. Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin
Pract. Jun 2007;22(3):286-296.
64. Dickerson RN. Treatment of hypocalcemia in critical illness--part 1. Nutrition. Apr 2007;23(4):358-361.
Copyright © 2017 University of Wisconsin Hospitals and Clinics Authority
Contact: Lee Vermeulen, CCKM@uwhealth.org Last Revised:
12/2017
